Abstract
Present study was under taken to predict the possible DNA damages (genotoxicity) and carcinogenicity caused by radiofrequency radiations (RF) to living tissue. Dry seeds of chickpea were treated with GSM cell phone (900 MHz) and laptop (3.31 GHz) as RF source for 24 and 48 h. Untreated seeds were used as (0 h) negative control and Gamma rays (250 Gray) as positive control. Plant chromosomal aberration assay was used as genotoxicity marker. All the treatment of RF inhibits seed germination percentage. 48 h laptop treatment has the most negative effect as compared to untreated control. A decrease was observed in mitotic index (M.I) and increase in abnormality index (A.I) with the increase in exposure duration and frequency in (Hz). Cell membrane damages were also observed only in 48 h exposure of cell phone and laptop (RF). Maximum nuclear membrane damages and ghost cells were again recorded in 48 h exposure of cell phone and laptop. The radiofrequency radiations (900 MHz and 3.31 GHz) are only genotoxic as they induce micronuclei, bi-nuclei, multi-nuclei and scattered nuclei but could be carcinogenic as 48 h incubation of RF induced fragmentation and ghost cells. Therefore cell phones and laptop should not be used unnecessarily to avoid possible genotoxic and carcinogenic effects.
Keywords: Radio frequency radiation, Genotoxicity, Carcinogenicity, Chromosomal aberrations
1. Introduction
Electromagnetic radiation (EMR) can be classified into two types: Ionizing radiation and Non-ionizing radiation. Non-ionizing radiation refers to any type of electromagnetic radiation that doesn’t carry enough energy per quantum to ionize atoms or molecules. (www.wikipedia.com). Non-ionizing radiation includes Ultra Violet (UV), Microwave and radiofrequency radiation. Probably the most important use of radiofrequency (RF) energy is in providing telecommunication services. Radio and television broadcasting, cellular telephones, laptops, radio communication for police and fire departments, amateur radio, microwave point-to-point links, and satellite communication. Besides being so useful these radiofrequency radiations have many biological effects on living tissues. Recent studies link exposure to health problem that includes lower sperm counts (Avendano et al., 2012), memory loss (Koivisto et al., 2000, Cech et al., 2008), sleep disruption (Loughran et al., 2005), decreased immune function, dizziness, headaches, higher blood pressure and reduced DNA repair capacity (Braune et al., 1998, Trimmel and Bachmann, 2004, James, 2008, Tyagi et al., 2011).
Genotoxicity is the property possessed by some substances that make them harmful to the genetic information contained in organism. Physical and chemical agents having ability to damage deoxyribonucleic acid (DNA) are called genotoxic (Galloway, 1994). Segment breaks in DNA molecule are called chromosomal aberrations. These are only visible in cell divisions. Mitosis is widely used for the study of genotoxic compounds using chromosomal aberration assay.
The damage of DNA or genotoxicity is an important consideration, because it has a potential to cause irreversible changes to genes and even cancer (M-boh, 2003). Mainly Allium cepa chromosomal aberration assay was used as bioassay plant since 1938 (Levan, 1938) for investigating environmental pollution factors, toxicity of chemical compounds, and evaluating potential anticancer properties (Bakare et al., 2000, Majewska et al., 2003, Babatunde and Bakare, 2006, Kuraś et al., 2007) but now researchers are also using Visia Faba, Vigna mungo and Cicer arietinum L. (Rank and Nielsen, 1997, Unyayar et al., 2006, Chahal et al., 2012, Siddiqui, 2012, Arain and Maqbool, 2011).
Non-thermal level of radiofrequency exposure has genotoxic effects in the form of chromosomal instability, altered gene expression, gene mutation, DNA fragmentation and DNA structure break. Some other genotoxic effects are documented to occur on neurons, blood lymphocytes, sperms, Red Blood Cell (RBC), epithelical cells, hematopoietic tissues, lung cells and bone marrow (Mashevich et al., 2003). Microwave frequencies ranging between 375 and 36.64 GHz can increase cell membrane permeability to staining dye used to study cytological aspects in living human buccal epithelium cells (Shckorbatov et al., 2002, Shckorbatov et al., 2011). EMFs can change secondary structure of cell membrane proteins by causing reversible changes to peptide linkage (Ikehara et al., 2003). EMFs have ability to influence usual oxidation and reduction inside a cell (Kovacic and Somanath, 2010) and their long time exposure can alter celular balance resulting in oxidative stress (Scaiano et al., 1994, Repacholi and Greenebaum, 1999, Jajte et al., 2002, Akdag et al., 2007, Simkó, 2007).
Chromosomal aberrations have been used as a measure of reproductive success in plants for many years but now they are also used as measure of co-relation between reduction in fertility, mutagenesis and carcinogenesis (Kostoff, 1934). Cytogenetic abnormalities are a characteristic attribute of cancer cells. To date, chromosome aberrations have been found in all major tumor types of cancer. Translocations and double stranded breaks (deletions) are more commonly found chromosomal aberration in tumor cells (Hindus and Weinberg, 1994, Knudson, 2001, Keen-Kim et al., 2008, Stratton et al., 2009).
Chromosomal aberrations in plants serve as excellent monitoring system for the detection of environmental chemicals that may pose a genetic hazard. The plant systems have proven most useful for this purpose (Nilan and Vig, 1976, Gustavino et al., 2015). Use of C. arietinum L. as assay plant is reported by many workers (Arain and Maqbool, 2011, Qureshi et al., 2014, Parihar and Mawal, 2015).
