Abstract
Introduction
Eosinophilic esophagitis (EoE) is one of the most prevalent esophageal diseases and the leading cause of dysphagia and food impaction in children and young adults. This underlines the importance of optimizing diagnosys and treatment of the condition, especially after the increasing amount of knowledge on EoE recently published. Therefore, the UEG, EAACI ESPGHAN, and EUREOS deemed it necessary to update the current guidelines regarding conceptual and epidemiological aspects, diagnosis, and treatment of EoE.
Methods
General methodology according to the Appraisal of Guidelines for Research and Evaluation (AGREE) II and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used in order to comply with current standards of evidence assessment in formulation of recommendations. An extensive literature search was conducted up to August 2015 and periodically updated. The working group consisted of gastroenterologists, allergists, pediatricians, otolaryngologists, pathologists, and epidemiologists. Systematic evidence-based reviews were performed based upon relevant clinical questions with respect to patient-important outcomes.
Results
The guidelines include updated concept of EoE, evaluated information on disease epidemiology, risk factors, associated conditions, and natural history of EoE in children and adults. Diagnostic conditions and criteria, the yield of diagnostic and disease monitoring procedures, and evidence-based statements and recommendation on the utility of the several treatment options for patients EoE are provided. Recommendations on how to choose and implement treatment and long-term management are provided based on expert opinion and best clinical practice.
Conclusion
Evidence-based recommendations for EoE diagnosis, treatment modalities, and patients’ follow up are proposed in the guideline.
Keywords: Eosinophilic esophagitis, guidelines, consensus development conferences, evidence-based practice
Introduction
Eosinophilic esophagitis (EoE) is an inflammatory condition of the esophagus that, today, constitutes the most prevalent cause of chronic esophagitis after gastroesophageal reflux disease (GERD) and the leading cause of dysphagia and food impaction in children and young adults. The first EoE cases appeared in the late 1970s and EoE was defined as a distinct clinicopathologic syndrome in the early 1990s.1,2 From that moment, its growing recognition and exponential increase in the number of identified patients from many continents makes EoE both a scientific and health challenge.
A growing amount of literature on EoE has been published from the identification of the disease, including several consensus documents by groups of experts and clinical practice guidelines developed under the auspices of American and European scientific societies for children and adult patients.3–6 However, the huge amount of knowledge achieved in the last 5 years, including several randomized controlled trials (RCTs) and systematic reviews, have determined that published guidelines on EoE could be currently outdated. In this regard, no previous guidelines have used specific methods suited to the purpose of establishing the quality of the evidence and the weight of the statements and recommendations provided.7 The use of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) technology has been recommended in recent years as a standard tool for the development of clinical practice guides.8 The present guide is the first one developed in EoE using this methodology.
The statements and recommendations in the present document are meant to be used by physicians and other health professionals involved in the management of EoE. Epidemiological, etiological, and pathogenic aspects are also reviewed, and the currently preferred approach to diagnosis and treatment of the disorder is defined. Whenever possible, the specific statements or recommendations were based on the best available evidence, and when such evidence was either not available or was found to be inconsistent, the recommendations were established by consensus among the authors according to expert opinion and best clinical practice.
Aims and methodology
This practical guide aims to provide a structured framework for the integrative management of EoE in children and adults, for clinicians involved in their management, including gastroenterologists, allergists, pediatricians, otorhinolaryngologists, pathologists, primary care practitioners, and dietitians.
Participants in the consensus
A task force of 21 physicians and researchers with recognized expertise in the clinical evaluation, endoscopy, histopathology, epidemiology, physiopathology, allergy, and treatment of EoE was gathered to address specific clinically relevant topics.
First, a preliminary list of topics to be covered by the guidelines and its general goals was set by the Steering Committee (AJL, JM-I, AJB, JG-C, AMS, UVA, AS, SA) and surveyed to a panel integrated for adult patients with EoE, as well as parents of affected children, in order to collect their opinions on the importance of each statement, the need of being included in a practice guide and its potential impact on clinical practice. The results of the survey were considered to refine the final list of topic, but EoE patients and parents did not participate in guidelines development. The refined list of topics was then submitted to all the participating authors, integrated as a Working Committee, and participating on behalf of the United European Gastroenterology (UEG), The European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), the European Academy of Allergy and Clinical Immunology (EAACI), and the European Society of Eosinophilic Oesophagitis (EUREOS).
Secondly, the Working Committee drafted the first document that was revised in depth by the Steering Committee in a face to face meeting, revising the terms of statement wording, strength of recommendation and quality of evidence. It was submitted to be reviewed by the Working Committee, who prepared a second version of the document. This document was submitted to further review by an External Review Committee, which included gastroenterologists, pediatricians, allergists, pathologist and EoE patients and parents. Finally, the Working Committee drafted the final document.
Literature search
A formal systematic review of the literature was carried out for every statement. MEDLINE (accessed via PubMed) and EMBASE electronic databases, as well as The Cochrane Database of Systematic Reviews (The Cochrane Library) and the Cochrane Central Register of Controlled Trials (CENTRAL), were consulted covering the period up until August 2015, with no restriction of languages, and periodically updated (Annex A). A review of the citations to identify potentially relevant articles was also carried out. Priority was placed on the identification of systematic reviews and other documents offering a critical synthesis of the scientific literature, as well as randomized clinical trials, whenever possible. Librarian and methodological support was provided by experts at the Spanish Society of Digestive Diseases (SEPD).
Review and assessment of evidence
The Working Committee followed the GRADE methodology (see www.gradeworkinggroup.org) to assess the quality (certainty) of evidence, 8 and classified the recommendations for the different clinical scenarios into four clear and easy-to-understand final categories:9 strong recommendation for an intervention, implying for the clinician to do it; weak recommendation for an intervention, which implies to probably do it; weak against an intervention, implying to probably do not do it; and strong against an intervention, implying not to do it.
Defined using the GRADE methodology, these recommendations were mainly, but not solely, based on the strict assessment of the quality of the evidence (high, moderate, low, or very low quality). The quality of the evidence could be downgraded as a result of limitations in the study design or in its implementation, imprecision of estimates (wide confidence intervals), variability in the results, indirectness of the evidence, or publication bias; or upgraded because of a very large magnitude of effects, a dose-response gradient, or if all the plausible biases would reduce an apparent treatment effect. Furthermore, the recommendations were also based on some other factors, such as desirable and undesirable consequences of alternative management strategies, variability in values and preferences, and the use of resources (costs). GRADE assessments were then reviewed and agreed upon by voting members of the Working Committee at a final face to face meeting. Finally, the Agree II instrument (www.agreecollaboration.org) was used to ensure the high quality of our Clinical Practice Guideline, which was evaluated by the authors and the Steering Committee.
Consensus process
A full-day consensus meeting was held in Vienna, Austria, on 16 October 2016 in order to vote the statements and recommendations based on the nominal group technique.10 The participants decided whether they considered the statement/recommendation to be adequate, based on a six-point Likert scale (1: strongly disagree; 2: quite disagree; 3: somewhat disagree; 4: somewhat agree; 5: quite agree; 6: strongly agree), and suggested changes or new ones. After voting, the work groups revised the statement and recommendations according to the comments received, and a second vote was then held. The statements and recommendations resulting from the second vote were discussed and approved during this physical presence meeting. A statement/recommendation was approved if over 75% of the participants agreed with it (Likert score of 4–6).
The voting group was composed by 15 members integrated in the steering and Working Committees, including gastroenterologists, pediatricians, allergists, and methodologists, all of them settled in Europe with expertise in EoE. Although there were no dietitians or EoE patients’ representatives, the impact of the recommendations on dietitians, as well as community resources and local availability, was discussed prior to voting for each statement.
Statements and recommendations
Each statement/recommendation is accompanied by the level of evidence (LE: high, moderate, low or very low), the result of the vote (percentage agreement) at the consensus meeting, and discussion of the corresponding evidence. The strength of recommendation (SR: strong or weak) using the GRADE approach was only given for studies on the accuracy of diagnostic procedures, or which assessed the efficacy of a treatment, as mentioned above. This kind of classification is easy to understand and is flexible, since it can be applied to the different clinical scenarios.
Role of the funding sources
The SEPD administered all aspects of the meeting, which was funded by the UEG as a part of the Link Award program, with no external funding sources.
Recommendation statements
Epidemiological and clinical-focused questions formed the basis of the systematic literature reviews (see Appendix A in the online supplementary material). The working group formulated 45 recommendations based on these reviews (see Table 1).
Table 1.
