Abstract
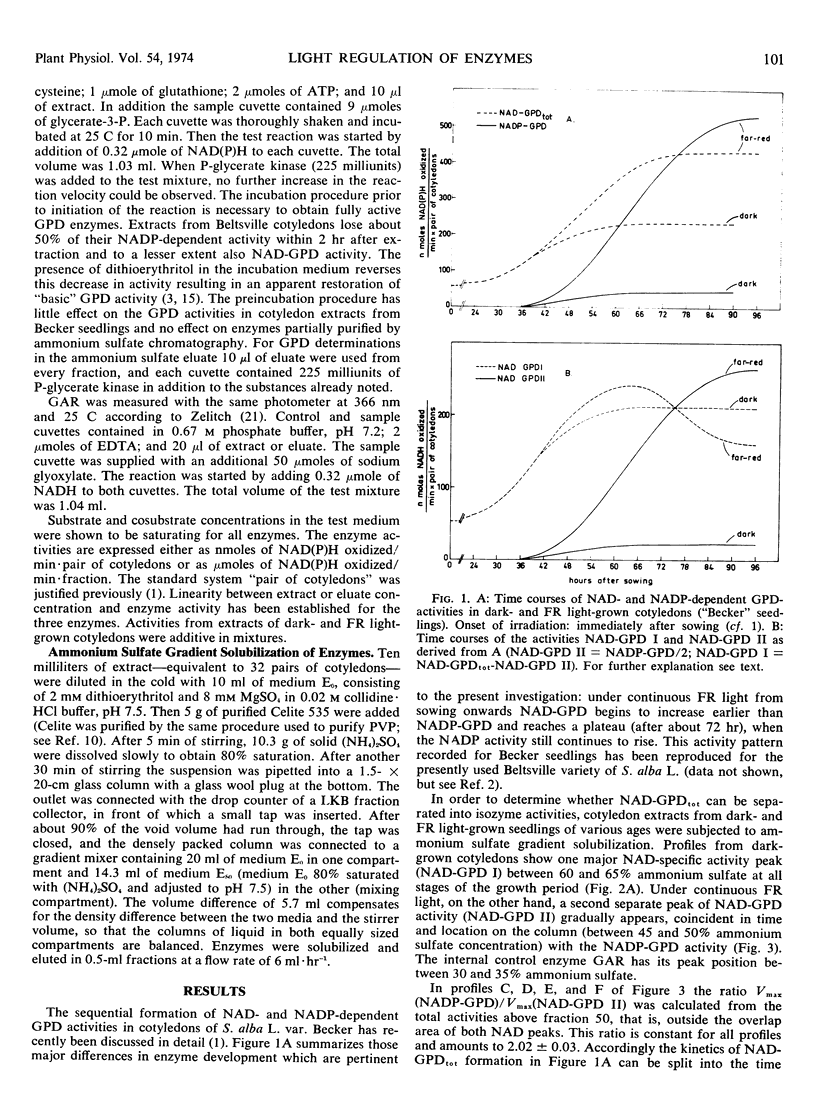
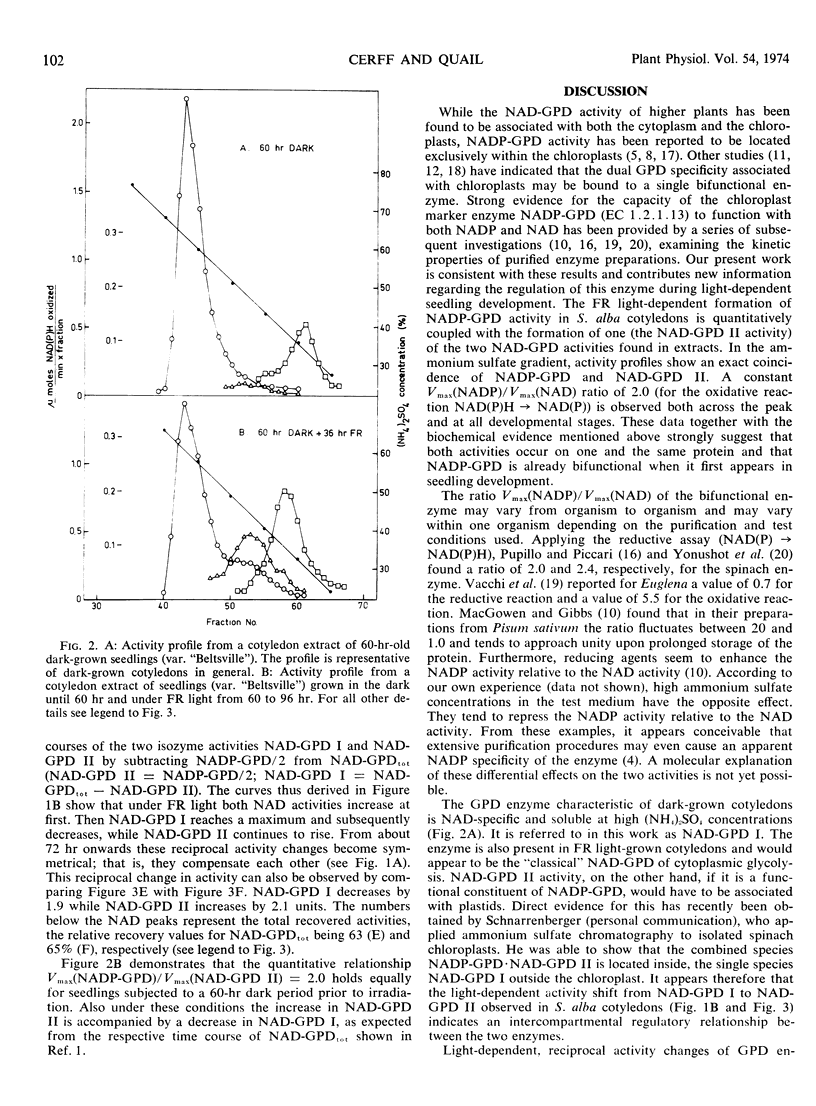
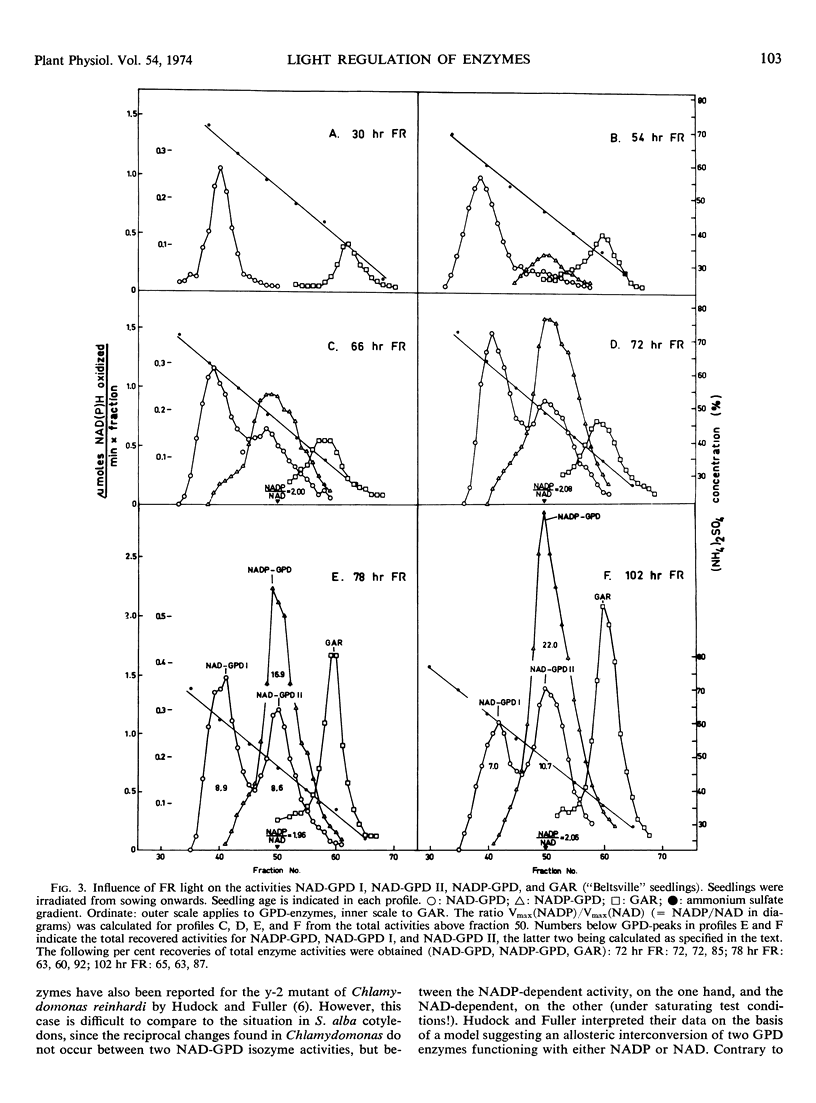
Ammonium sulfate chromatography has been employed to separate glyceraldehyde 3-phosphate dehydrogenases (GPD) of Sinapis alba cotyledons of various developmental stages. Cotyledons of dark-grown seedlings possess one major NAD-specific enzyme designated NAD-GPD I. Irradiation with continuous far red light leads to a strong increase in NADP-GPD activity and to the formation of a second NAD activity designated NAD-GPD II. These two activities occur in a constant ratio during cotyledon development, and they are eluted together in ammonium sulfate chromatography. In a later stage of cotyledon development the light-dependent increase in NAD-GPD II is matched by an equivalent decrease in NAD-GPD I. These data suggest that the chloroplast marker enzyme NADP-GPD (EC 1.2.1.13) also has NAD activity and that the light-dependent formation of this bifunctional enzyme is correlated with activity changes of the NAD-GPD of cytoplasmic glycolysis (EC 1.2.1.12).