The usage of GSM cell phone and laptop has increased many folds over the last few years. It is therefore a matter of great concern. Prolonged use of GSM cell phones due to free call packages and use of laptops on our laps during travel and leisure expose humans to more intense radiation. More portable devices with RF are to be expected in future that may be operated near the body. This will further increase the exposure of people to high frequency electromagnetic fields. Many researchers worked on effects of far field RF on plants but no study is carried out on near field effects. Therefore present study is first attempt to predict possible radiofrequency radiation induced genotoxic and carcinogenic effects.
2. Material and methods
Plant chromosomal aberration assay was used as genotoxicity marker as suggested by (Grant, 1978). Kabuli chickpea genotype NCS 0530 was obtained from National Agriculture Research Center, Islamabad (NARC) was used in assay.
2.1. RF source and treatment plan
In order to predict possible cytotoxic and genotoxic effects by near field RF, Nokia GSM set, model N0# X2-00 (900 MHz) and HP laptop, model N0# 430 core i5 (3.31 GHz) were used as RF sources. 150 healthy dry seeds of chickpea were distributed in two petri plates. Each petri plate was placed at distances of 1 inch to cell phone and laptop for 24 and 48 h (Fig. 1). Untreated seeds were used as (0 h) negative control and Gamma rays 250 (Gray) as positive control.
Figure 1.
RF treatment of chickpea seeds.
2.2. Seed germination
Seeds were soaked in distilled water for 2 h before sowing in the sand pots. The number of roots recovered was expressed in percentage.
2.3. Root fixation and slide preparation
2 cm root samples were collected and fixed in Carnoy solution (3:1 alcohol and glaciered acetic acid) for 24 h. Roots were then transferred to 70% alcohol until used. Root tips were spread using the squash technique (Dille and King, 1983, Dille et al., 1986) and stained with 2% Acetocarmine (2% in 45% glacial acetic acid).
2.4. Microscopy of mitotic slide
Slides were studied and photographed with Olympus 1X51 Microscope at 100 × magnification. Five slides per treatment were used to score number of cells for each chromosomal aberration (DNA damages).
2.5. Data analysis
2.5.1. Mitotic Index (M.I.)
Mitotic index was calculated as described by Racuciu (2009). It was calculated by the following formula:
2.5.2. Abnormal index (A.I.)
Abnormal index was calculated by the method of (Racuciu, 2009) according to the following formula.
3. Results
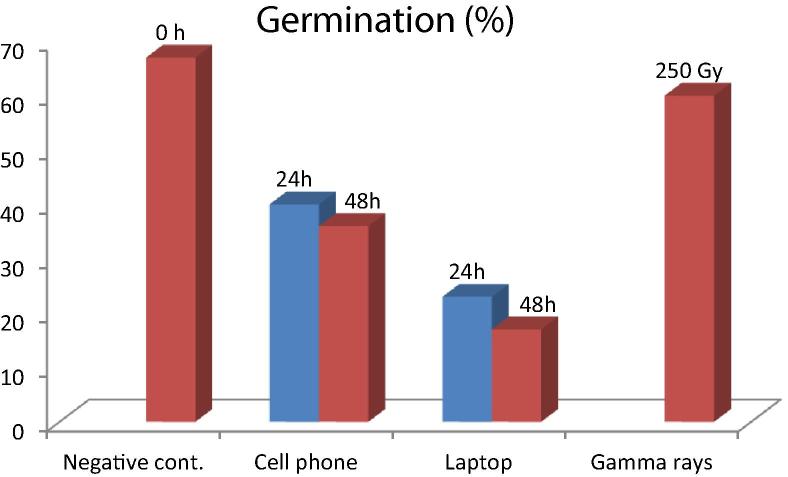
The effect of radiofrequency radiations on the germination percentage of chickpea is presented in (Fig. 2). All the treatment of RF inhibits seed germination percentage. Cell phone RF (900 MHz) has less inhibitory effect than laptop (3.31 GHz). 48 h laptop exposure has the most negative effect with 17% germination as compared to negative control with 67% germination. Present results are consistent with Racuciu et al. (2015) and Kumar et al. (2015) who reported electromagnetic radiation of mobile phone induced root and coleoptiles growth inhibition in Zea mays and Cammaerts and Johansson (2015) in Lepidium sativum. Possible reasons for reduced growth observed by these researchers were retarded in chlorophyll pigments and nucleic acid content, interference in starch and sucrose metabolism and lack of imbibitions by germinal cells. Parihar and Mawal (2015) working with radiations emitted by 2G and 3G mobile phones also observed growth retardation and diminished fresh and dry weight of roots in pulses. On the contrary Brozouei et al. (2010) reported that only high dose of ionization radiation can depress germination percentage.
Figure 2.
Effect of radiofrequency radiations on germination of chickpea (h = hours; Gy = Gray).
The results indicate negative association between radiation exposure duration and germination percentage except 24 h cell phone exposure (40%). This may be due to random mutation induced by RF. A slight mutation in genes responsible for cell division may cause germination inhibition. The possible reason behind decline in germination, growth and survival are generally metabolic disorders of which cytokinine breakdown or lack of synthesis is most common (Gandhi et al., 2014, Gustavino et al., 2014).