Summary of European statements and recommendations on the management of EoE
| Section and number | Statements | Level of evidence | Strength of recommendation | Key references |
|---|---|---|---|---|
| Section A | EoE concept and epidemiology | |||
| 1 | EoE represents a chronic, local immune-mediated esophageal disease, characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation. Other systemic and local causes of esophageal eosinophilia should be excluded. Clinical manifestations or pathologic data should not be interpreted in isolation | NA | NA | Expert opinion |
| 2 | Adult patients achieving clinical and histological remission on PPI therapy are part of the EoE continuum, rather than a separate entity. Responders and non-responders to PPI therapy show overlapping phenotypic, genetic, and mechanistic features. More data are required in children. | Moderate | NA | 11, 12, 17, 19, 21, 22 |
| 3 | EoE and GERD are different entities and may coexist, either unrelated or interacting bidirectionally. | Moderate | NA | 26, 30 |
| 4 | The incidence of EoE has increased and currently varies widely from 1 to 20 new cases per 100,000 inhabitants per year (mean value 7). Prevalence rates ranges between 13 and 49 cases per 100,000 inhabitants | Moderate | NA | 44, 45 |
| 5 | The frequency of EoE in adults with esophageal symptoms undergoing an upper endoscopy is 7%. This frequency may rise up to 23% and 50% in patients with dysphagia and food impaction, respectively. Further data in children are required. | Moderate | NA | 46, 53, 54, 56, 60, 61 |
| 6 | EoE may occur at any age with a rising incidence in children with age and a peak in adults at 30–50 yrs | Moderate | NA | 62, 63, 64 |
| 7 | Male gender is a strong risk factor for EoE both in children and adults. | High | NA | 45 |
| 8 | Rhinitis, asthma and eczema are significantly more common in EoE patients compared to the general population. However, it remains unproven that atopy predisposes to EoE. | Moderate | NA | 79 |
| 9 | EoE is a distinct form of food allergy. IgE-mediated food allergies are common in EoE patients. | High | NA | 91 |
| 10 | EoE and celiac disease are independent disorders. | High | NA | 95 |
| 11 | EoE appears to have no causal or temporal relationship with hypereosinophilic syndromes, inflammatory bowel disease, esophageal atresia, and connective tissue disorders. | Low | NA | 99, 108, 110, 116 |
| Section B | Diagnosis | |||
| 12 | In older children and adults with EoE solid food dysphagia, food impaction, and non-swallowing associated chest pain are the most commonly reported symptoms. In younger children and infants the most common symptoms reported are reflux-like symptoms, vomiting, abdominal pain, food refusal and failure to thrive. | High | NA | 64, 87, 118, 119, 120 |
| 13 | Al least six biopsies should be taken from different locations, focusing on areas with endoscopic mucosal abnormalities. | Moderate | Strongly in favor | 121, 122, 129, 131 |
| 14 | The accepted threshold for eosinophil density for the diagnosis of EoE is 15 eosinophils per high power field (standard size of ∼0.3 mm2) in esophageal mucosa, taken as the peak concentration in the specimens examined. | Moderate | Strongly in favor | 132, 135, 136, 140 |
| 15 | Hematoxylin-eosin staining is sufficient for histological assessment of EoE in routine clinical practice. | Low | Weakly against | 141, 144, 147 |
| 16 | Besides peak eosinophil count, additional histological features may include eosinophil microabscesses, basal zone hyperplasia, dilated intercellular spaces, eosinophil surface layering, papillary elongation, and lamina propria fibrosis. | Moderate | Weakly in favor | 149, 150 |
| 17 | Currently, noninvasive biomarkers are not accurate to diagnose or monitor EoE. Some minimal invasive diagnostic tools show promise and merit further evaluation | Moderate | Strongly against | 159, 161, 163, 164, 165 |
| 18 | Symptoms do not correlate accurately with histologic disease activity, so histology currently continues to be necessary to monitor the disease. | Moderate | Weakly in favor | 166, 172 |
| 19 | Endoscopic findings alone do not reliably establish a diagnosis of EoE. Their value to assess disease activity needs further evaluation. | Low | Weakly in favor | 131, 183, 184 |
| Section C | Natural history | |||
| 20 | Untreated EoE is usually associated with persistent symptoms and inflammation, leading to esophageal remodeling resulting in stricture formation and functional abnormalities. There is some evidence that effective anti-inflammatory treatment may limit progression. | Moderate | NA | 64, 99, 185, 188, 189, 192, 151, 152 |
| 21 | EoE significantly impacts health-related quality of life of patients, impairing their social and psychological functioning. | Moderate | NA | 194, 195, 197, 198 |
| 22 | There is no evidence so far that EoE is a pre-malignant condition. | Moderate | NA | 207 |
| Section D | Treatment | |||
| 23 | PPI therapy induces clinical and histological remission in a proportion of pediatric and adult patients with EoE. | Moderate | Strongly in favor | 211, 212, 215 |
| 24 | In PPI responders, long-term PPI therapy is effective in maintaining remission | Low | Strongly in favor | 214, 219, 220 |
| 25 | Systemic steroids are not recommended in EoE | Moderate | Strongly against | 221 |
| 26 | Topical corticosteroids are effective for induction of histological remission in both pediatric and adult EoE patients. | High | Strongly in favor | 226, 227, 228, 229 |
| 27 | In steroids responsive patients, long-term therapy with topical corticosteroids is effective in maintaining remission in a proportion of patients. | Low | Strongly in favor | 224, 230, 231 |
| 28 | Swallowed topical corticosteroids seem to have a favorable safety profile in the treatment of EoE, with no serious side effects reported. Esophageal candidiasis, mostly incidental, may occur in up to 10% of patients. | Moderate | NA | 22, 230, 231 |
| 29 | There is a limited place for elemental diet in EoE, which should only be considered after failure of properly performed medical treatment and/or elimination diet. Elemental diet induces histologic remission in up to 90% of pediatric and adult EoE patients. There is limited information regarding symptoms. | Low | Weakly against | 63, 80, 235, 238, 239 |
| 30 | Food allergy testing-based elimination diet induces histologic remission in less than one third of adult patients. This rate may be higher in pediatric patients. | Moderate | Strongly against | 245, 239, 248, 250 |
| 31 | The utility of allergy tests in the identification of food triggers of EoE is consistently low in adults and variable in children. | Low | Strongly against | 237, 245, 255, 256 |
| 32 | An empiric six-food group elimination diet induces histologic remission in around three quarters of pediatric and adult patients. | Moderate | Weakly in favor | 84, 85, 239, 260 |
| 33 | In adult patients, an empiric four-food elimination diet achieves remission in half of the patients, whereas a two-food elimination diet (animal milk and gluten-containing cereals) may be still effective in 40% of patients. | Moderate | Weakly in favor | 250, 261, 265 |
| 34 | Prolonged avoidance of triggering foods may lead to drug-free sustained clinical and histological remission of EoE. | Low | Strongly in favor | 85, 260, 268 |
| 35 | Endoscopic dilation improves dysphagia in up to three quarters of adult EoE patients with reduced esophageal caliber, without having an effect on the underlying esophageal inflammation. | Moderate | Strongly in favor | 269, 274 |
| 36 | Endoscopic dilation in EoE is a safe procedure, with a risk of esophageal perforation smaller than 1%. | Moderate | NA | 269 |
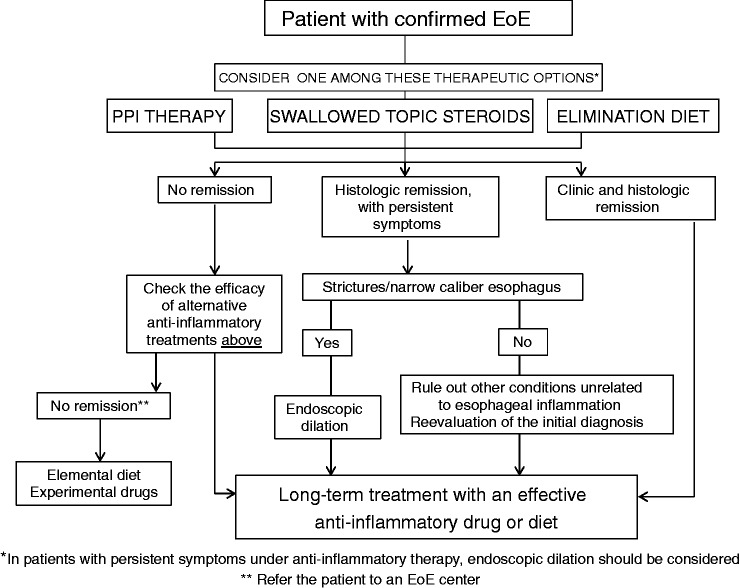
| 37 | PPIs, diet or topical steroids might be offered as first line anti-inflammatory therapy. The choice of therapy should be individually discussed with the patient and might be potentially interchangeable over time. The efficacy of any therapy should be checked by a follow-up endoscopy after a 6- to 12-week initial course. Endoscopic dilation should be considered in patients with dysphagia/food impaction unresponsive to anti-inflammatory treatment. | Low | Strongly in favor | Expert opinion |
| 38 | Azathioprine and 6-mercaptopurin might play a role in inducing and maintaining long-term remission in EoE in limited cases. | Low | Weakly in favor | 278 |
| 39 | Sodium cromoglicate and antihistamines have no effect on symptoms or esophageal eosinophilia. | Low | Strongly against | 63 |
| 40 | There is insufficient evidence to recommend montelukast, a leukotriene receptor antagonist, in patients with EoE. | Moderate | Strongly against | 283, 284 |
| 41 | First generation chemoattractant receptor- homologous molecule on Th2 cells (CRTH2) antagonists induces modest clinic and histologic improvement in EoE. | High | Weakly against | 285 |
| 42 | The anti-IL5 antibodies mepolizumab and reslizumab have no effect on symptoms and modestly reduce esophageal eosinophilia. | High | Strongly against | 286, 287, 288 |
| 43 | QAX576, an anti-IL13 antibody, has no effect on symptoms but reduces esophageal eosinophilia and downregulates EoE transcripts in a sustained manner. | High | Weakly against | 289 |
| 44 | Omalizumab, an anti-IgE antibody, has no effect on symptoms or esophageal eosinophilia. | High | Strongly against | 256 |
| 45 | Infliximab, an anti-tumor necrosis factor alpha antibody, has no effect on symptoms or esophageal eosinophilia. | Low | Strongly against | 292 |
EoE: eosinophilic esophagitis; PPI: proton pump inhibitor.
Section A. EoE concept and epidemiology
What is the current definition of EoE?
Statement 1: EoE represents a chronic, local immune-mediated esophageal disease, characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation. Other systemic and local causes of esophageal eosinophilia should be excluded. Clinical manifestations or pathologic data should not be interpreted in isolation.
LE: NA; Agreement: 100%, votes: strongly agree (100%).
Summary of the evidence: EoE as a distinct entity was first described in 1993 and 1994.1,2 Guidelines for the disease, which were originally written in 2007,3 were updated in 2011 when a first formal definition of the disease was provided.4 EoE was defined as a chronic immune/antigen-mediated disease, isolated to the esophagus, and characterized by symptoms of esophageal dysfunction and eosinophil-predominant inflammation on esophageal biopsy. It is important to stress that other systemic and local causes of esophageal eosinophilia should be excluded (see Supplementary table 1).
From 2011, the most relevant advances in the definition of EoE have been related to evolving considerations of the trial of proton pump inhibitor (PPI) therapy as a diagnostic criteria and the disease phenotype termed PPI-responsive esophageal eosinophilia (PPI-REE). Aside from clinic and histologic features, the original definition diagnostic criteria for EoE in 2007 included a PPI trial:3 only patients unresponsive to PPI therapy (or alternatively with a normal esophageal pH monitoring) could be diagnosed of EoE. These criteria were based on the assumption that only GERD, as an acid-related disorder, could respond to the acid suppressive effect of PPIs, and considered GERD and EoE as mutually exclusive disorders. The description of a new potential disease phenotype in 2011, termed PPI-REE, was acknowledged as one of the major additions to previous knowledge.4 It refers to patients with clinic, endoscopic and histologic features of EoE which completely remit on PPI therapy, albeit not necessarily associated to GERD.11 Consequently, PPI-REE replaced GERD as the main differential diagnosis of EoE and pH monitoring was retracted as a diagnostic criterion. However, all iterations of guidelines since 2011 have systematically maintained a PPI trial as a diagnostic criterion,4–6 since PPI-REE and EoE were considered distinct disorders as they showed a different response to the PPI trial.
Since 2011, solid evidence, mostly from adult patients, has highlighted that PPI-REE and EoE are virtually indistinguishable from one another, even at the genetic level, and very different from GERD.12 No other inflammatory disease than PPI-REE is defined by its response to a single medication, instead of by its clinic, endoscopic, bioptic, molecular, genetic, and therapeutic overlap with EoE. Therefore, the main novelty in these guidelines is the retraction of the term PPI-REE and the consideration of PPI therapy not as a diagnostic criterion for EoE, but rather as a therapeutic agent. Consequently, these guidelines consider that clinical and histological features suggestive of EoE may remit with treatment of PPI therapy, topical steroids or elimination diets. Since it still remains unknown whether the esophageal immune response in patients who respond to PPI therapy is triggered by GERD, food allergens or the combination of both factors, the term “antigen” has now been removed from the definition.
Does response to PPI therapy rule out EoE?
