Abstract
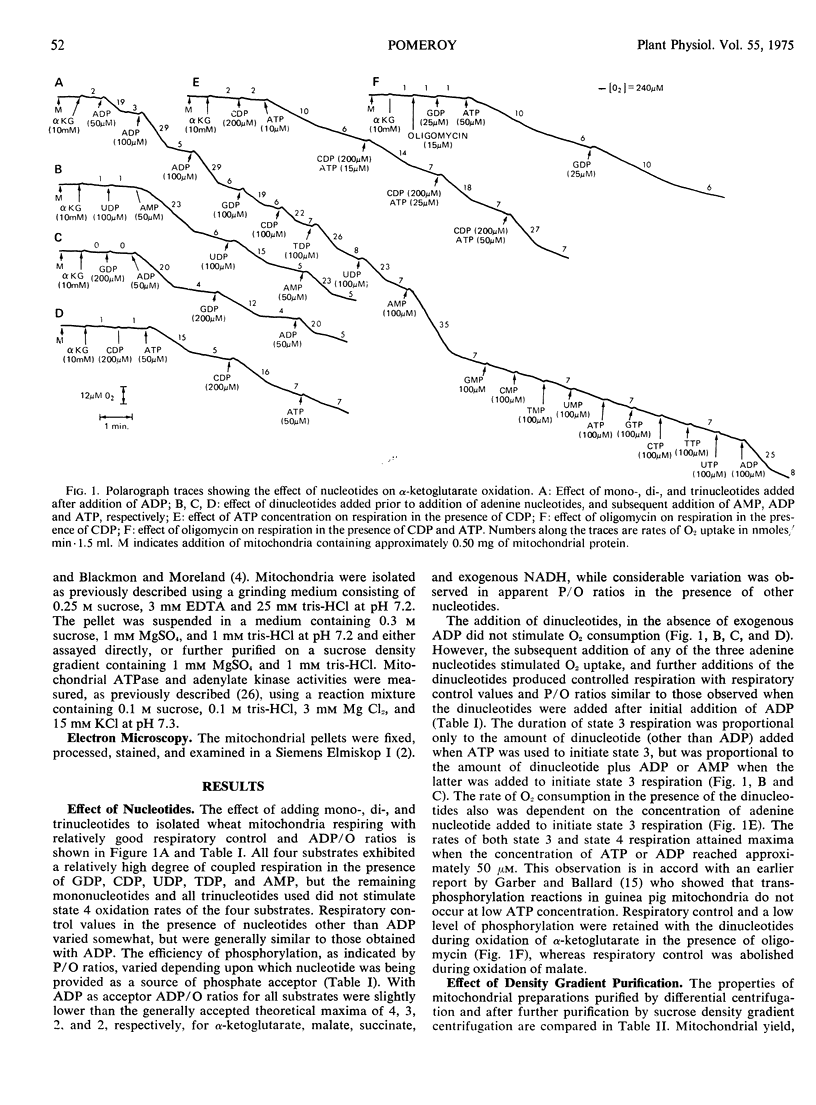
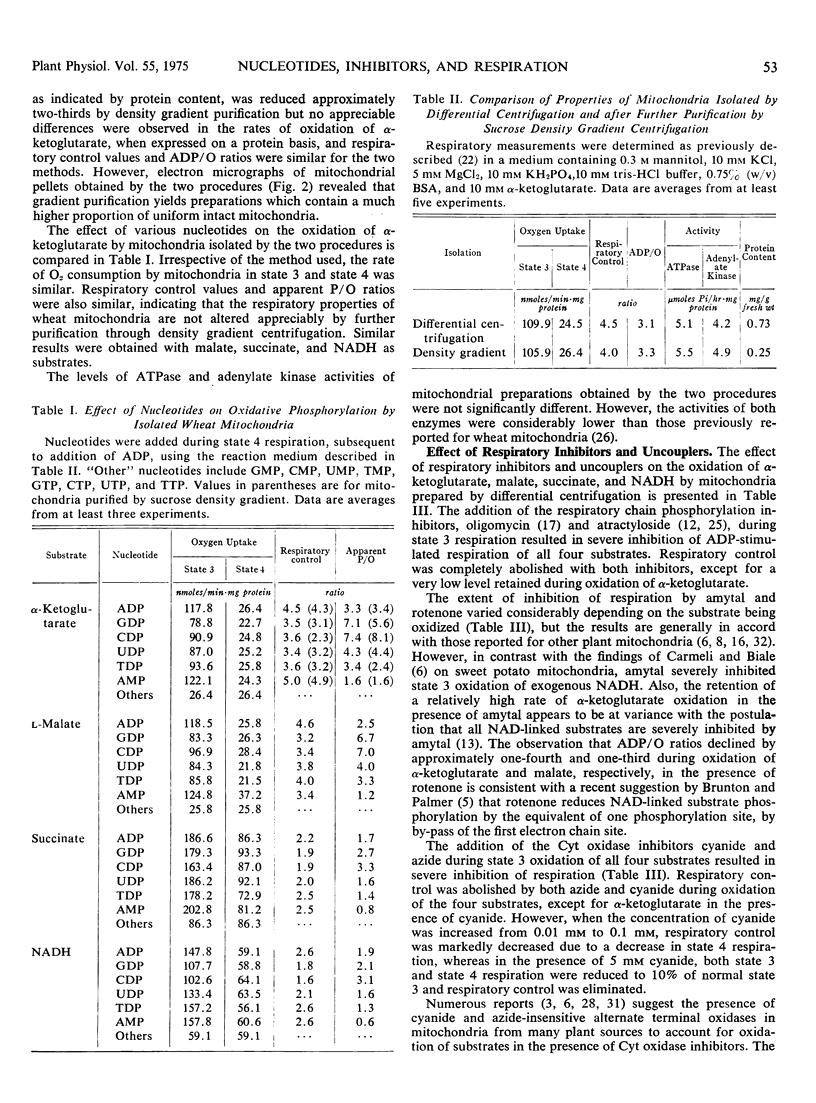
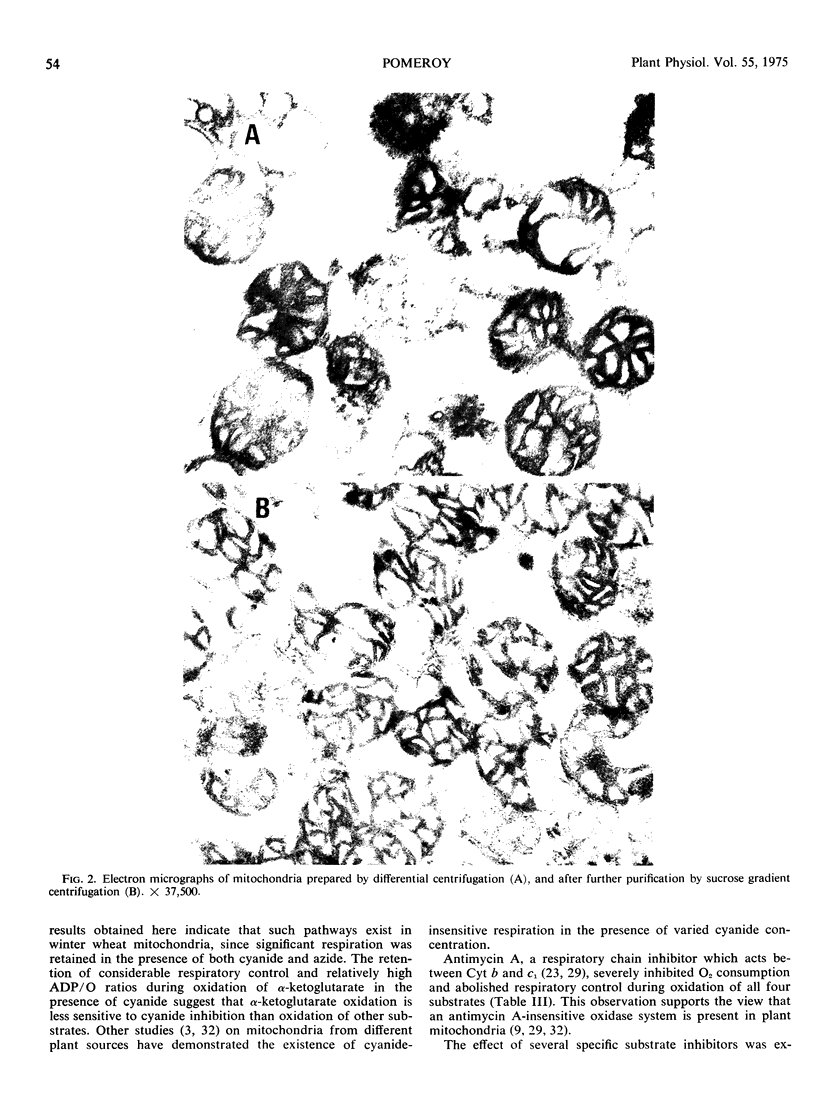
The effect of mono-, di-, and trinucleoside phosphates and respiratory inhibitors on respiration in winter wheat (Triticum aestivum L. cv. Rideau) mitochondria has been examined. When added during state 4 respiration, subsequent to addition of ADP, all of the dinucleotides stimulated oxidation and induced respiratory control with all substrates examined. Similar results were obtained with AMP, but other mononucleotides and all trinucleotides did not affect the rate of oxidation. Nucleoside