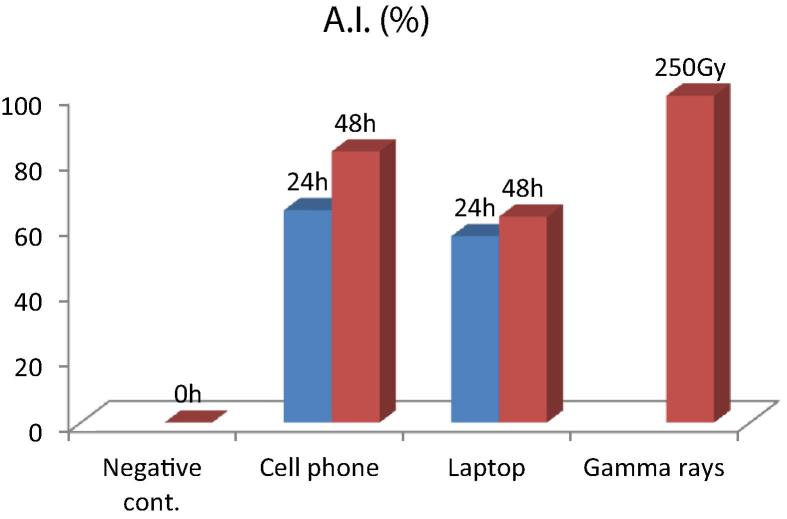
The results of effect of radiofrequency on abnormality index and mitotic index on chickpea root tip cells are compiled in Table 1. The abnormality index showed a linear increase with exposure and frequency in Hz (Fig. 3). Whereas mitotic index (%) exhibited a linear decrease with increased RF exposure (Fig. 4). Similar trends were observed by Lamsal et al. (2010) in Allium cepa root tip cells treated with agricultural insecticide. Results showed that mitotic index decreases as abnormality index increased. The altered mitotic rate of the plant subjected to EMR is mostly attributed to interference in normal steps of mitosis and spindle formation (Moisescu et al., 2008, Tkalec et al., 2009), failure of DNA replication and proteins synthesis (Lia and Singh, 2004), enzyme production, function and regulation and low level of ATP generation due to decreased oxidative photophosphorylation (Hao et al., 2015) (see Figure 5, Figure 6).
Table 1.
Effect of radiofrequency on abnormality index (A.I.) and mitotic index (M.I.) in chickpea root tip cells.
| Treatments | Hours | Number of cells |
No. of abnormal cells in stages of mitosis |
A.I. (%) | M.I. (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dividing | Abnormal | Normal | Non-dividing | Metaphase | Anaphase | Telophase | ||||
| Negative control | 0 | 500 | 0 | 500 | 0 | 75 | 0 | 141 | 0 | 100 |
| Cell phone (900 MHz) | 24 | 419 | 276 | 143 | 81 | 42 | 143 | 92 | 65 | 84 |
| 48 | 316 | 263 | 56 | 184 | 27 | 117 | 116 | 83 | 63 | |
| Laptop (3.31 GHz) | 24 | 450 | 260 | 140 | 50 | 169 | 76 | 0 | 57 | 90 |
| 48 | 397 | 318 | 78 | 103 | 243 | 75 | 0 | 63 | 79 | |
| Positive control Gamma ray (Gy) | 250 | 211 | 211 | 0 | 189 | 128 | 37 | 0 | 100 | 52 |
Figure 3.
Effect of radiofrequency radiations on abnormality index (%) (h = hours; Gy = Gray).
Figure 4.
Effect of radiofrequency radiations on mitotic index (%) (h = hours; Gy = Gray).
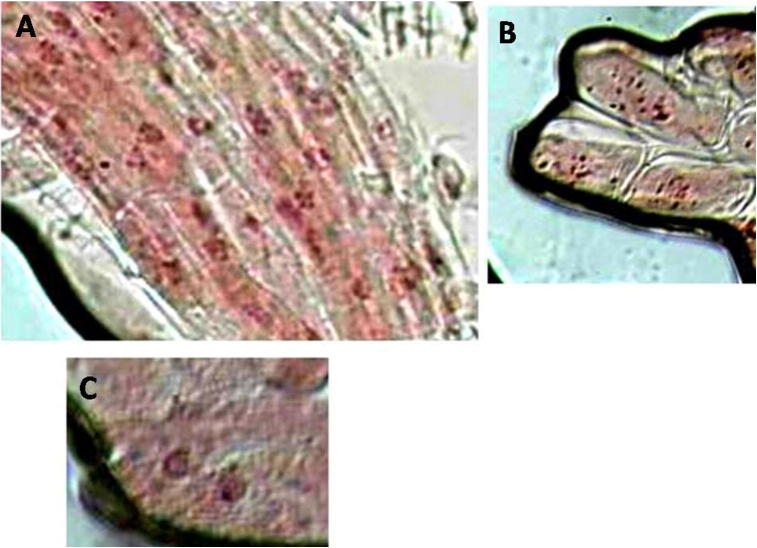
Figure 5.
Negative control showing normal mitotic cells (A and B = pro-metaphase; C = prophase).
Figure 6.