Statement 2: Adult patients achieving clinical and histological remission on PPI therapy are part of the EoE continuum, rather than a separate entity. Responders and non-responders to PPI therapy show overlapping phenotypic, genetic, and mechanistic features. More data are required in children.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Over the past few years, multiple studies have closed the gap between PPI-REE and EoE. Both disorders have been repeatedly reported to be phenotypically indistinguishable, in terms of clinical, endoscopic, pH monitoring, histological, and molecular data.11–22 A major contribution in this field has been the identification of a similar pattern of gene up- and down-regulation comprising the EoE hallmark gene signature in EoE and PPI-REE patients, but not in GERD patients.21 After in vitro demonstration of potential anti-inflammatory effects of PPIs,22 recent clinical studies have shown PPI monotherapy effectively down-regulates allergic Th2 inflammation in PPI-REE patients,17,20,22 in a similar way to that seen in EoE after topical steroid therapy. Moreover, PPI therapy in PPI-REE patients can almost normalize the overall genetic signature, similar to that found in EoE patients.21 In addition, two recent series have reported that EoE patients responsive to diet/topical steroids may also achieve remission on PPI therapy.23,24 Collectively, growing evidence underscores that it may be counterintuitive to separate PPI-REE from EoE depending on a different response to a single medication, when both disorders exhibit overlapping phenotypic, genetic, and mechanistic features. These evolving considerations have been recently compiled in a Position Paper endorsed by an international panel of experts.12 Recently, the first series of three patients with symptoms and histologic features of EoE responsive to vonoprazan has been published.25 Vonoprazan is a new potassium-competitive acid blocker with a more potent and sustained acid suppression than PPI therapy. These novel findings underscore the importance of GERD and targeting gastric acid output in a subset of EoE patients, although it does not necessarily exclude an antigen-mediated Th2 inflammatory response.
What is the relationship between GERD and EoE?
Statement 3: EoE and GERD are different entities and may coexist, either unrelated or interacting bidirectionally.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: GERD and EoE are the most frequent esophageal diseases. Both conditions are more common in young males, so their coexistence is plausible. Consequently, they are not mutually exclusive disorders and may coexist in a single patient, albeit not necessarily interacting. In a patient with symptoms of esophageal dysfunction, a well-established diagnosis of GERD based on endoscopic findings or pH monitoring may not necessarily rule out EoE.
The interacting relationship between GERD and EoE might be bidirectional and complex.26 It has been suggested that GERD may contribute to the pathogenesis of EoE by causing esophageal mucosal integrity changes, promoting trans-epithelial allergen permeation and subsequent allergic immune activation.20 However, this hypothesis remains unproven. EoE patients have been shown to have hypersensitivy to the presence of intra-esophageal acid, with lower thresholds for onset of symptoms and pain after esophageal acid infusion when compared to healthy volunteers.27 Esophageal mucosal integrity at baseline is markedly impaired in EoE patients compared to healthy controls.20 Since acid hypersensitivity is strongly related to impaired esophageal mucosal integrity, these structural changes may justify the observed acid hypersensitivity. Acid hypersensitivity might also explain symptom improvement or remission on PPI therapy despite persistent esophageal inflammation in pediatric and adult EoE patients.11,13,17,28–31 Likewise, EoE may potentially induce architectural and functional changes in the esophagus that can induce GERD.26,30
What is the current incidence and prevalence of EoE?
Statement 4: The incidence of EoE has increased and currently varies widely from 1 to 20 new cases per 100,000 inhabitants per year (mean value 7). Prevalence rates range between 13 and 49 cases per 100,000 inhabitants.
LE: Moderate. Agreement: 100%, votes: strongly agree (93%).
Summary of evidence: Several studies aimed to estimate the incidence and prevalence of the disease with different designs, including prospective and retrospective registries of cases, series of endoscopies and esophageal biopsies, and population-based studies, have consistently shown that the incidence and prevalence of EoE have risen rapidly. Different studies with different methodologies have shown that the current incidence rate ranges between 6 and 13 new cases per 100,000 inhabitants-year in Europe,32–35 USA,36–38 and Canada.39,40
Regarding prevalence, an increase from 9.91 in 2000 to 42.96/100,000 inhabitants in 2003 was reported for children.38 These last prevalence rates are consistent with other recent studies in patients of all ages carried out in USA,36,37,41,42 and in Europe (Spain, Switzerland, and Denmark),32–35,43 which have shown a gradual and significant increase of up to 40–56 cases per 100,000 inhabitants.
A pediatric population-focused systematic review has estimated that the incidence of EoE in children varied from 0.7 to 10/100,000 per person-year,44 while prevalence ranged from 0.2 to 43/100,000.
A recent systematic review with meta-analysis of population-based studies has shown an increase in the overall incidence rates of EoE after 2008, being currently of 7.2 (95% CI 0.8–20.2) new patients/100,000 inhabitants yearly. The best estimates for current pooled prevalence were 28.1 (95% CI 13–49) patient per 100,000 inhabitants. It was also observed that the incidence and prevalence rates were significantly higher in adults than children and in studies carried out in America compared to Europe.45
What is the frequency of EoE in patients with esophageal symptoms?
Statement 5: The frequency of EoE in adults with esophageal symptoms undergoing an upper endoscopy is 7%. This frequency may rise up to 23% and 50% in patients with dysphagia and food impaction, respectively. Further data in children are required.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: This question has been addressed mostly in adult patients. The prevalence of EoE was prospectively determined in 400 consecutive adult patients with esophageal symptoms undergoing routine upper endoscopy in the USA. The prevalence of EoE in this cohort was 6.5% (25/385; 95% CI, 4.3–9.4%) if the histologic cut-off was >20 eosinophils per high power field (eos/hpf) and increased to 7.3% when the cut-off changed into >15 eos/hpf.46
The prevalence of EoE may vary depending on the evaluated symptom.47 EoE is uncommon in patients with refractory GERD symptoms (0.9–8%).48–52 In adults with non-cardiac chest pain, EoE was found in 6% of the patients.53 EoE prevalence may rise up to 23% and 46% in patients undergoing upper endoscopy for dysphagia and food impaction, respectively.15,54–59
In pediatric populations who underwent an upper endoscopy for any indication, the prevalence of EoE ranged from 2.3% to 6.8%, with a pooled prevalence of 3.7% (95% CI 2.4–5.1).44 In children under 18 years of age undergoing upper endoscopy for abdominal pain, the frequency of EoE was 6%,60 and 14 of 376 children with refractory aerodigestive symptoms (3.7%) were diagnosed as having EoE refractory to medical treatment.61
What is the age of presentation of EoE?
Statement 6: EoE may occur at any age with a rising incidence in children with age and a peak in adults at 30–50 yrs.
LE: Moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of available evidence: EoE has been reported throughout the life span, from infancy to almost 100 years of age.62 However, most cases occur in children, adolescents, and adults younger than 50 years. Available data mostly come from retrospective studies in both pediatric and adult populations. As for children, most studies coincide with peak incidence in older children.37,38,63,64 Studies in adults have shown that the majority of cases is clinically apparent at the age of 30–50 years.33,36,37
Is male gender a risk factor for EoE?
Statement 7: Male gender is a strong risk factor for EoE both in children and adults.
LE: High. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Nearly all studies have shown that the prevalence of EoE is higher in males than in females both in children and in adults.44,62,65–69 Male predominance is shown in Europe,32,35,43,70,71 in the US and Canada,37,46,62,67,72 and in some reports from the East,73–75 with a low number of EoE cases. In a retrospective Australian study on children, 30 of 42 cases who were reclassified as EoE were boys but the difference was not statistically significant.76 In contrast, in another general population based endoscopy study from northern Sweden with 1000 randomly selected subjects with or without gastrointestinal symptoms the prevalence of EoE was 1.1% and there was a male predominance.77 In a recent meta-analysis of EoE in children and adults in population-based studies by Arias et al. there was a clear male predominance, odds ratio (OR) 2.01 (95% CI 1.63–2.48).45 Although twin and family studies have revealed strong environmental and weaker genetic cues explaining heritability of EoE,72 a gender-specific association between single nucleotide polymorphisms (SNP) in thymic stromal lymphopoietin (TSLP) gene as well as a nonsynonymous SNP in the TSLP receptor has been suggested as a mechanism for the male predilection of EoE.78 In conclusion, male gender is a two- to threefold risk factor for EoE.
Is EoE associated with atopy?
Statement 8: Rhinitis, asthma, and eczema are significantly more common in EoE patients compared to the general population. However, it remains unproven that atopy predisposes to EoE.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: EoE patients usually suffer from a high number of concomitant atopic disorders including rhinitis, asthma and eczema. A limited number of studies have provided direct comparisons of atopy between EoE and series of patients with GERD, upper gastrointestinal symptoms or healthy volunteers endoscopically assessed to exclude EoE, or database-registered subjects.
A recent systematic review of 21 studies overall including 53,592 adult and pediatric EoE patients and 54,759 controls found that the criteria for defining a diagnosis of atopy in either EoE patients or controls was not structurally considered in most of the studies. Despite this limitation, overall allergic rhinitis was significantly more common among EoE patients compared to control subjects (OR 5.58; 95% CI 3.27–9.53), as were bronchial asthma (OR 3.06; 95% CI 2.01–4.66) and eczema (OR 2.86; 95% CI 1.88–4.36).79
Is EoE a food allergy and how does it relate with other food allergies?
Statement 9: EoE is a distinct form of food allergy. Immunoglobulin E (IgE)-mediated food allergies are common in EoE patients.
LE: High. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: From its early descriptions, EoE was considered as a particular form of food allergy in which the esophageal inflammatory infiltration remitted after exclusively feeding patients with amino acids-based elemental diets.80 A personal history of atopy is documented prior to EoE diagnosis in 50–60% of cases.36,81,82 Most EoE patients are sensitized to aeroallergens or food allergens, identified by serum IgE measurements or by skin prick tests.83 On the other hand, a small proportion of EoE patients do not suffer from concomitant atopy, and therefore they are not sensitized to foods nor aeroallergens. EoE shows no relevant differences in these particular patients, among whom offending foods can be also identified after empiric elimination diet and sequential food reintroduction.84,85
The criteria used to define “food allergy” were extremely variable, ranging from food sensitization exclusively to food-induced anaphylaxis and even celiac disease.79 Likewise, allergy tests used for defining and diagnosing food allergy have yield variable results when used in patients with EoE: 15–43% of EoE patients also have IgE-mediated food allergies,36,81 and even a high prevalence of anaphylaxis has been documented in EoE patients,86 giving rise to the hypothesis that the presence of IgE-mediated food allergy might be considered a predictive factor for the subsequent development of EoE in adult and pediatric patients.87 Several case series have reported patients undergoing oral immunotherapy for progressive desensitization from IgE-mediated food allergy that eventually developed EoE,88–90 whose risk has been summarized in a meta-analysis to be 2.72% (95%CI 1.7–4.0%).91 However, there is insufficient evidence on the relative risk of oral immunotherapy for food allergies to induce de novo EoE. Once EoE is accidentally triggered by oral immunotherapy, most cases remit after discontinuation of oral immunotherapy.88,92 The decision of whether discontinuing immunotherapy or maintaining it while treating pharmacologically EoE may depend on the severity of the food allergy episodes (e.g. anaphylaxis) intended to prevent.
Is EoE related with celiac disease?
Statement 10: EoE and celiac disease are independent disorders.
LE: High. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: An association between celiac disease and EoE has not been demonstrated in large cross-sectional and population-based epidemiological studies.93,94 A systematic review on this topic found no support for the hypothesis of a true association,95 but evidence pointed toward the independent course of both diseases, including the lack of a common genetic basis and an effectiveness for gluten-free diet in achieving histological remission of EoE in celiac patients of only 32%,96 similar to that expected for wheat elimination in EoE patients. A significant publication bias in favor of short studies reporting positive associations between both diseases was also documented.95
Does EoE predispose to other associated disorders?
Statement 11: EoE appears to have no causal or temporal relationship with hypereosinophilic syndromes, inflammatory bowel disease (IBD), esophageal atresia, and connective tissue disorders (CTDs).
