Abstract
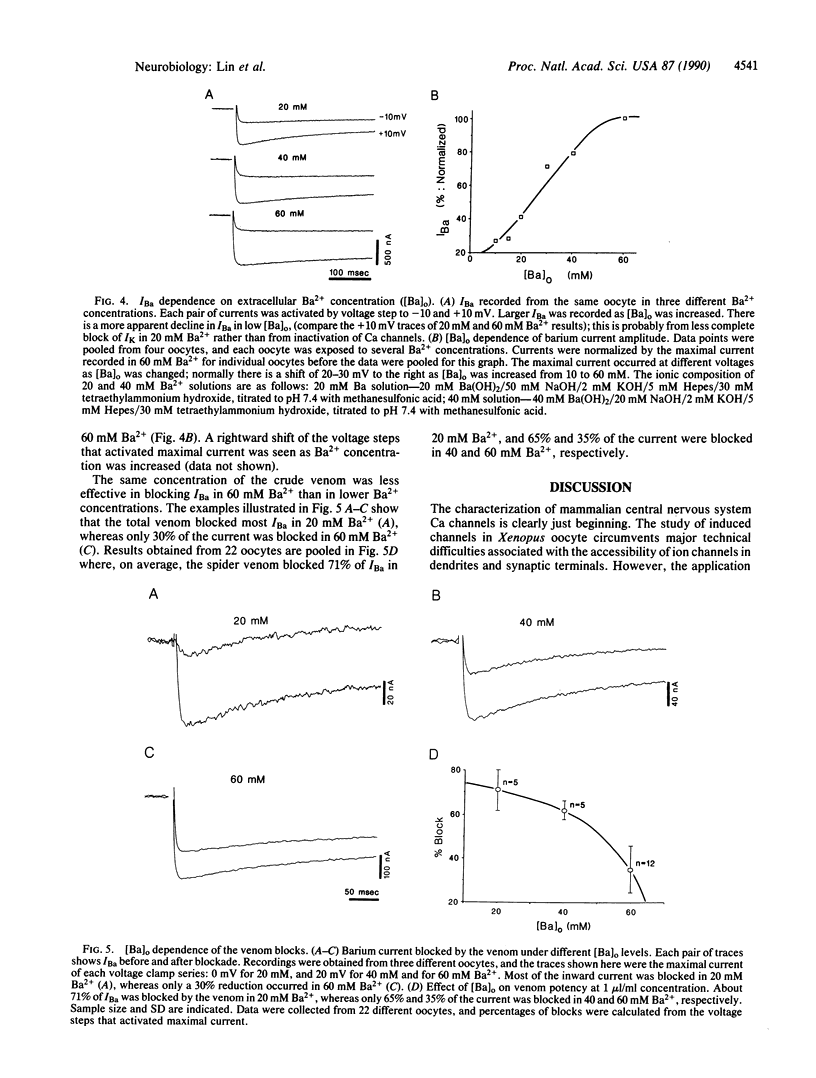
Injection of rat brain mRNA into Xenopus oocytes has been shown to induce a calcium current (ICa) that is insensitive to dihydropyridine and omega-conotoxin. We examined the effect of funnel-web spider venom on two aspects of this expressed ICa: (i) the calcium-activated chloride current [ICl(Ca)] and (ii) the currents carried by barium ions through calcium channels (IBa). In the presence of 1.8 mM extracellular calcium, ICl(Ca) tail current became detectable between -30 and -40 mV from a holding potential of -80 mV and reached a maximal amplitude between 0 and +10 mV. Total spider venom partially (83%) and reversibly blocked the calcium-activated chloride current without changing its voltage sensitivity. A chromatographic toxin fraction from the venom also blocked this current (64%). The venom had a minimal effect on INa and IK. Direct investigation of inward current mediated by calcium channels was carried out in high-barium solution. IBa had a higher threshold of activation (-30 to -20 mV) and reached its maximal amplitude at about +20 mV. Total venom or a partly purified chromatographic toxic fraction blocked IBa partially and reversibly without changing its current-voltage characteristics. Furthermore, the extent of the total venom block depended on the concentration of extracellular barium. Only 35% of the IBa was blocked in 60 mM Ba2+, whereas the block increased to 65% and 71%, respectively, for 40 and 20 mM Ba2+. On the basis of these results, we propose that the calcium channels expressed from rat brain mRNA in Xenopus oocytes is similar to the recently discovered P-type channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. S., MacKinnon R., Smith C., Miller C. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol. 1988 Mar;91(3):317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner D., Pitler T. A., Alger B. E. Protein kinase C activators block specific calcium and potassium current components in isolated hippocampal neurons. J Neurosci. 1988 Nov;8(11):4069–4078. doi: 10.1523/JNEUROSCI.08-11-04069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Intracellular Ca2+ activates phospholipase C. Trends Neurosci. 1988 Dec;11(12):517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Umbach J. A., Swartz B. E. Barbiturates depress currents through human brain calcium channels studied in Xenopus oocytes. J Pharmacol Exp Ther. 1988 Dec;247(3):824–829. [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Nomura Y. Cyclic AMP facilitates slow-inactivating Ca2+ channel currents expressed by Xenopus oocyte after injection of rat brain mRNA. Neurosci Lett. 1987 Dec 16;83(1-2):123–127. doi: 10.1016/0304-3940(87)90227-8. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H. T., Branton W. D., Phillips H. S., Jan L., Byerly L. Spider toxins selectively block calcium currents in Drosophila. Neuron. 1989 Dec;3(6):767–772. doi: 10.1016/0896-6273(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Murawsky M. M., Shi M. Further characterization of phasic calcium influx in rat cerebrocortical synaptosomes: inferences regarding calcium channel type(s) in nerve endings. J Neurochem. 1989 Apr;52(4):1260–1269. doi: 10.1111/j.1471-4159.1989.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., O'Leary M. E., Murawsky M. M., Wang T. Presynaptic calcium channels in rat cortical synaptosomes: fast-kinetics of phasic calcium influx, channel inactivation, and relationship to nitrendipine receptors. J Neurosci. 1986 May;6(5):1349–1357. doi: 10.1523/JNEUROSCI.06-05-01349.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. J., Rezazadeh S. M., Leslie S. W. Differential sensitivity of synaptosomal calcium entry and endogenous dopamine release to omega-conotoxin. Brain Res. 1988 Dec 13;475(1):141–145. doi: 10.1016/0006-8993(88)90207-7. [DOI] [PubMed] [Google Scholar]