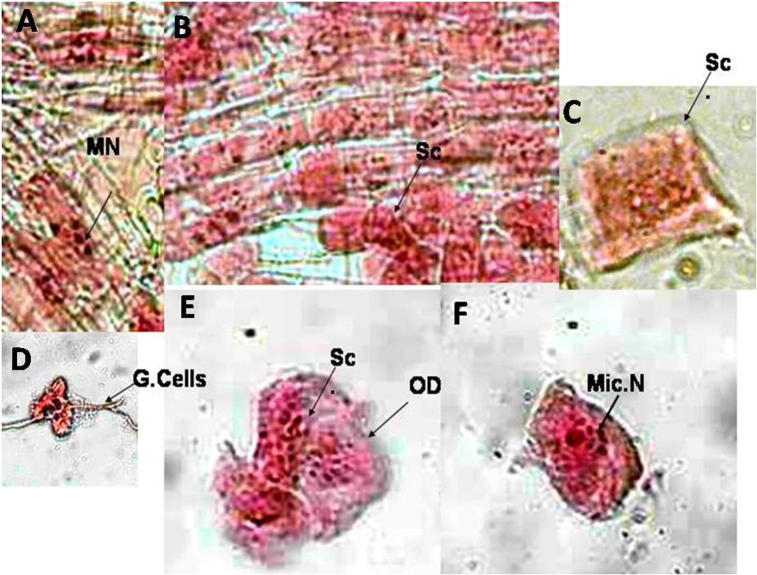
Positive control Gamma rays (250 Gy) induced chromosomal aberrations (A) Multinuclei (MN); (B, C and E) sticky metaphase (Sc) and oxidative cell membrane damage (OD); (D) ghost cell with pilus; (F) micro-nuclei (Mic. N).
The types of chromosomal aberrations induced by radiofrequency are compiled in (Table 2). During 24 h with cell phone treatment most frequent abnormality was scattered nuclei (118 cells) while least frequent abnormality was micronuclei (25 cells) (Fig. 7).
Table 2.
Types of chromosomal aberrations induced by radiofrequency radiations.
| Mitosis stage | S. No | Abnormalities | Control (%) |
Cell phone (900 MHz) |
Laptop (3.31 GHz) |
|||
|---|---|---|---|---|---|---|---|---|
| Negative control (0 H) | Positive control Gamma rays (250 Gy) | 24 (H) | 48 (H) | 24 (H) | 48 (H) | |||
| Metaphase | 1 | Sticky metaphase | 0 | 31 | 0 | 27 | 33 | 0 |
| 2 | Translocations | 0 | 0 | 42 | 0 | 0 | 0 | |
| 3 | Distributed metaphase | 0 | 15 | 0 | 0 | 0 | 0 | |
| Anaphase | 4 | Scattered nuclei | 0 | 34 | 118 | 0 | 130 | 60 |
| 5 | Laggard | 0 | 43 | 0 | 2 | 0 | 0 | |
| 6 | Fragmentation | 0 | 36 | 25 | 117 | 39 | 183 | |
| Interphase | 7 | Micronuclei | 0 | 0 | 25 | 43 | 16 | 25 |
| 8 | Multinuclei | 0 | 37 | 0 | 0 | 33 | 0 | |
| 9 | Dinuclei | 0 | 0 | 67 | 73 | 27 | 50 | |
Figure 7.
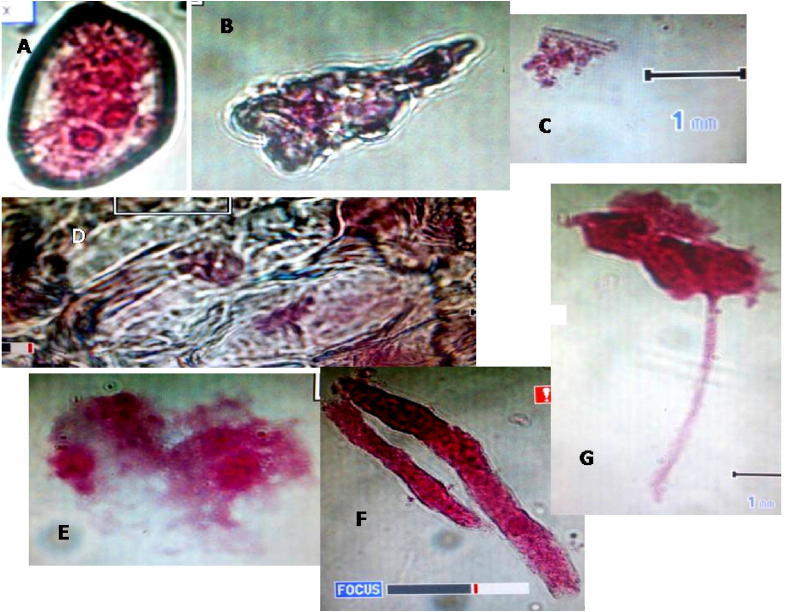
Cell phone 24 h treatment induced chromosomal aberrations (Showing (A and E) Di-nuclei; (D) sticky metaphase; (B and C) ghost cells; (G) ghost cell with proliferation pilus); (F) fragmentation.
In 48 h cell phone treatment fragmentation (117 cells) was the most frequent abnormality, while least frequent abnormality was sticky metaphase (27 cells) (Fig. 8). In 24 h laptop exposure most frequent abnormality was scattered nuclei (130 cells) while the least frequent abnormality was micronuclei (16 cells) (Fig. 9). In 48 h laptop exposure, the most frequent abnormality was fragmentation (183 cells) while the least frequent abnormality was micronuclei (25 cells) (Fig. 10).
Figure 8.
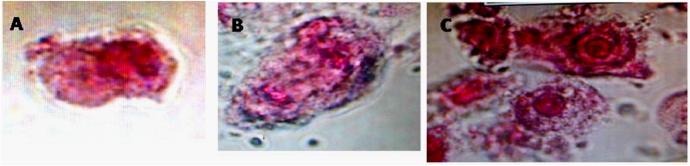
Cell phone 48 h treatment induced chromosomal aberrations (A and B) Laggard; (C) micronuclei.
Figure 9.