LE: Low. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Esophageal involvement has been described in some cases of eosinophilic gastroenteritis and other eosinophilic gastrointestinal disorders (EGID).97,98 However, EoE largely remains as a disorder indefinitely restricted to the esophagus with no cases later extending to distal GI segment described. An anomalous T-lymphocyte clone expansion toward the development of hypereosinophilic syndrome has neither being described in the natural history of EoE.99
The concurrence of EoE and Crohn’s disease in two patients led to speculation on the true relationship between both disorders characterized by an idiopathic dysregulated mucosa immune response causing inflammation.100,101 In the first reported case,100 EoE was diagnosed 8 years after the patient had been suffering from Crohn’s disease, while the second presented with EoE 3 years before the onset of Crohn’s disease.101 IBD and EoE are highly prevalent disorders in Westernized countries, respectively affecting 137–241 and 45–56 patients/100,000 inhabitants in Europe and US;32,33,37,102–107 concomitances of both diseases in the same patients are so rarely described that, from an epidemiological point of view, they should be considered as completely independent disorders. The expression of surface markers in blood eosinophils from patients allow distinguishing IBD and EoE from each other and from healthy controls,108 which provided additional evidence on the independence of both diseases by the distinct patterns of activation signals from the inflamed tissues.
Several case reports and short series of children and adolescents with esophageal atresia have suggested that EoE appears as a concomitant problem among them.109–113 A male predominance, frequent sensitization to food and/or aeroallergens, and peripheral eosinophilia are also observed in patients who share both conditions, in whom eosinophilic esophageal infiltration and symptoms also reverse after topic or systemic steroids and after dietary therapy. Some genetic connections between esophageal atresia and EoE have been suggested by mice models that involves microdeletions in the Forkhead box (FOX) transcription factor gene cluster. Specifically, the FOXF1 gene has been involved in esophageal atresia and other anomalies,114 and binding sites for the FOXF1 protein include promoter regions of proinflammatory genes as those for eotaxins.115 Further research should establish the etiological association between both conditions.
Retrospective database analyses have shown a link between EoE and CTDs, including Marfan’s syndrome (MFS), hypermobile Ehlers–Danlos syndrome (EDS), and joint hypermobility syndrome (JHS), by the finding of an unexpectedly higher than expected (8-fold) prevalence of EoE among patients with CTDs (relative risk: 8.1; 95% CI 5.1–12.9).116 The investigation of the molecular connection of this association found mutations in fibrillin-1 (FBN1) and TGFBR1 genes, which were related to an impaired epithelial barrier function and excessive TGF-β signaling,117 respectively, with both contributing to the EoE-CTD proposed phenotype. Further prospective research must confirm the aforementioned syndromic association and to establish the particularities of EoE among these patients.
Section B. Diagnosis
What are the most common symptoms in EoE?
Statement 12: In older children and adults with EoE solid food dysphagia, food impaction, and non-swallowing associated chest pain are the most commonly reported symptoms. In younger children and infants the most common symptoms reported are reflux-like symptoms, vomiting, abdominal pain, food refusal, and failure to thrive.
LE: high. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Several studies describing presenting symptoms of EoE clearly show a different pattern of clinical presentation between adults and young children. In adults, dysphagia (70–80%) and food impaction (33–54%) constitute the most common symptoms. Other associated symptoms are heartburn, regurgitation, chest discomfort and exercise-induced chest pain.87,118–120
Clinical manifestations of EoE in infants and toddlers mainly consist of non specific symptoms such as reflux-like symptoms, vomiting, nausea, abdominal pain, food refusal, or failure to thrive.64 Older children, usually over 10 years old, and adolescents might exhibit dysphagia and food impaction like adult patients.
What is the appropriate biopsy protocol for diagnosing and monitoring EoE?
Statement 13: At least six biopsies should be taken from different locations, focusing on areas with endoscopic mucosal abnormalities.
LE: Moderate; SR: Strong in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Because inflammatory changes in EoE are frequently patchy and may not be present in all biopsies,121–125 it is recommended that at least 6 biopsies should be obtained from at least two different locations in the esophagus, typically in the distal and proximal halves of the esophagus. Diagnostic sensitivity increases with the number of biopsies and is maximized after taking at least six biopsies.126–128
Esophageal biopsies should be targeted to areas of endoscopic abnormality, mainly white exudates and longitudinal furrows, which are associated with higher peak eosinophil counts.121,122,129,130 Biopsies should also be taken despite a normal endoscopic appearance of the esophagus, which has been reported in up to 10–32% of adult and pediatric patients, respectively.63,131 It is also advisable to obtain duodenal and gastric mucosal biopsies at the moment of initial diagnosis in order to exclude eosinophilic gastroenteritis.
What is the accepted threshold for eosinophil mucosal density for the diagnosis of EoE?
Statement 14: The accepted threshold for eosinophil density for the diagnosis of EoE is 15 eos/hpf (standard size of ∼0.3 mm2) in esophageal mucosa, taken as the peak concentration in the specimens examined.
LE: Moderate; SR: Strong in favor. Agreement: 100%, votes: strongly agree (87%).
Summary of evidence: Having a histologic threshold for the diagnosis of EoE is useful to make a distinction from other inflammatory esophageal diseases. The 15 eos/hpf threshold was set to increase the uniformity of EoE diagnosis,132–134 upon its capacity to reliably distinguish between EoE and GERD.87,135–139 GERD is associated to low eosinophil counts, usually <5 eos/hpf, but it is important to re-emphasize that GERD and EoE are not mutually exclusive disorders and may coexist. A sensitivity of 100% and a specificity of 96% for the diagnosis of EoE have been recently shown with the cut-point of 15 eos/hpf.140
The selected 15 eos/hpf threshold, however, is somewhat arbitrary and clinical judgment is required to interpret the significance of borderline counts, as well as counts compatible with EoE in asymptomatic patients. Additional limitations to quantifying eosinophil counts are variability in the definition of an intraepithelial eosinophil in hematoxylin stained tissue sections and the lack of standardization of the size of a high-power field, since different microscopes may have different high-power field areas.132 Therefore, it may be useful to report eosinophil density (eos/mm2) together with the eosinophil count (eos/hpf); communication with the pathologist upon questionable findings can also be helpful in clinical practice.
As for histological assessment of EoE, are there other techniques apart from hematoxylin-eosin staining?
Statement 15: Hematoxylin-eosin (HE) staining is sufficient for histological assessment of EoE in routine clinical practice.
LE: Low. SR: Weakly against. Agreement: 100%, votes: strongly agree (87%).
Summary of evidence: HE staining is sufficient for the assessment of eosinophil counts and associated histologic features in clinical practice. Specific techniques, including immunochemistry,141–144 electron microscopy,145–147 and confocal microscopy following immune fluorescence staining,148 are currently performed for research purposes only.
Which additional histological markers besides peak eosinophil counts could be considered in the histological assessment of EoE?
Statement 16: Besides peak eosinophil count, additional histological features may include eosinophil microabscesses, basal zone hyperplasia, dilated intercellular spaces, eosinophil surface layering, papillary elongation, and lamina propria fibrosis.
LE: low. SR: Weakly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Currently, the histologic diagnosis of EoE relies on a peak count ≥15 eos/hpf assessed within the epithelial stratum. However, other histologic features that can be assessed in HE-stained slides include eosinophil abscesses, basal zone hyperplasia, dilated intercellular spaces, eosinophil surface layering, and papillary elongation of the squamous epithelium.149 These histologic abnormalities are not specific for EoE and might be found in other esophageal diseases, but tend to be more severe in EoE patients.
An EoE-specific histologic scoring system (EoEHSS) has been recently developed, and in-site validated, to provide a standardized method to evaluate esophageal biopsies for features in addition to peak eosinophil count (Supplementary table 2).150 Histologic abnormalities were scored for severity (grade) and extent (stage) in a four-point scale (0 normal; 3 maximum change) for eight EoE-associated features, including eosinophil density, basal zone hyperplasia, eosinophil abscesses, eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis. A strong-to-moderate agreement was found among the three pathologists who evaluated the biopsy samples. The EoEHSS composite score better discriminated treated from untreated patients than peak eosinophil count. Interestingly, this score might be utilizable by pathologists after minimal training, consisting of less than one minute per biopsy slide. Lamina propria fibrosis was not included in the EoEHSS score because it was not present in the majority of biopsies. However, it should be also evaluated when available, since several EoE therapies have demonstrated a potential ability to reverse existing remodeling fibrotic changes of the esophagus.151–154
Are there less invasive diagnostic tests useful for diagnosing or monitoring EoE?
Statement 17: Currently, noninvasive biomarkers are not accurate to diagnose or monitor EoE. Some minimal invasive diagnostic tools show promise and merit further evaluation.
LE: moderate. SR: Strongly against. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Finding a reliable none or minimally invasive method to monitor disease activity of EoE, therefore avoiding repeat endoscopies with biopsies, has been largely pursuit. Only absolute serum eosinophil count has been consistently shown to significantly correlate with the degree of esophageal eosinophilia, and to significantly decrease after steroid- or PPI-induced histologic remission.155–161 However, its diagnostic accuracy was only 0.754.160 Potential biomarkers, including total IgE, eosinophil cationic protein,159–161 eosinophil-derived neurotoxin,156,161,162 mast cell tryptase,160 several chemokines,156,160 and fractionated exhaled nitric oxide,163 have all failed to diagnose or monitor the disease. Likewise, a comprehensive diagnostic panel of inflammatory factors known to be associated with EoE pathogenesis was not increased in the serum or correlated with response to topic steroid treatment.164
As for minimally invasive devices, both the String Test (a capsule filled with approximately 90 cm of string) and the Cytosponge (an ingestible gelatin capsule comprising compressed mesh attached to a string) have shown preliminary good correlations with esophageal eosinophilia degree and eosinophil-derived proteins.165,166 These results should be further corroborated in larger studies.
Are symptoms alone accurate to monitor disease activity in EoE?
Statement 18: Symptoms do not correlate accurately with histologic disease activity, so histology currently continues to be necessary to monitor the disease.
LE: moderate, SR: weakly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Assessment of activity in EoE includes patient-reported outcome (PRO) measures (symptoms and quality of life), clinician-reported outcome (ClinRO), and objective measures (laboratory, endoscopic, and histologic findings).167 The lack of validated instruments to assess EoE activity has definitely led to an inconsistent relationship between symptoms and esophageal inflammation in EoE patients.29,157,168–170 In adult patients, EoE activity can be currently assessed using the validated Eosinophilic Esophagitis Activity Index (EEsAI) PRO instrument.171 This index quantifies the difficulties foreseen by patients with different food consistencies, as well as dietary or behavioral modifications for the same food consistencies. Recently, a prospective multicenter study has shown a modest predictive capacity of distinct EEsAI cutoff values to predict either histologic or endoscopic remission.172 Therefore, clinicians should not make assumptions about the biological activity of EoE exclusively upon symptoms, and endoscopic esophageal biopsies currently continue to be necessary to accurately monitor the disease activity.
Alternative validated instruments for symptom assessment are the Dysphagia Symptom Questionnaire in adults and the pediatric EoE symptom score (PEESS).173–175 Evaluation of EoE-related quality of life can also be helpful when assessing disease activity. Currently validated questionnaires are the EoO-QoL-A in adults and children and the PedsQL in pediatric patients.176,177
A new endoluminal functional lumen imaging probe, the EndoFLIP system, has initially demonstrated a significant reduction in esophageal distensibility in EoE patients.178 This functional test has shown a lack of correlation of eosinophil counts and esophageal distensibility, partially explaining the dissociation between inflammatory activity and symptoms in EoE.179 Furthermore, reduced esophageal distensibility predicted risk for food impaction,179 and correlated with endoscopically identified ring severity.180 Whether the addition of the EndoFLIP system to PRO measures can enhance our accuracy to predict the real biological activity of EoE warrants further investigation.