diphosphates did not stimulate respiration when added prior to the addition of ADP, but subsequent addition of AMP, ADP, or ATP re-established coupled respiration in the presence of the dinucleotides.
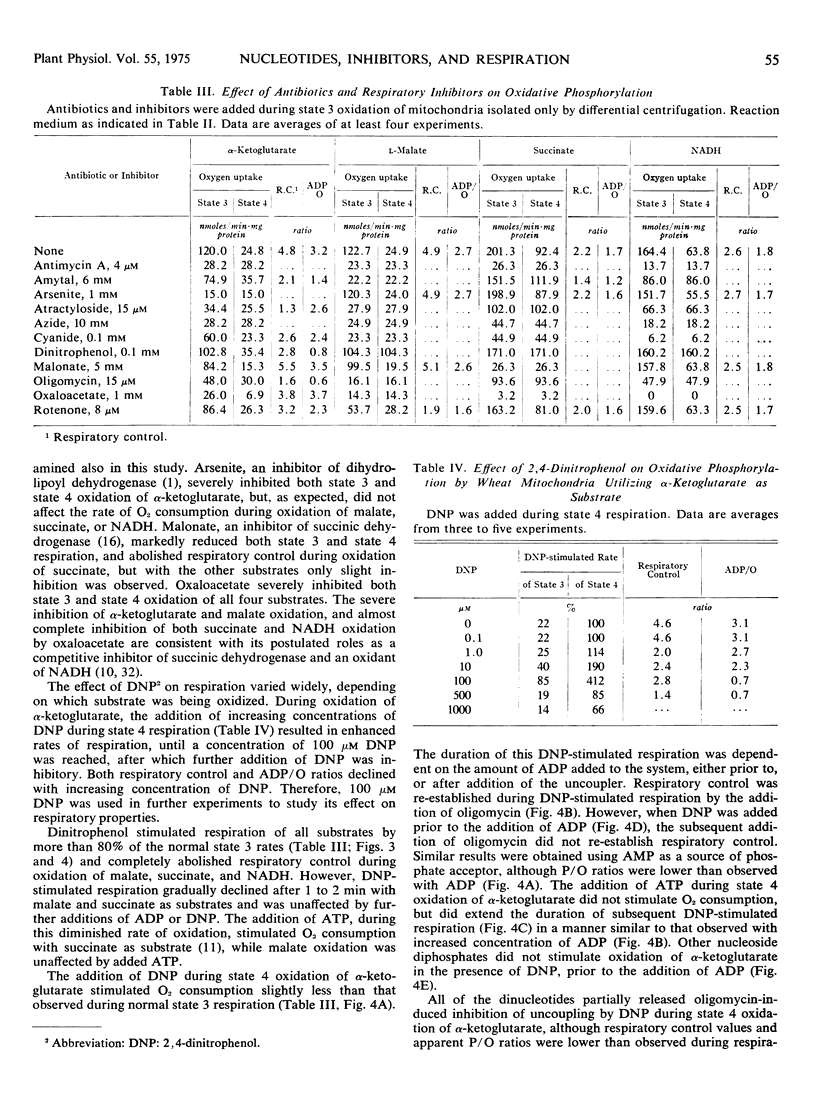
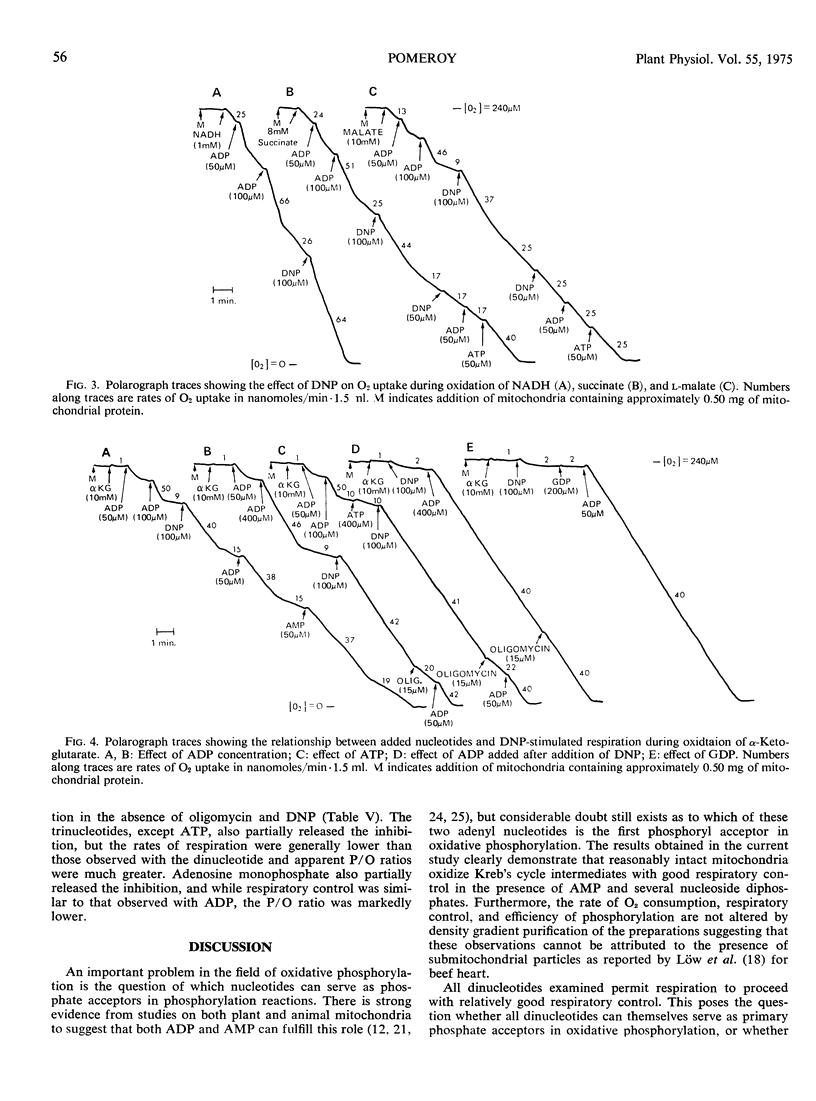
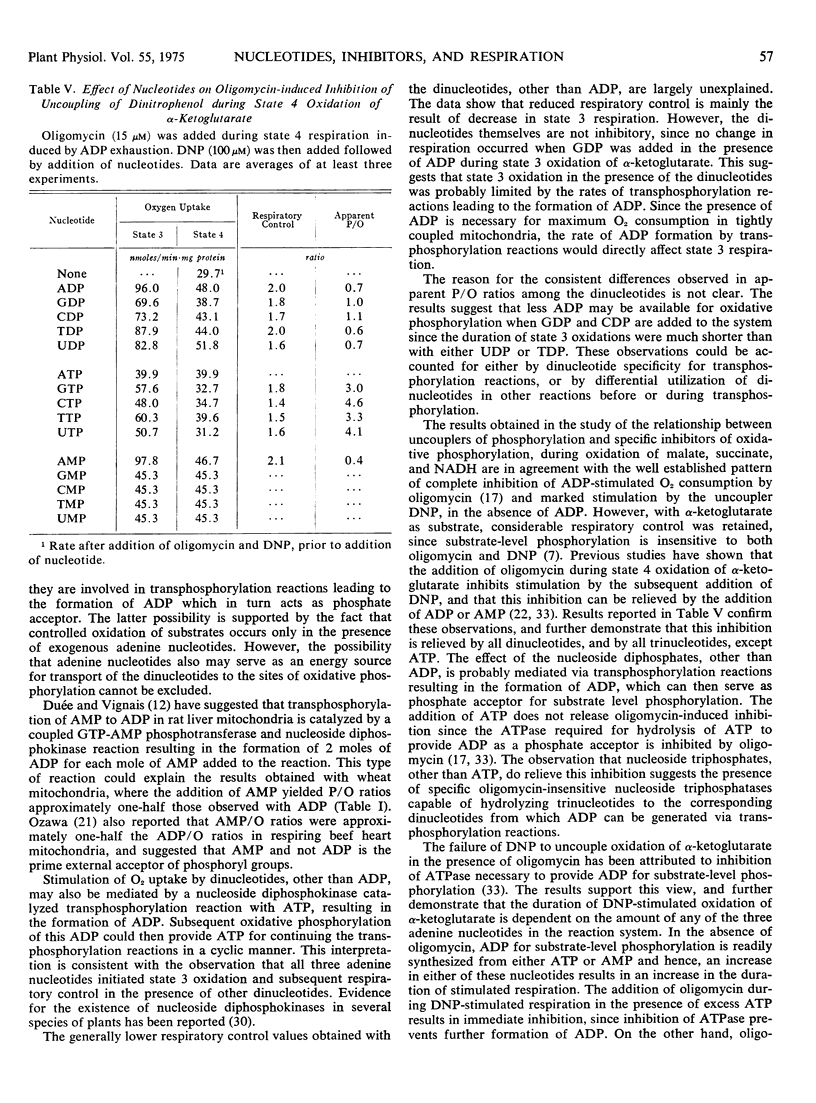
The duration of 2, 4-dinitrophenol stimulated respiration during oxidation of α-ketoglutarate was found to be dependent on the amount of AMP, ADP, or ATP added, either prior, or subsequent to, addition of the uncoupler. The addition of oligomycin during 2, 4-dinitrophenol stimulated respiration reestablished