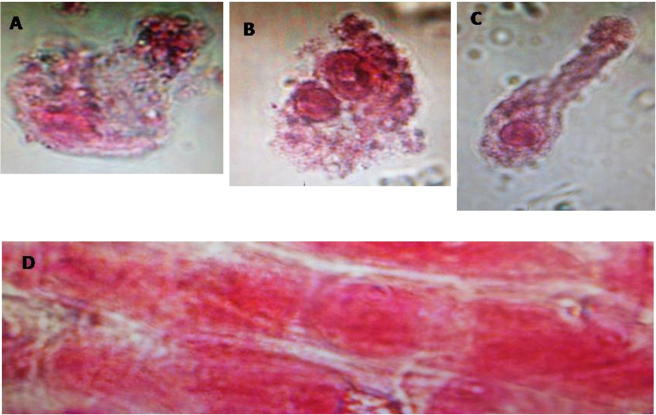
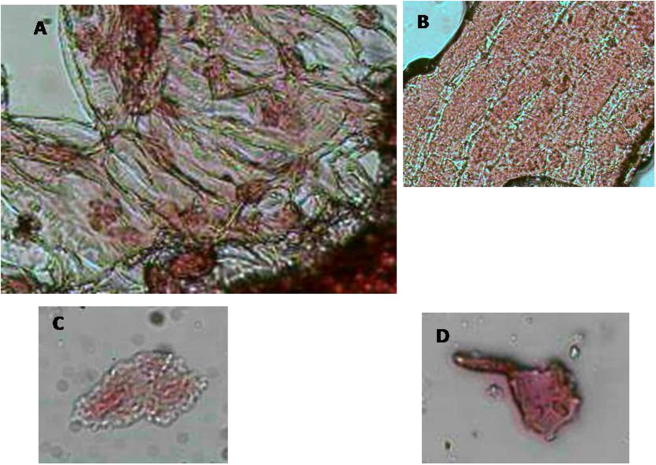
Laptop 24 h treatment induced chromosomal aberrations (A and C) abnormal telophase with nuclear membrane damage (B) Di-nuclei with cell membrane damage and (D) chromosomal fragmentation.
Figure 10.
Laptop 48 h treatment induced chromosomal aberrations (A and C) normal prophase (B) fragmentation (C) scattered nuclei and (D) ghost cells with pilus.
Present research reveals increased DNA damages with increasing duration of RF exposure. Chavdoula et al. (2010) reported mobile phone radiations (900 MHz–1800 MHz) induced DNA fragmentation in the egg chamber cells resulting in decreased fertility and apoptosis in Drosophila melanogaster. Gustavino et al. (2015) evaluated mutagenic potential of radiofrequency radiation of 915 MHz continuous wave radiation for 72 h on secondary root tips of Vicia faba and recorded dose dependent increase in micronucleus frequency. Similarly Zotti-Martelli et al. (2005) assess the micronucleus (MN) induction capability of microwaves (1800 MHz), on peripheral blood lymphocytes of humans and found statistically significant increase of MN, in exposure time and applied power density dependent manner. Presence of pilus like tube in some cells treated with cell phone 24 and laptop 48 h is evidence of cellular connection that may lead to proliferation of apoptotic cells and nuclear aggregation commonly found in cancerous cells. This may be due to error of repair machinery and high level of fragmentation that leads to defected chimeric gene (Shaffer and Pandolfi, 2006, Meyerson, 2007) expressing pilus like tubular out growth. Therefore it is suggested to carry out PCR amplifications with all type of pilin promoters in such cells (see Fig. 8).
The results of oxidative damages induced by radiofrequency are presented in Table 3. Maximum cell membrane damages were observed in 48 h exposure with cell phone (100 cells) and laptop (20 cells). Maximum nuclear membrane damages were again recorded in 48 h exposure with cell phone (342 cells) and laptop (254 cells).
Table 3.
Oxidative damages induced by radiofrequency radiations in chickpea root tip cells.
| Treatments | Hours | Type of oxidative damage (No. of cells) |
||
|---|---|---|---|---|
| C.M. damage | N. M. damage | Ghost cells | ||
| Negative control | 0 | 0 | 0 | 0 |
| Cell phone (900 MHz) | 24 | 0 | 164 | 60 |
| 48 | 100 | 342 | 132 | |
| Laptop (3.31 GHz) | 24 | 0 | 200 | 200 |
| 48 | 20 | 254 | 250 | |
| Gamma ray 250 Gy | 17 | 32 | 255 | |
| Grand mean | 137 | 992 | 897 | |
(C.M. = Cell membrane; N.M. = Nuclear membrane).
Maximum numbers of ghost cells were found in 48 h cell phone (432 cells) and laptop (255 cells) RF. It can be inferred from the results that increase in exposure duration and frequency (Hz) of RF increased the number of cells with oxidative damages. Overall cell membrane damage (137 cells) was the least frequent oxidative damage while nuclear membrane damage (992 cells) was most frequent. All the treatments with RF induced more oxidative stress than positive control (250 Gy). The disruption of membrane integrity may be due to interference of RF with membrane permeability or membrane proteins leading to oxidative stress (Livingstone, 2003). Afzal and Mansoor (2012) reported mobile phone emitted radiofrequency radiation induced oxidative stress in mung bean and wheat crops. Burlaka et al. (2013) observed significant overproduction of free radicals/reactive oxygen species and oxidative damage of DNA in quail embryo cells exposed to GSM 900 MHz for one hundred and fifty-eight hours. They relate oxidative changes to health effects up to oncogenesis.
Similar findings are reported by Xu et al. (2010) working with cultured neurons irradiated with 1800 MHz RF radiation. They reported that RF is capable of causing oxidative damage to mtDNA that leads to the neurotoxicity of RF radiation in the brain. In another study with 1.8 GHz Global system for mobile communication (GSM) Avci et al. (2012) concluded that RF exposure can enhance protein oxidation in rat brain cells as compared to control group (p < 0.001).