Are endoscopic findings important for diagnosing or monitoring EoE?
Statement 19: Endoscopic findings alone do not reliably establish a diagnosis of EoE. Their value to assess disease activity needs further evaluation.
LE: low. SR: weakly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Several endoscopic findings have been associated with EoE, either affecting the mucosal surface [edema or decreased vascularity (also referred as loss of vascular pattern), longitudinal furrowing, rings (also called trachealization), white plaques (also referred as spots or exudates), and fragile (or crêpe paper) mucosa] or the esophageal caliber [strictures and narrow caliber esophagus]. A first meta-analysis including 4678 patients with EoE and 2742 non-EoE controls revealed modest sensitivity and negative and positive predictive values for endoscopic features to predict esophageal inflammation.131 Of note, the prevalence of endoscopic features was substantially heterogeneous among the included studies.
Consequently, the EoE endoscopic reference score EREFS (acronym for exudates, rings, edema, furrows, and strictures) was proposed as a standardized tool to classify and grade the presence and severity of the five major endoscopically features of EoE.181 The EREFS classification system was validated in adult patients in a prospective multicenter study, with good interobserver agreement among practicing and academic gastroenterologists.182 External validation of the EREFS system showed consistent scoring between experts and trainee endoscopists.183 Regarding the accuracy of the EREFS system to diagnose and monitor EoE activity, conflicting results have been shown in two recent single-center studies.182,184 As such, larger multicenter studies are required to ascertain the utility of the EREFS system for disease activity assessment. All these data indicate that endoscopists should not base a diagnosis of EoE, neither to make assumption on the activity or remission of this disease exclusively based on endoscopic findings.
Section C. Natural history
Is EoE clinically, endoscopically, histologically, and functionally a progressive disorder?
Statement 20: Untreated EoE is usually associated with persistent symptoms and inflammation, leading to esophageal remodeling resulting in stricture formation and functional abnormalities. There is some evidence that effective anti-inflammatory treatment may limit progression.
LE: moderate. Agreement: 100%, votes: strongly agree (47%).
Summary of evidence: The first prospective assessment of a series of 30 adult patients with EoE with an average of 7.2 years of follow-up documented that, in the absence of anti-eosinophil treatment or elimination diets, dysphagia and esophageal eosinophilic infiltration persisted over time. Subepithelial fibrosis also developed.99 For pediatric EoE, the first data on its natural history was evaluated in 89 children with an 8-year follow-up,185 founding EoE to be a chronic and relapsing condition, as confirmed in a large chart review of 620 EoE patients (where 330 patients had a greater than 1 year follow-up for analysis) at the Children’s hospital of Philadelphia.64 While the majority of young adults diagnosed with EoE during childhood continued to require pharmacologic treatment and/or dietary modification for EoE,186 recurrent symptoms were present in 91% of the 32 adults retrospectively followed for a 3.3 year period at Mayo Clinic, leading to 61% of them to repeated treatment with swallowed topical steroids at least once.187
The duration of an untreated disease, expressed as diagnostic delay, constitute the major risk factors for esophageal remodeling and stricture formation in EoE, as nicely shown in retrospective studies. The analysis of 200 Swiss adult EoE patients show that delay in diagnosis determined the prevalence of fibrotic esophageal features, which increased from 46.5% in diagnostic delay up to 2 years to 87.5% when it was >20 years.188 Similarly, a significant difference in esophageal diameter was determined by a delayed diagnosis in adult EoE patients, ranging from <10 mm if EoE diagnosis was reached after 14.8 years to ≥17 mm when it lasted only 5 years.189 Disease duration has been also identified as the leading association for abnormalities in high-resolution manometry in EoE patients.190 On the other hand, patient’s age significantly increases both subepithelial collagen deposits and the likelihood of fibrostenotic disease.191 The OR for fibrostenosis for each 10-year increase in age was found to be 2.1 (95% CI 1.7–2.7).192 All these findings suggested that the natural history of EoE represents a progression from an inflammatory to a fibrostenotic phenotype.
Some preliminary data are showing the ability of both topic steroids and dietary treatment to reverse esophageal remodeling in children, potentially avoiding esophageal strictures by reversing epithelial mesenchymal transition.151–154
Does EoE affect quality of life of patients?
Statement 21: EoE significantly impacts health-related quality of life of patients, impairing their social and psychological functioning.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: EoE may have a profound effect on the quality of life and psychosocial adjustment of affected children and their families, including social difficulties, anxiety, sleeping difficulties, depression, and school problems.193,194 Scores measuring health-related quality of life are significantly worse in pediatric patients with more pronounced esophageal symptoms,195 in children with active histologic disease and those treated with dietary restrictions.177 Impairment of quality of life persists over time, even 15 years after following the initial diagnosis,196 and improves during the course of evaluation and treatment.195 Patients with less symptom severity were those who showed the most improved quality of life scores.195
As for adult patients, psychosocial domains are affected, but not physical wellbeing or mental functioning.197 Anxiety mainly derives from concerns related to the disease itself (uncertainty about the long-term consequences of a chronic illness, fears of disease progression, long-term medication), highly restrictive dietary modifications and swallowing difficulties/choking hampering social interactions.197 Similar to those findings observed in children, quality of life in adult patients with EoE is worse in patients with more symptom severity and biological disease activity.198
Does EoE progress into malignancy?
Statement 22: There is no evidence that EoE is a pre-malignant condition.
LE: Moderate. Agreement: 100%, votes: strongly agree (93%).
Summary of evidence: Anecdotal case reports had suggested a relationship between esophageal neoplasia and esophageal eosinophilia,199,200 but neither patient fulfilled diagnostic criteria for EoE. Therefore, esophageal eosinophilia was likely tumor-associated inflammation. Esophageal eosinophilia (not necessarily EoE) has been also associated with Barrett's esophagus.201–206 In a series of 13 adult patients, no patient developed esophageal dysplasia or carcinoma after follow-up for a mean period of 13.6 years (range 5–24 years).207 As for children with EoE, the expression of p53 and Ki-67 (early markers of esophageal dysplasia) on immunochemistry was found to be significantly increased compared to both children with normal esophagus and GERD, but was reduced following medical therapy and not associated with dysplasia.208
Section D: Treatment
Is PPI therapy effective in inducing remission of EoE?
Statement 23: PPI therapy induces clinical and histological remission in a proportion of pediatric and adult patients with EoE.
LE: moderate, SR: strong in favor. Agreement: 100%, votes: strongly agree (93%).
Summary of evidence (see Supplementary table 3): From 2006 to 2010, several retrospective case series and studies highlighted the existence of patients with clinical, endoscopic and histological features compatible with EoE showing clinicopathological response to PPI therapy.28,57,209,210 In 2011, a first large prospective study in adult patients with a similar profile reported a 50% response after an 8 week course of PPI therapy.11 Of note, response to PPI therapy was observed in 80% and 33% of patients with pathological and normal esophageal acid exposure on pH monitoring, respectively.11
Since then, several RCTs and prospective studies have fully corroborated remission rates on PPI therapy ranging from 33% to 36%,14,15, 211,212 when histological remission was defined by <5 to 7 eos/hpf. Of note, remission rates increased to 50% and 57% when histological remission was redefined as <15 eos/hpf.14,211 A first review article in 2013 revealed response to PPI therapy was significantly commoner with documented GERD when compared to patients with negative pH monitoring (70% vs. 29%, p < 0.001).213 The first prospective study conducted in pediatric patients has lately shown a 47% rate of histological remission on PPI therapy.214
A recent systematic review with meta-analysis, including 33 studies with 619 patients with suspected EoE, has shown that PPIs led to histological remission (defined by <15 eos/hpf) in 50.5% (95% CI 42.2–58.7%) and symptomatic improvement in 60.8% (95%CI 48.38–72.2%) of cases.215 No significant differences were noted in patients’ age, study design, and type of PPI assessed. A trend towards increase efficacy was observed when PPI was administered twice daily compared to once daily, and among patients with a pathological pH monitoring.215 However, the authors cautioned about the interpretation of these findings, due to poor-quality evidence (a majority of case reports and retrospective studies, with no placebo-controlled trials so far), heterogeneity in results and publication bias in favor of studies reporting histological responses to PPI therapy. Recommended PPIs doses in adults are omeprazole 20–40 mg twice daily or equivalent; in children, 1–2 mg/kg or equivalent.
Is PPI therapy effective to maintain remission in EoE?
Statement 24: In PPI responders, long-term PPI therapy is effective in maintaining remission.
LE: low, SR: Strong in favor. Agreement: 100%, votes: strongly agree (87%).
Summary of evidence: When pharmacological treatment for EoE is stopped, symptoms and/or esophageal eosinophilia typically recur over a 3–6 month period.4 However, the long-term therapeutic strategy and best maintenance doses for pharmacologic therapies are yet to be defined. An approach where the dose is progressively decreased to the lowest dose that keeps the disease in remission seems reasonable until more data are available.216
Until recently, the sustained efficacy of PPIs in children was limited to two retrospective series only comprising six patients with PPI-REE, all with recurrence of esophageal eosinophilia and symptoms over time while on maintenance PPI therapy.217,218 A recent prospective study has first shown that most PPI-REE pediatric patients (78%) remain in clinic-pathologic remission at one-year follow up on maintenance PPI low doses.214 As for adults, a first long-term follow-up multicenter study including 75 patients has been lately published.219 All patients who temporarily discontinued PPI therapy had symptom and/or histological relapse. The majority of patients (73%) maintained histological remission after at least 1 year on tapering PPI dosage to the minimum effective clinical dose. Among relapsers, most regained histological remission after dose escalation, suggesting some patients continue to require maintenance high-dose PPI. Another series from Spain has lately corroborated these findings, with 80% of patients keeping response to PPI therapy after tapering doses.220 No data for >1 year of follow up are available yet.
Are systemic steroids recommended in EoE?
Statement 25: Systemic steroids are not recommended in EoE.
LE: moderate, SR: Strongly against. Agreement: 93%, votes: strongly agree (87%).
Summary of evidence: An RCT conducted in children compared the efficacy and safety of oral prednisone (1 mg/kg/dose twice a day) with swallowed fluticasone propionate (2 puffs 4 times/day; 110 µg per puff for ages 1–10 years and 220 µg per puff for ages 11 years or older,) for 12 weeks.221 Both agents were equally effective in achieving initial histological and clinical improvement at week 4, after which drugs were progressively tapered. However, systemic effects (hyperphagia, weight gain, and/or cushingoid features) were noted in 40% of patients in the oral prednisone arm, whereas the only relevant side effect for topical steroids was esophageal candidiasis, present in 15% of patients.
Are topical steroids effective in inducing remission of EoE?
Statement 26: Topical corticosteroids are effective for induction of histological remission in both pediatric and adult EoE patients.
