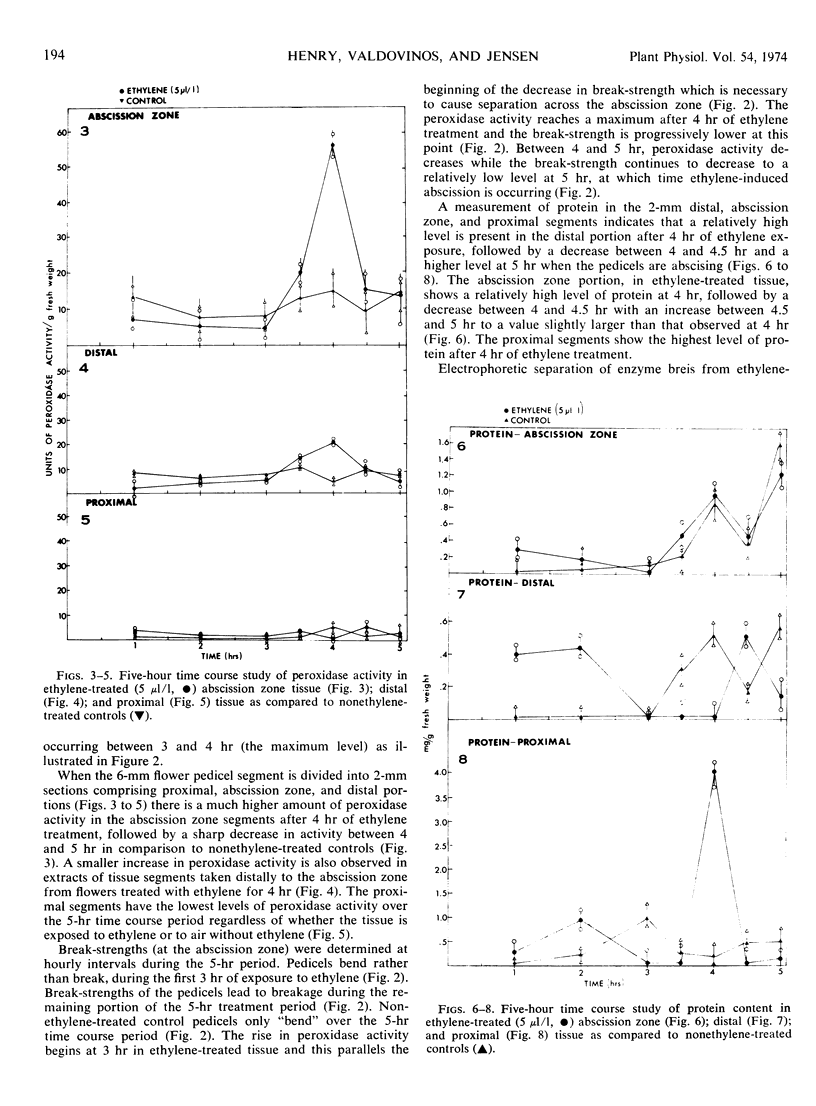
Abstract
Ethylene-induced abscission in flower pedicels of Nicotiana tabacum L. cv. Little Turkish causes a progressive increase in peroxidase activity during the first 4 hours of a 5-hour time course ethylene treatment period, with decrease in peroxidase activity occurring between 4 hours and 5 hours, when the supernatant extracts of abscission zone segments are tested spectrophotometrically for peroxidase activity, using guaiacol and hydrogen peroxide. Nonethylene-treated tissue has a much lower level of peroxidase activity over the same time course period. In ethylene-treated tissue the decline in break-strength correlates with the beginning of increase in peroxidase activity (3 hours). When the abscission zone area of the pedicel is further divided into proximal, abscission zone, and distal portions, respectively, the ethylene-treated tissue has the highest peroxidase activity in the abscission zone portion, with the maximum peak occurring at 4 hours and decreasing between 4 hours and 5 hours. Acrylamide gel electrophoresis of enzyme breis from ethylene-treated aand nonethylene-treated plants reveals that no new peroxidase isozymes are formed in response to ethylene, indicating an increase in the amount of one or in both of the two already existing isozyme banding patterns. The measurement of protein in the proximal, abscission zone, and distal segments, over a 5-hour ethylene treatment period, indicates that it is being translocated in a distal to proximal direction in the abscission zone pedicel. The possible participatory role for peroxidase in ethylene-induced tobacco flower pedicel abscission are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Henry E. W., Jensen T. E. Peroxidases in tobacco abscission zone tissue. I. Fine-structural localization in cell walls during ethylene-induced abscission. J Cell Sci. 1973 Sep;13(2):591–601. doi: 10.1242/jcs.13.2.591. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis L. N., Varner J. E. Synthesis of Cellulase during Abscission of Phaseolus vulgaris Leaf Explants. Plant Physiol. 1970 Aug;46(2):194–199. doi: 10.1104/pp.46.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W. Peroxidase Activity in the Abscission Zone of Bean Leaves during Abscission. Plant Physiol. 1973 Sep;52(3):263–267. doi: 10.1104/pp.52.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge I., Osborne D. J. Role of peroxidase when hydroxyproline-rich protein in plant cell walls is increased by ethylene. Nat New Biol. 1971 Feb 17;229(7):205–208. doi: 10.1038/newbio229205a0. [DOI] [PubMed] [Google Scholar]
- Schwertner H. A., Morgan P. W. Role of IAA-Oxidase in Abscission Control in Cotton. Plant Physiol. 1966 Nov;41(9):1513–1519. doi: 10.1104/pp.41.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Jensen T. E., Sicko L. M. Ethylene-induced Rough Endoplasmic Reticula in Abscission Cells. Plant Physiol. 1971 Jan;47(1):162–163. doi: 10.1104/pp.47.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