4. Conclusion
It is concluded that radiofrequency radiations are genotoxic as they induced chromosomal aberrations in chickpea mitotic cells and the presence of ghost cells is clear indication of their carcinogenic potential. To avoid reported DNA damages in this work cell phones should always be used either for short duration or with handsfree for long duration and they should not be kept in pockets or near body. Laptops should not be used unnecessarily for enjoyment purpose. It must be placed on desk top rather lap to minimize their exposure to human body. Further assay of carcinogenity are recommended on mouse and human cell lines.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afzal M., Mansoor S. Effect of mobile phone radiation on morphological and biochemical parameters of mung bean (Vigna radiata) and wheat (Triticum aestivum) Asian J. Agric. Sci. 2012;4(2):149–152. [Google Scholar]
- Akdag M.Z., Bilgin M.H., Dasdag S., Tumer C. Alteration of nitric oxide production in rat exposed to a prolonged, extremely low-frequency magnetic field. Electromagn. Biol. Med. 2007;26(2):99–106. doi: 10.1080/15368370701357866. [DOI] [PubMed] [Google Scholar]
- Arain, A., Maqbool F., 2011. Gross mutations and oxidative stress induced by high doses of Gamma rays on Chickpea (Ciecer arietinum L.) root tip cells (M.Sc.Thesis). Submitted to the University of Sindh, Jamshoro.
- Avci B., Akar A., Bilgici B., Tunçel Ö.K. Oxidative stress induced by 1.8 GHz radio frequency electromagnetic radiation and effects of garlic extract in rats. Int. J. Radiat. Biol. 2012;88(11):799–805. doi: 10.3109/09553002.2012.711504. [DOI] [PubMed] [Google Scholar]
- Avendano C., Mata A., Sarmient C.A.S., Doncel G.F. Use of laptop computer connected to internet through wi-fi decrease human sperm motility and increase sperm DNA fragmentation. Fertil. Steril. 2012;97(1):39–45. doi: 10.1016/j.fertnstert.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Babatunde B.B., Bakare A.A. Genotoxicity screening of wastewaters from Agbara industrial estate, Nigeria evaluated with the Allium test. Pollut. Res. 2006;25(2):227–234. [Google Scholar]
- Bakare A.A., Mosuro A.A., Osibanjo O. Effect of simulated leachate on Chromosomes and mitosis in roots of Allium cepa (L) J. Environ. Biol. 2000;21(3):263–271. [Google Scholar]
- Braune S., Wrocklage C., Raczed J., Gailus T., Lucking C.H. Resting blood pressure increase during exposure to radio frequency electromagnetic field. Lancet. 1998;351:1857. doi: 10.1016/s0140-6736(98)24025-6. [DOI] [PubMed] [Google Scholar]
- Brozouei A., Kafi M., Khazaei H., Naseriyan B., Majdabadi A. Effect of Gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pak. J. Bot. 2010;42(4):2281–2290. [Google Scholar]
- Burlaka A., Tsybulin O., Sidorik E., Lukin S., Polishuk V., Tsehmistrenko S., Yakymenko I. Overproduction of free radicals species in embryonal cells exposed to low intensity radiofrequency radiation. Exp. Oncol. 2013;5:219–225. [PubMed] [Google Scholar]
- Cammaerts M.C., Johansson O. Effect of man-made electromagnetic fields on common Brassicaceae Lepidium sativum (cress d’ Illinois) seed germination: a preliminary replication study. Int. J. Exp. Bot. 2015;84:132–137. [Google Scholar]
- Cech R., Leitgeb N., Pediadiaditis M. Current densities in a pregnant women model induced by simultaneous ELF electric and magnetic field exposure. Phys. Med. Biol. 2008;53(1):177–186. doi: 10.1088/0031-9155/53/1/012. [DOI] [PubMed] [Google Scholar]
- Chahal V., Nagpal A., Katnoria J.K. Genotoxicity evaluation of soil sample from agricultural field under wheat cultivation. Bot. Res. Int. 2012;5(1):01–03. [Google Scholar]
- Chavdoula E.D., Panagopoulos D.J., Margaritis L.H. Comparison of biological effects between continuous and intermittent exposure to GSM-900- MHz mobile phone radiation: detection of apoptotic cell-death features. Mutat. Res. 2010;700:51–61. doi: 10.1016/j.mrgentox.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Dille J., King N. Changes in mitotic indexes in roots of cereal exposed to di-methyl sulphide (DMJO) Cytologica. 1983;48:659–662. [Google Scholar]
- Dille J., King N., Brigth M. Morphological, cytological and cytogenetic effect of 50 to × 25 seed treater (f) (lindane and captan) on roots and chromosomes of rye (S.cereal L.) Cytologia. 1986;5:489–492. [Google Scholar]
- Galloway S.M. Genotoxicity testing. Mutat. Res. 1994;312:195–322. doi: 10.1016/0165-1161(94)00007-7. [DOI] [PubMed] [Google Scholar]
- Gandhi G., Kaur G., Nisar U. A cross-sectional case control study on genetic damage in individuals residing in the vicinity of a mobile phone base station. Electromagn. Biol. Medi. 2014;9:1–11. doi: 10.3109/15368378.2014.933349. [DOI] [PubMed] [Google Scholar]