LE: High, SR: strongly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence (see Supplementary table 4): Currently, 11 randomized trials conducted in children and adults,157,168–170,211,212,221–225 which have been summarized in several systematic reviews and meta-analyses,226–229 confirm the efficacy of topical steroid therapy for histologic remission in EoE patients (Supplementary table 5). Variability regarding inclusion criteria, agents (fluticasone/budesonide), daily dosages, length of treatment (from 2 to 12 weeks), delivery system (swallowed puffs from inhalers, suspension, viscous slurry, effervescent tablets), and definition of histologic remission (from <1 to <20 eos/hpf) notably hampers comparative analyses among these studies. The currently recommended drugs and doses are summarized in Supplementary table 6.
As for the delivery system, a milestone study compared budesonide 1 mg twice daily for 8 weeks given in nebulized and viscous preparations.223 Complete histologic remission was significantly higher (64% vs. 27%) in the oral viscous budesonide group. Overall drug mucosal contact time, measured by means of nuclear scintigraphy, was significantly longer in patients treated with the oral viscous budesonide and this difference was significantly higher in the distal esophagus. Therefore, this important study pointed out the histologic improvement was directly related to higher mucosal contact time and highlighted the importance of appropriate drug delivery methods in the treatment of EoE. Presently, the higher histologic remission rates in randomized control trials have been accomplished with effervescent tablets and oral viscous budesonide.225
Unlike histologic remission, data on symptom resolution are less clear. Several clinical trials have not been able to demonstrate a statistically significant advantage of topical corticosteroids over placebo,168–170,224,225 or even performed worse than PPI therapy.211 Furthermore, two recent meta-analysis could not elucidate a clear trend in symptom improvement with topical steroids as compared with placebo.228,229 Several reasons can explain this discrepancy between histologic and clinic outcomes, including differences in patient selection, definitions of symptom response, steroid formulations and duration of treatment. Symptom assessment in EoE can be troublesome due to use of different non-validated symptom-scoring tools, the subjective nature of assessment of clinical response, changes in symptom profile in the transition from childhood to adulthood, behavioral adaptations masking symptoms (solid food avoidance, prolonged meal times and excessive mastication), and symptoms related to fibrostenotic features or lack of esophageal distensibility, which may not be influenced by inflammation healing.
Are long-term topical corticosteroids effective in maintaining EoE in remission?
Statement 27: In steroids responsive patients, long-term therapy with topical corticosteroids is effective in maintaining remission in a proportion of patients.
LE: low, SR: Strongly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Up to now, a single randomized, double-blind, placebo-controlled trial has evaluated the efficacy of long-term topical steroid treatment for EoE.230 Twenty-eight adult patients were randomized to oral budesonide suspension 0.5 mg/day or placebo for 50 weeks. At the end of the study period, complete remission (<5 eos/hpf) was documented in 36% of patients in the budesonide group, while no patient in the placebo group remained in complete remission. Possibly, low-dose maintenance budesonide dosage in this trial underrated the long-term effectiveness of topical steroid therapy.
As for children, an extension of treatment in a randomized, double-blind, placebo-controlled trial has been lately reported.224 Those patients who achieved complete remission (<1 eos/hpf in both distal and proximal esophagus) with high-dose fluticasone (1760 mcg/day) received a 50% dose reduction for three months and were re-evaluated. Sustained response was documented in 73% of initial responders. A more recent prospective study in children responders (<15 eos/hpf) to swallowed fluticasone from metered dose inhaler revealed that long-term administration of similar doses led to sustained remission to 59% and 63% of patients during months 13–24 and >2 yrs of follow-up, respectively.231
Are topical steroids safe drugs in the treatment of eosinophilic esophagitis?
Statement 28: Swallowed topical corticosteroids seem to have a favorable safety profile in the treatment of EoE, with no serious side effects reported. Esophageal candidiasis, mostly incidental, may occur in up to 10% of patients.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Although the numbers of patients with EoE involved in individual trials with fluticasone propionate and budesonide are low, the overarching number of recruited patients allows a perspective on a large cohort of treated patients. No statistically significant different adverse events (AEs) compared to placebo were found in the majority of RCTs comparing topical steroids to placebo, except for esophageal candidiasis, which were reported in 5–26% of EoE patients assigned to active interventions in short term/induction of remission,157,168,170,211,221,223,225 and in 0–5% in long-term/maintenance trials.222,230 Esophageal candidiasis were asymptomatic in all cases and constituted an incidental finding in scheduled endoscopies, which may also imply the higher monitoring level of patients in short compared to long-term trials. As a treatment (when reported) oral nystatin or oral fluconazole were used. There were no differences in the risk of esophageal candidiasis for nebulized versus viscous topic budesonide.223 According to a recent meta-analysis of RCTs, a NNH of 9 for developing an asymptomatic esophageal candidiasis was calculated.229 No RCTs reported severe AEs.
Some uncertainty ranges around suppression of systemic cortisol levels induced by topical steroid treatment, especially in children. In short-term RCTs 24 h urine and/or serum cortisol levels were not suppressed.168,224,225 Information on long-term effects of swallowed topical steroids on adrenal suppression in children is being provided by observational studies including short series of EoE patients with disagreeing results: While no differences in serum cortisol levels were found following treatment with swallowed fluticasone propionate (range 220–880 µg daily) and budesonide (range 0.5–1 mg daily), along treatment lengths of 8–43 weeks,232 an additional study showed that adrenal suppression was present in 10% of children treated with swallowed glucocorticoids for ≥6 months and was found only in those treated with FP >440 µg daily.233 Finally, 43% of children presented suboptimal stimulated cortisol, independently of treatment duration.234 No clinical sign of adrenal insufficiency or growing impairment have been reported so far.231
Until more information is available, cortisol monitoring to prevent adrenal insufficiency could be advisable for children with EoE if they are receiving high doses of swallowed topic steroids for long periods, or concomitant use of inhaled/nasal corticosteroids for associated atopic diatheses.
Is there a place for elemental diet in the clinical management of EoE patients?
Statement 29: There is a limited place for elemental diet in EoE, which should only be considered after failure of properly performed medical treatment and/or elimination diet. Elemental diet induces histologic remission in up to 90% of pediatric and adult EoE patients. There is limited information regarding symptoms.
LE: Low, SR: Weakly against. Agreement: 100%, votes: strongly agree (83%).
Summary of evidence (see Supplementary table 7): The first evidence on the efficacy of a dietary intervention for inducing remission of EoE was provided with elemental diet. In ten children with severe esophageal eosinophilia attributed to GERD and refractory to other therapies, exclusive feeding with an amino acid-based formula devoid of antigenic capacity was given for a minimum of six weeks,80 while avoiding all kind of table foods. Clinic and histologic remission was observed in 8 children, whereas the remaining two patients showed symptomatic and histologic improvement. Subsequent reports have repeatedly confirmed the efficacy of elemental diet in patients of all ages.63,235–238 Despite the absence of any RCT, a recent meta-analysis has shown that the overall effectiveness of elemental diet among observational studies in inducing histological remission of EoE is 90.8% (95% CI 84.7–95.5%), with no differences between age groups.239 In contrast, there is limited evidence on the ability of elemental diets to achieve symptomatic improvement: In a large prospective pediatric series elemental diets induced clinical improvement of EoE after only 8.5±3.8 days;235 in adults, elemental diet achieved histological remission in around 2 weeks.238
However, and despite outperforming all other dietary or topic steroid-based treatments in terms of efficacy,239,240 several disadvantages impact on the use of elemental diets in clinical practice, including its poor palatability, which requires using nasogastric tubes in most of children,63 and lack of adherence in up to one-third of adults recruited for a 4-week trial.238
A complete avoidance of all kind of table food determines feelings of being different from their family and peers in children and aggravates the eating/diet and social impacts of EoE,241 which are major determinants of health-related quality of life (QoL) in adults.176,242 The cost of elemental formulas is also high and not universally covered by health insurances. All these drawbacks would increase in long-term use.
A potential role for elemental diets has been proposed after failure to empiric six-food elimination diets in patients who wish to further investigate the causality of unusual foods and potential involvement of aeroallergens in EoE.243 However, this option has not been assessed yet in clinical practice or research. Therefore, the multiple disadvantages of amino acid formulas in EoE relegate their only realistic utility to small children who are not yet taking solid food if symptoms and inflammation persist and no narrowing is appreciated, especially if a rapid clinical improvement is required.
What is the efficacy of food allergy testing-based elimination diet for inducing histologic remission in EoE?
Statement 30: Food allergy testing-based elimination diet induces histologic remission in less than one third of adult patients. This rate may be higher in pediatric patients.
LE: moderate, SR: Strongly against. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence (see Supplementary table 7): Food allergy testing-based elimination diet stands for eliminating foods with positive results on skin prick tests (SPT) and atopy patch tests (APT). In 2002, an elimination diet combining SPT and APT, excluding an average of five foods, first induced clinic and histologic remission in 49% of pediatric patients.244 Causative foods were exclusively attributed by symptom recurrence after food reintroduction, with no biopsy evaluation. A decade later, the same research group updated their results with an overall efficiency of 53%.245 Nevertheless, several studies have further reported worse results with this strategy in children and adult patients.63,236,246–248 A meta-analysis revealed that this dietary approach led to histologic remission in 45.5% of patients (95% CI 35.4–55.7%), with wide heterogeneity (I2: 75%) indicating a low reproducibility.239 The efficacy rates were significantly lower in adults than in children (32.2% vs. 47.9%).
More recently, a pilot study in adults evaluating an elimination diet guided by blood IgE microarrays (e.g. measuring IgE levels to food protein components) was interrupted early due to poor efficacy (7% histologic remission).249 A recent study also in adults evaluated the accuracy of the combination of multiple allergy skin and blood tests, measuring either immediate or delayed hypersensitivity responses, to detect offending foods in adult EoE patients.250 Similarly, no allergy test could accurately predict food triggers identified through food challenge with histologic reassessment in responders to a six-food elimination diet.
Should allergy testing be used to identify causative food triggers of EoE?
Statement 31: The utility of allergy tests in the identification of food triggers of EoE is consistently low in adults and variable in children.
LE: Low, SR: Strongly against. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Because food-specific serum IgE can be detectable in some individuals even in the absence of clinical reactions to the particular food, SPT assessing both the presence and the function of mast cell-bound IgE represents the standardized and validated technique to study immediate allergic reactions.251 In contrast, APT is used to assess the presence of non-IgE, cell-mediated reactions.252 No study has incorporated skin biopsies to verify the presence of an immunologic infiltrate at the cutaneous site of a positive APT result for food to validate this technique in EoE;253 besides, APT performance is not standardized and interpretation is subjective with a significant interobserver variation.
As also described in IgE-mediated food allergy,254 negative predictive values (NPV) of skin testing for foods are generally superior to the positive predictive values (PPV) in EoE patients. Assessment of diagnostic accuracy of skin allergy testing in pediatric EoE has provided PPV for SPT ranging from 26.3% to 86.3% depending on the food (average of 47%),245 while NPV were >90% for multiple foods, with the exception of egg, wheat, soy (range 79–90%), and milk (30%). Predictive values for APT followed a similar trend, with PPV ranging from 12% to 86.2% (average 44%), and NPV >90% with the exception of milk (31%). A NPV >90% for SPT means that there is a greater than 90% chance that the patient will not have an IgE-mediated reaction; a NVP >90% for APT almost excludes a delayed food hypersensitivity reaction. The combination of SPT and APT for building an elimination diet increased the sensitivity rates of skin testing (65–95%, with the exception of milk and pork, which were in the 50% range) as well as specificity rates for all foods (78–90%).237 That combination yielded an averaged PPV poor (44%), but increased the average NPV (92%), with the exception of milk (44%).245 However, others have found slightly lower NPV values for milk, egg, and wheat – the most common food triggers for EoE – (being 40%, 56%, and 67%, respectively).237
The fact that EoE is defined as a food allergy in a vast majority of patients excludes the validity of such methods in identifying EoE food triggers. Besides, as ATP has not been validated in food allergy, variability in NPV in APT results can be attributable to the different thresholds established by different authors for considering a result as positive. SPT in EoE might also reflect cross-reactivity with environmental allergens, and not directly a food sensitization (grass allergy may present wheat-specific IgE). An extremely low concordance between SPT results and food triggers of EoE identified by biopsy-monitored sequential food reintroduction has been repeatedly provided.84,85 Additionally, cumulative data support the current thought that EoE is primarily non-IgE mediated,255 but associated to IgG4,256–258 and according to available evidences, IgE testing via SPT or serum is very unlikely to provide meaningful data for generating a foundation for elimination diets.