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Cerff R. Glyceraldehyde 3-Phosphate Dehydrogenases and Glyoxylate Reductase: I. Their Regulation Under Continuous Red and Far Red Light in the Cotyledons of Sinapis alba L. Plant Physiol. 1973 Jan;51(1):76–81. doi: 10.1104/pp.51.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudock G. A., Fuller R. C. Control of Triosephosphate Dehydrogenase in Photosynthesis. Plant Physiol. 1965 Nov;40(6):1205–1211. doi: 10.1104/pp.40.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melandri B. A., Baccarini A., Pupillo P. Glyceraldehyde-3-phosphate dehydrogenase in photosynthetic tissues: kinetic evidence for competitivity between NADP and NAD. Biochem Biophys Res Commun. 1968 Oct 10;33(1):160–164. doi: 10.1016/0006-291x(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Pupillo P., Baccarini-Melandri A. D-glyceraldehyde-3-phosphate dehydrogenase in photosynthetic cells. I. The reversible light-induced activation in vivo of NADP-dependent enzyme and its relationship to NAD-dependent activities. Biochim Biophys Acta. 1970 Nov 11;220(2):178–189. doi: 10.1016/0005-2744(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Müller B., Ziegler I., Ziegler H. Lichtinduzierte, reversible Aktivtätssteigerung der NADP-abhängigen Glycerinaldehyd-3-phosphat-Dehydrogenase in Chloroplasten. Zum mechanismus der reaktion. Eur J Biochem. 1969 May 1;9(1):101–106. doi: 10.1111/j.1432-1033.1969.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Schulman M. D., Gibbs M. D-glyceraldehyde 3-phosphate dehydrogenases of higher plants. Plant Physiol. 1968 Nov;43(11):1805–1812. doi: 10.1104/pp.43.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonuschot G. R., Ortwerth B. J., Koeppe O. J. Purification and properties of a nicotinamide adenine dinucleotide phosphate-requiring glyceraldehyde 3-phosphate dehydrogenase from spinach leaves. J Biol Chem. 1970 Aug 25;245(16):4193–4198. [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]