- Grant W.F. Chromosome aberrations in plants as a monitoring system. Environ. Health Perspect. 1978;27:4–7. doi: 10.1289/ehp.782737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavino B., Carboni G., Petrillo R., Santovetti E., Rizzoni M. Micronucleus induction by 915 MHz Radiofrequency radiation in Vicia Faba root tips. Mutagenesis. 2014:1409–1431. doi: 10.1093/mutage/gev071. arXiv. [DOI] [PubMed] [Google Scholar]
- Gustavino B., Carboni G., Petrillo R., Paoluzzi G., Santovetti E., Rizzoni M. Exposure to 915 MHz radiation induces micronuclei in vicia faba root tips. Mutagenesis. 2015 doi: 10.1093/mutage/gev071. gev071. [DOI] [PubMed] [Google Scholar]
- Hao Y., Zhao L., Peng R. Effect of Microwaver ralliation on brain energy metabolism and related mechanisms. Military Med. Res. 2015;2(4) doi: 10.1186/s40779-015-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindus P.W., Weinberg R.A. Tumor suppressor gens. Curr. Opin. Genet. Dev. 1994;4:15–141. doi: 10.1016/0959-437x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Ikehara T., Yamaguchi H., Hosokawa K., Miyamoto H., Aizawa K. Effect of ELF magnetic field on membrane protein structure of living HeLa cells studied by Fourier transform infrared spectroscopy. Bioelectromagnetics. 2003;24:457–464. doi: 10.1002/bem.10120. [DOI] [PubMed] [Google Scholar]
- Jajte J., Grzegorczyk M., Zmysacute, Rajkowska E. Effect of 7 mT static magnetic field and iron ions on rat lymphocytes: apoptosis, necrosis and free radical processes. Bioelectrochemistry. 2002;57(2):107–111. doi: 10.1016/s1567-5394(02)00059-2. [DOI] [PubMed] [Google Scholar]
- James R.J. Effect of low level radio frequency (3 KHz to 30 GHz) energy on human cardiovascular reproductive immune and other system a review of the recent literature. Int. J. Hyg. Environ. Health. 2008;211(1–2):1–29. doi: 10.1016/j.ijheh.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Keen-Kim D., Nooraie F., Rao P.N. Cytogenetic biomarkers for human cancer. Front. Biosci. 2008;1:5928–5949. doi: 10.2741/3127. [DOI] [PubMed] [Google Scholar]
- Knudson A.G. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- Koivisto M., Kravese C.M., Revonsvo A., Laine M., Hamaalainen H. The effect of electromagnetic field emitted by GSM phone on working memory. Neuroreport. Cognit. Neurosci. 2000;11(8):1641–1643. doi: 10.1097/00001756-200006050-00009. [DOI] [PubMed] [Google Scholar]
- Kostoff D. Heteroploidy in Nicotiana tobaccum and Solanum melongena caused by fumigation with nicotine sulphate. Bull. Soc. Bulg. 1934;87(8):10. [Google Scholar]
- Kovacic P., Somanath R. Electromagnetic fields: mechanism, cell signaling, other bioprocesses, toxicity, radicals, antioxidants and beneficial effects. J. Recept. Signal Transduction. 2010;30(4):214–226. doi: 10.3109/10799893.2010.488650. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh H.P., Batish D.R., Kaur S. EMF radiations (1800 MHz) inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose. Protoplasma. 2015 doi: 10.1007/s00709-015-0863-9. Online Publication. [DOI] [PubMed] [Google Scholar]
- Kuraś M., Augustynowicz J., Śliwińska E., Pilarski R., Ilasz R., Tykarska T., Zobel A., Gulewicz K. Changes in chromosome structure, mitotic activity and nuclear DNA content from cells of Allium test induced by bark water extract of Uncaria tomentosa (willd.) J. Ethnopharmacol. 2007;107(2):211–221. doi: 10.1016/j.jep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Lamsal K., Ghimire B.K., Sharma P., Ghimiray A.K., Kim S.W., Yu C.Y., Chung I.M., Lee Y.S., Kim J., Shakya S.R. Genotoxicity evaluation of the insecticide ethion root of Allium cepa. Afr. J. Biotechnol. 2010;9(27):4204–4210. [Google Scholar]
- Levan A. The effect of colchicine on root mitoses in Allium. Hereditas. 1938;24:471–486. [Google Scholar]
- Lia H., Singh N.P. Magnetic-field induced DNA strand breaks in rain cells of the rat. Environ. Health Perspect. 2004;112(6):687–694. doi: 10.1289/ehp.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone D.R. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Médecine Vétérinaire. 2003;154:427–430. [Google Scholar]
- Loughran S.P., Wood A.W., Barton T.M., Croft R.J., Thompson B., Stough C. The effect of electromagnetic field emitted by mobile phone on human sleep. Neuroreport. 2005;16(17):1973–1976. doi: 10.1097/01.wnr.0000186593.79705.3c. [DOI] [PubMed] [Google Scholar]
- Majewska A., Wolska E., Śliwińska E., Furmanowa M., Urbańska N., Pietrosiuk A., Zobel A., Kuraś M. Anti-mitotic effect, G2/M accumulation, chromosomal and ultra structure changes in meristimatic cells of Allium cepa L. root tips treated with the extract from Rhadiola rosea roots. Caryologia. 2003;56:337–351. [Google Scholar]
- Mashevich M., Folkman D., Kesar A. Exposure of human peripheral blood lymphocyte to electromagnetic field associated with cellular phones lead to chromosomal instability. Bioelectromagnetics. 2003;24:82–90. doi: 10.1002/bem.10086. [DOI] [PubMed] [Google Scholar]