As a consequence, the diagnostic accuracy of skin allergy tests is insufficient to design effective diets for EoE patients, or to support the development of dietary advancement in EoE.259
What is the efficacy of empiric six-food elimination diet for inducing histologic remission in EoE?
Statement 32: An empiric six-food group elimination diet (SFED) induces histologic remission in around three quarters of pediatric and adult patients.
LE: moderate, SR: weakly in favor. Agreement: 100%, votes: strongly agree (87%).
Summary of evidence (see Supplementary table 7): The efficacy of an empiric elimination diet in EoE was first reported in 2006, aiming at overcoming both the unfeasibility of elemental diet in clinical practice and the low sensitivity/specificity of allergy skin testing to identify EoE food triggers. It consisted of eliminating the six foods most commonly associated with food allergy in the pediatric population in Chicago (cow’s milk protein, wheat, egg, soy, peanut/tree nuts, fish, and seafood).236 In this first seminal study, the so-called SFED achieved clinical and histologic remission in 74% of children (74%). Similar results have been further obtained in patients of all ages.84,85,237,247 A recent meta-analysis on seven observational studies provided an extremely homogenous (I2 statistic = 0) histologic remission rate of around 72% (95% CI 66–78%) in children and adult patients.239 The high level of dietary restriction and the large number of endoscopies after individual food reintroduction that this six-food elimination diet requires counteract its effectiveness and wide reproducibility in clinical practice.
Aside from a six-food elimination diet, are there simpler empiric dietary strategies for EoE?
Statement 33: In adult patients, an empiric four-food elimination diet (FFED) achieves remission in half of the patients, whereas a two-food elimination diet (TFED; animal milk and gluten-containing cereals) may be still effective in 40% of patients.
LE: moderate, SR: weakly in favor. Agreement: 100%, votes: strongly agree (87%).
Summary of evidence: The moderate-to-high effectiveness and wide reproducibility of SFEDs are counteracted by several drawbacks,239 namely the high level of dietary restriction and the large number of endoscopies after individual food reintroduction. Of note, the majority (65%–85%) of patients responders to a SFED were found to have just 1 or 2 causative foods after six food challenges and endoscopies (Supplementary table 8).84,85,260,261 The most common causative foods identified after a response to a SFED have been cow’s milk, wheat, egg, and, to a lesser extent, soy/legumes, with a negligible role for nuts, fish, and seafood. Upon this rationale basis, it was developed the FFED, avoiding the most common food triggers in EoE (cow’s milk, wheat, eggs, and legumes).261 A first prospective multicenter study in adult patients showed a 54% remission in adults,261 whereas an abstract in pediatric population revealed a 71% efficacy.250 In both studies, cow’s milk was the most common food trigger (especially in children). Half of adult responders were found to have cow’s milk, wheat or both as food triggers,262 whereas 74% of pediatric patients had a single food trigger.250 Accordingly, a step-up approach (in other words, eliminating at first the one or two most common food triggers and subsequently increasing the level of restriction in non-responders) should be further evaluated in EoE. In this respect, cow’s milk elimination diet in children has been recently reported to achieve histological remission in 65% and 61% of patients in two studies, albeit both studies were flawed by methodological issues.263,264 An upcoming study, only available in abstract form, has first evaluated the efficacy of a TFED (animal milk and gluten-containing cereals), stepping up to FFED and SFED.265 A TFED achieved EoE remission in 38 patients (40%), whereas remission rates increased to 52% and 65% with a FFED and SFED, respectively. Among responders to a TFED, the most common food triggers were animal milk (60%), gluten-containing cereals (25%) and both (15%). Compared to starting with a SFED, this step-up strategy allowed reducing endoscopic procedures and the diagnostic process time by 35%.
Is long-term avoidance of food triggers effective to maintain EoE in remission?
Statement 34: Prolonged avoidance of triggering foods may lead to drug-free sustained clinical and histological remission of EoE.
LE: Low; SR: Strong in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Once the food or foods responsible for EoE in each individual patient has been identified, long-term avoidance has been recommended in order to maintain disease remission.266,267 In contrast with the abundant literature that has evaluated the effectiveness of dietary modification in achieving EoE remission, the sustained efficacy of avoiding consumption of food responsible of the disease has been assessed limitedly, with no documents specifically targeted to report on this topic, so the available evidence on it has been provided with scarce details as secondary outcomes of observational and quasi-experimental research addressed to additional goals.
Two studies conducted in adults reported that all the patients who did not take the food(s) responsible for the disease remained asymptomatic,84,85 and with histologic remission in esophageal biopsies for a period of up to three years,85 making drug treatment unnecessary. Despite some persistent isolated eosinophils in the esophageal infiltration (being in all case its density below the diagnostic threshold) other histopathological features of active EoE, such as epithelial hyperplasia, were not present after one year on the dietary elimination.84 The involvement of two or more foods in the origin of EoE in adults was reported as hampering a complete adherence to a prolonged food avoidance maintenance therapy, that although effective, was substituted by a drug-based treatment allowing a liberalized diet.24
In children, a follow up of up to 4 years of maintenance under food triggers avoidance has been reported for five children,260 after which they were rechallenged with food antigens they had reacted to during the initial food reintroduction phase, showing EoE recurrence in most of cases. Empiric SFED-based therapy has showed no treatment-related complications and none of the children demonstrated nutrient deficiencies or growth deceleration during the dietary reintroduction phase,260 and neither after a year of progressive reintroduction of eliminated foods.268 No study has appropriately evaluated the effect of long-term food avoidance on the natural history of EoE, especially regarding the reversion of fibrous remodeling phenomena,152 and neither the impact of continuously avoiding common foods that usually cause the disease on the health-related QoL of EoE patients.
What is the efficacy of endoscopic dilation in treating EoE?
Statement 35: Endoscopic dilation improves dysphagia in up to three quarters of adult EoE patients with reduced esophageal caliber, without having an effect on the underlying esophageal inflammation.
LE: Moderate, SR: Strong in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: The effectiveness of esophageal dilation in patients with EoE have been mostly reported in retrospective and single-center studies, the results of which were summarized in a meta-analysis of nine studies overall including 525 adult patients who underwent to 992 dilation procedures.269 Clinical improvement was documented in 75% (95% CI 58–93%, I2 = 86%) of patients. The duration of the effect in terms of clinical relief was variable. Only four studies have reported post-dilation caliber that was 13 mm of average.270–273 No differences were reported according to the dilation device used. Available information on children is limited.274
Can endoscopic dilation be considered as a safe procedure in the treatment of EoE?
Statement 36: Endoscopic dilation in EoE is a safe procedure, with a risk of esophageal perforation smaller than 1%.
LE: moderate. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: A systematic review of the literature has assessed the rate of complications, including esophageal perforation, death, hemorrhage, and post-procedural chest pain, after 992 endoscopic dilations performed in 525 adult EoE patients.269 A total of three esophageal perforations (0.3%) and one hemorrhage (0.1%) were reported, all from the same institution,275 and no death was reported. Accordingly, the rate of major complications is consistent with that reported for endoscopic dilation in other esophageal diseases.276 Post-procedural chest pain has been reported in up to three quarters of patients when asked directly before discharge from the endoscopy room,270 but decreased to 2% by chart review.269 Deep mucosal rents or laceration should not be reported as endoscopic complications, seeing as they are the intended outcome. More studies are required in children.
How to choose a therapeutic option for an EoE patient?
Statement 37: PPIs, diet, or topical steroids might be offered as first line anti-inflammatory therapy. The choice of therapy should be individually discussed with the patient and might be potentially interchangeable over time. The efficacy of any therapy should be checked by a follow-up endoscopy after a 6- to 12-week initial course. Endoscopic dilation should be considered in patients with dysphagia/food impaction unresponsive to anti-inflammatory treatment.
LE: low, SR: strong in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Although there are no approved medications by regulatory authorities yet, solid data supporting all treatment categories have been published over the last decade. EoE is a chronic disease in which the esophageal inflammation progresses over time to esophageal fibrotic remodeling, leading to narrow caliber esophagus (caliber <13 mm) and esophageal strictures.188,192 Patients may present with an inflammatory, a fibrostenotic or a mixed phenotype. Histological features may remit with treatment of PPI therapy, topical steroids or elimination diets, whereas fibrostenotic features can be solved by means of endoscopic dilation. Making an analogy with therapeutic considerations on IBD,277 all EoE patient should receive a treatment targeted to cure esophageal inflammation (mucosal healing) plus endoscopic dilation in case of fibrostenotic endoscopic findings. The efficacy of any pharmacological/dietary therapy should be checked by means of a follow-up endoscopy after a 6- to 12-week initial course.4 In addition, all patients with fibrostenotic abnormalities (including narrow caliber esophagus <13 mm and strictures) should be offered endoscopic dilation, preferably after a trial of medical/pharmacological therapy.277 Endoscopic dilation should not be the only therapeutic intervention, as it has no effect on the underlying esophageal inflammation.207 As for patients with severe symptomatic esophageal strictures, initial therapy with topical steroids and endoscopic dilation might rapidly achieve remission of clinic, endoscopic, and histologic features.
The proposed therapeutic algorithm for EoE is summarized in Figure 1. At the present time, it is well known that PPI therapy leads to clinical and histological (<15 eos/hpf) remission in half of patients with suspected EoE.215 Due to its safety profile, ease of administration and high response rates. PPI therapy can be considered a first-line treatment, although the choice of therapy should be made after the patient is informed about the pros and cons of alternative therapies, such as diet and topical steroids.12 Treatments might be interchangeable, seeing as two recent series have reported that EoE patients responsive to diet/topical steroids may also achieve further remission on PPI therapy and viceversa.23,24 In case of unresponsiveness to PPI therapy, a choice between topical steroid or dietary therapy should be made. The choice should be once again individually discussed with the patient and their relatives and may depend on the age (adolescent and young adults usually show poor adherence to diet), the severity of the disease (severe symptoms should be treated with topical steroid therapy) or the patient’s lifestyle and preferences or their ability to understand food label information.277 Once the therapy is instituted, the choice might be changed over time due to treatment side effects or the unwillingness of the patient to continue the medication (topical steroid therapy) or negative impact on quality of life and family resources (dietary interventions).
Figure 1.
Therapeutic algorithm proposed for eosinophilic esophagitis in clinical practice.
What is the efficacy of immunomodulators in treating EoE?
Statement 38: Azathioprine and 6-mercaptopurin might play a role in inducing and maintaining long-term remission in EoE in limited cases.