- M-boh, 2003. Genotoxicity: there should or not, introduction of cases of industries chemicals. Toxicol. Let. 140–141. [DOI] [PubMed]
- Meyerson M. Cancer broken genes in solid tumors. Nature. 2007;448:545–546. doi: 10.1038/448545a. [DOI] [PubMed] [Google Scholar]
- Moisescu M.G., Leveque P., Betrand J.R., Kovacs E., Mir L.M. Microscopic observation of living cells during their exposure to modulated electromagnetic fields. Bioelectrochemistry. 2008;74(1):9. doi: 10.1016/j.bioelechem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Nilan R.A., Vig B.K. Plant test system for detection of chemical mutagen. In: Alexander Hollander., editor. Vol. 4. Plenum Publishing Corp.; New York: 1976. pp. 147–170. (Chemical Mutagens). [Google Scholar]
- Parihar L., Mawal P. Effect of 2G and 3G mobile phones radiations on germination of seed and growth of seedlings of pulses. J. Pharm. Res. 2015;7(3):268–271. [Google Scholar]
- Qureshi S.T., Soomro A.G., Bux H., Yasmeen A. Genotoxic and carcinogenic effects of house hold detergents using chromosomal aberration assay in Chickpea (Cicer arietinum L.) root tip cells. World Appl. Sci. J. 2014;32(7):1381–1387. [Google Scholar]
- Racuciu M. Effect of radiofrequency radiation on root tip cells of Zea mays. Roum. Biotechnol. Lett. 2009;14(3):4365–4369. [Google Scholar]
- Racuciu M., Iftode C., Miclaus S. Inhibitory effects of low thermal radiofrequency radiation on physiological parameters of Zea mays seedlings growth. Rom. J. Phys. 2015;60(3–4):603–612. [Google Scholar]
- Rank J., Nielsen M.H. Allium Cepa anaphase-telophase root tip chromosome aberration assay on N-methl-n-nitrosourea, maleic hydrazide sodium a zide, and ethyl methanerul fonate. Mutat. Res. 1997;390:1212–1227. doi: 10.1016/s0165-1218(97)00008-6. [DOI] [PubMed] [Google Scholar]
- Repacholi M.H., Greenebaum B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics. 1999;20(3):133–160. doi: 10.1002/(sici)1521-186x(1999)20:3<133::aid-bem1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Scaiano J.C., Mohtat N., Cozen F.L., McLean J., Thansandote A. Application of the radical pair mechanism to free radicals in organized systems: can the effect of 60 Hz be predicted from the studies under static fields? Bioelectromagnetics. 1994 doi: 10.1002/bem.2250150608. [DOI] [PubMed] [Google Scholar]
- Shaffer D.R., Pandolfi P.P. Breaking the rules of cancer. Nat. Med. 2006;12:14–15. doi: 10.1038/nm0106-14. [DOI] [PubMed] [Google Scholar]
- Shckorbatov Y.G., Shakhbazov V.G., Navrotskaya V.V., Grabina V.A., Sirenko S.P., Fisun A.I., Gorobets N.N., Kiyko V.I. Application of intracellular microelectrophoresis to analysis of the influence of the low-level microwave radiation on electrokinetic properties of nuclei in human epithelial cells. Electrophoresis. 2002;23:2074–2079. doi: 10.1002/1522-2683(200207)23:13<2074::AID-ELPS2074>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Shckorbatov Y.G., Pasiuga V.N., Kolhigin N.N., Grabina V.A., Ivanchenko D., Victor Bykov V., Dumin O. Cell nucleus and membrane recovery after exposure to microwaves. Proc. Latv. Acad. Sci. 2011;65:1–20. [Google Scholar]
- Siddiqui S. Lead induced genotoxicity in Vigna mungo var. HD-94. J. Saudi Soc. Agr. Sci. 2012;11:107–112. [Google Scholar]
- Simkó M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 2007;14(10):1141–1152. doi: 10.2174/092986707780362835. [DOI] [PubMed] [Google Scholar]
- Stratton M.R., Campbell P.J., Futreal P.A. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkalec M., Malaric K., Pavlica M., Pevalekkozlina B., Vidakovic-clfrek Z. Effects of radiofrequency electromagnetic field on seed germination and root meristematic cells of Allium cepa L. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009;672(2):76. doi: 10.1016/j.mrgentox.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Trimmel M., Bachmann J. Cognitive social motivational and health aspect of student in laptop classroom. J. Comp. Assisted Learn. 2004;20(2):151–158. [Google Scholar]
- Tyagi A., Duhan Bhatia D. Effect of mobile phone radiation on brain activity GSM Vs CDMA. Int. J. Sci. Technol. Manage. 2011;2(2):1–5. [Google Scholar]
- Unyayar S., Celik A., Cekic F.O.Z., Gözel A. Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis. 2006;21(1):77–81. doi: 10.1093/mutage/gel001. [DOI] [PubMed] [Google Scholar]
- Xu S., Zhou Z., Zhang L., Yu Z., Zhang W., Wang Y., Wang X., Li M., Chen Y., Chen C., He M., Zhang G., Zhong M. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res. 2010;22(1311):189–196. doi: 10.1016/j.brainres.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Zotti-Martelli L., Peccatori M., Maggini V., Ballardin M., Barale R. Individual responsiveness to induction of micronuclei in human lymphocytes after exposure in vitro to 1800-MHz microwave radiation. Mutat. Res. 2005;582:42–52. doi: 10.1016/j.mrgentox.2004.12.014. [DOI] [PubMed] [Google Scholar]