LE: low, SR: weakly in favor. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: To date, only one case series on the efficacy of azathioprine or 6-mercaptopurin for EoE has been published.278 These drugs exerted a positive steroid-sparing effect and induced and maintained long-term steroid-free remission in three steroid-dependent adults with active EoE (one patient with eosinophilic gastroenteritis and esophageal involvement).
What is the efficacy of anti-allergic drugs in EoE?
Statement 39: Sodium cromoglicate and antihistamines have no effect on symptoms or esophageal eosinophilia.
LE: low, SR: strongly against. Agreement: 100%, votes: strongly agree (100%).
Statement 40: There is insufficient evidence to recommend montelukast, a leukotriene receptor antagonist, in patients with EoE.
LE: moderate, SR: strongly against. Agreement: 100%, votes: strongly agree (38%).
Statement 41: First generation chemoattractant receptor-homologous molecule on Th2 cells (CRTH2) antagonists induce modest clinic and histologic improvement in EoE.
LE: high, SR: weakly against. Agreement: 100%, votes: strongly agree (93%).
Summary of evidence: Several anti-allergic drugs effective in rhinitis and asthma have failed to show a relevant impact on EoE-related symptoms or esophageal inflammation. Despite a role for mast-cells has been recognized in EoE,279,280 a 4-week trial of sodium cromoglicate, a mast cell inhibitor, did not prove either symptomatic or histologic improvement in 14 children with EoE.63
Montelukast, a leukotriene D4 receptor antagonist, use at high doses (10–100 mg) in adults,281 and standard ones in chidren,282 led to some symptomatic improvement in open-labeled trials, with no patients achieved histologic response. In a recent RCT, montelukast was not superior to placebo to maintain remission from EoE after steroid therapy at 20 mg/daily doses.283 Montelukast also failed to maintain topic steroid-induced remission in a prospective series of adults with EoE, with reappearance of symptoms and eosinophilic inflammation within a 3-month period.284
Finally, the efficacy of OC000459, a selective antagonist of CRTH2, has been recently assessed in a randomized, double-blind, placebo-controlled trial conducted in 26 adult EoE patients. Compared to placebo, OC000459 100 mg bid for 8 weeks led to a significant decrease in symptom scores and esophageal inflammation density, but no normalization of esophageal biopsies was documented.285
Is there a role for biologic drugs in the treatment of EoE?
Statement 42: The anti-IL5 antibodies mepolizumab and reslizumab have no effect on symptoms and modestly reduce esophageal eosinophilia.
LE: high, SR: strongly against. Agreement: 100%, votes: strongly agree (79%).
Statement 43: QAX576, an anti-IL13 antibody, has no effect on symptoms but reduces esophageal eosinophilia and downregulates EoE transcripts in a sustained manner.
LE: high, SR: weakly against. Agreement: 100%, votes: strongly agree (86%).
Statement 44: Omalizumab, an anti-IgE antibody, has no effect on symptoms or esophageal eosinophilia.
LE: high, SR: strongly against. Agreement: 100%, votes: strongly agree (93%).
Statement 45: Infliximab, an anti-tumor necrosis factor alpha antibody, has no effect on symptoms or esophageal eosinophilia.
LE: low, SR: strong against. Agreement: 100%, votes: strongly agree (100%).
Summary of evidence: Several biologic agents, approved or under investigation for severe asthma, have also been evaluated in EoE. The efficacy of mepolizumab and reslizumab, anti-IL5 monoclonal antibodies, has been assessed in three randomized, double-blind, placebo controlled trials involving children, adolescents and adults with active EoE.286–288 Compared to placebo, no symptomatic improvement was observed in two in three studies.286,288 All trials documented a significant reduction of esophageal eosinophilic infiltration, but with no histologic remission observed.
QAX576, an anti-IL13 antibody, has been recently evaluated in a randomized, double-blind, placebo controlled trial in adult EoE patients.289 No significant symptomatic improvement was observed, albeit QAX576 led to a reduction of mean eosinophil count by 60% and downregulated gene expression of EoE-relevant esophageal transcripts for up to 6 months after treatment. Other anti-IL-13 drugs are currently under evaluation.
Despite some observational studies reporting clinical benefit from omalizumab, an anti-IgE antibody, in EoE patients,290,291 a recent randomized, double-blind, placebo controlled trial in adult patients demonstrated no relevant effects on esophageal symptoms or eosinophilia compared to placebo.256
Finally, infliximab, an anti-tumor necrosis factor administered at doses 5 mg/kg body weight, at weeks 0 and 2, did not lead to symptom or histologic improvement at week 6 in a case series of three adult EoE patients.292
Conclusions and future perspectives
The value of these guidelines lies in widespread use and implementation. Follow-up on the implementation process will be made one year after commencement by monitoring the degree of adherence to proposed statements and recommendations through information at EoE connect (www.eoeconnect.eu), the European registry and database of EoE.
Content of these guidelines will be translated into the different European languages and published in the national medical journals, which are routinely read by the members of the national medical societies. Educational meetings, pocket cards and guideline apps are known to be useful tools and will be used in the implementation process.
The rapid development in this area, especially regarding therapeutic procedures and concerning biomarkers motivates the working group’s aim to review and update the guideline after three years from the publication date. The unmet needs that have arisen during the development of these guidelines should be addressed in the upcoming years and are summarized in Supplementary table 9.
Finally, education is a key feature in the management of EoE and should be heavily promoted to health professionals and caregivers, as well as to patients and families. Developing and validating educational tools will further the establishment of vertical and horizontal networks between Centers of Excellence, gastroenterology and allergy specialists, pediatricians, pathologists, nutritionists, and primary care practitioners. Implementation at the community level should be in partnership with the patient organizations.
Supplementary Material
(For references 21–292, see Reference list (continuation) in supplementary material.)
Declaration of conflicting interests
No, I do not have any industry or government relationships to report: AA, AS, CB, MAS, JR-S, JR.
Advisory boards: ALK (IT), Meda Pharma (IT), MSD (UvA), Nutricia (AJB), Takeda (UvA).
Consulting: Dr Falk Pharma (SEA), Receptos Inc (AP).
Educational support: Abbvie (JAD), Danone (JAD), MSD (JAD), Prospectus (JAD).
Research grants/clinical trial funding: Abbvie (AP), Astra Zeneca (SEA), Biogaia (AP), Dr Falk Pharma (AJB, AJL, AMS, JM-I, JG-C), Nutricia (AJB, AP).
Speaker’s bureau: Abbvie (UvA, SM), Allergan (SM), Aptalis (SM, AMS), AstraZeneca (AJB, SM), Dr Falk Pharma (UvA, SM), Kibion (SM), MSD (UvA), Nestlé (AP, AMS), Nutricia (AP), Olympus (SM), Reckitt Benckiser (SEA, SM), Shire (UvA), Schwabe (SM), Takeda (UvA).
Funding
These guidelines have been developed and funded within the United European Gastroenterology 2014 Link Award program “Harmonizing diagnosis and therapy of Eosinophilic Oesophagitis across Europe” (HaEoE-EU).
Author contributions
The steering committee (AJL, JM-I, AJB, JG-C, AMS, UVA, AS, SA) designed the preliminary list of topics to be covered. All authors systematically reviewed the literature and drafted the statements. The steering committee assessed the evidence and provided GRADE evaluations. After three rounds of reviews by all members, the steering committee then drafted the initial manuscript, which was reviewed, revised and approved by all members of the consensus group and all authors. All members voted on the recommendations. Subsequently it was made available to all members for final comments prior to submission for publication.
Collaborators
Teresa Caballero1, Mirna Chehade2, Nicholas Dawe3, Bethany Doefler4, Ikuo Hirano4, Celia Pinto5, Evan Dellon6, Miriam Espinosa7, Javier Júdez8, Inmaculada Lujan7, Antonella Muraro9, Roberto Penagini10, Olga Redondo-González11, Edoardo Savarino12, Michael Vieth13, and Victor Vila-Miravet14.
1Allergy Department, Hospital La Paz Institute for Health Research (IdiPaz), Madrid, Spain
2Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY, USA
3Department of Otolaryngology, The Freeman Hospital, Newcastle upon Tyne, UK
4Division of Gastroenterology and Hepatology, Northwestern University, Chicago, IL, USA
5ALPEDIA Medical Team, Madrid, Spain
6Center for Esophageal Diseases and Swallowing, Division of Gastroenterology and Hepatology, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC, USA
7Spanish Association of Eosinophilic Esophagitis (AEDESEO), Spain
8Departamento de Gestión del Conocimiento, Sociedad Española de Patología Digestiva (SEPD), Spain
9Food Allergy Referral Centre Veneto Region, Department of Women and Child Health, Padua General University Hospital, Padua, Italy
10Gastroenterology and Endoscopy Unit, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico and Università degli Studi of Milan, Milan, Italy
11Research Support Unit. Hospital General La Macha Centro. Alcázar de San Juan, Spain
12Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, Italy
13Institute for Pathology, Klinikum Bayreuth, Bayreuth, Germany
14Department of Pediatric Gastroenterology, Hospital Sant Joan de Déu. Barcelona, Spain
References
- 1.Attwood SE, Smyrk TC, Demeester TRJJ. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38: 109–116. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Spichtin HP, Bernoulli R, et al. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr 1994; 124: 1419–1429. [PubMed] [Google Scholar]
- 3.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–1363. [DOI] [PubMed] [Google Scholar]
- 4.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou A, Koletzko S, Heuschkel R, et al. Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr 2014; 58: 107–118. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108: 679–692. [DOI] [PubMed] [Google Scholar]
- 7.Lucendo AJ, Arias Á, Redondo-González O, et al. Quality assessment of clinical practice guidelines for eosinophilic esophagitis using the AGREE II instrument. Expert Rev Gastroenterol Hepatol 2017 (in press). [DOI] [PubMed]
- 8.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falck-Ytter Y, Kunz R, Guyatt GH. How strong is evidence? Am J Gastroenterol 2008; 103: 1334–1338. [DOI] [PubMed] [Google Scholar]
- 10.Delbecq AL, VandeVen AH, Gustafson DH. Group techniques for program planning: a guide to nominal group and Delphi processes, Glenview, IL: Scott Foresman and Company, 1975. [Google Scholar]
- 11.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9: 110–117. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 2016; 65: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35: 300–307. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez-Elizondo G, Ngamruengphong S, Khrisna M, et al. The outcome of patients with oesophageal eosinophilic infiltration after an eight-week trial of a proton pump inhibitor. Aliment Pharmacol Ther 2013; 38: 1312–1319. [DOI] [PubMed] [Google Scholar]
- 15.Dellon ES, Speck O, Woodward K, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol 2013; 108: 1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moawad FJ, Schoepfer AM, Safroneeva E, et al. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther 2014; 39: 603–608. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Infante J, Rivas MD, Hernandez-Alonso M, et al. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther 2014; 40: 955–965. [DOI] [PubMed] [Google Scholar]
- 18.Dellon ES, Speck O, Woodward K, et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: a prospective study. Clin Gastroenterol Hepatol 2014; 12: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moawad FJ, Wells JM, Johnson RL, et al. Comparison of eotaxin-3 biomarker in patients with eosinophilic oesophagitis, proton pump inhibitor-responsive oesophageal eosinophilia and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2015; 42: 231–238. [DOI] [PubMed] [Google Scholar]
- 20.Van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014; 12: 1815–1823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.