coupled respiration with low ADP/O ratios, when added after addition of ATP or conditions which allow formation of ATP from added ADP. The nucleoside diphosphates, other than ADP, did not stimulate oxidation of α-ketoglutarate in the presence of 2, 4-dinitrophenol until a small amount of adenine nucleotide was added to the system. The results suggest that dinucleotides other than ADP, are able to participate in the energy conversion processs of the mitochondria, probably via transphosphorylation reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. III. The Operation of the Tricarboxylic Acid Cycle. Plant Physiol. 1957 Mar;32(2):100–105. doi: 10.1104/pp.32.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Elfvin L. G., Biale J. B., Honda S. I. Studies on ultrastructure and purification of isolated plant mitochondria. Plant Physiol. 1968 Dec;43(12):2001–2022. doi: 10.1104/pp.43.12.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon W. J., Moreland D. E. Adenosine triphosphatase activity associated with mung bean mitochondria. Plant Physiol. 1971 Apr;47(4):532–536. doi: 10.1104/pp.47.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton C. J., Palmer J. M. Pathways for the oxidation of malate and reduced pyridine nucleotide by wheat mitochondria. Eur J Biochem. 1973 Nov 1;39(1):283–291. doi: 10.1111/j.1432-1033.1973.tb03125.x. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effects of oligomycin on respiration and swelling of isolated liver mitochondria. Nature. 1961 May 6;190:502–504. doi: 10.1038/190502a0. [DOI] [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Bonner W. D., Jr Oxalacetate control of Krebs cycle oxidations in purified plant mitochondria. Biochem Biophys Res Commun. 1972 May 12;47(3):619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Duée E. D., Vignais P. V. Kinetics of phosphorylation of intramitochondrial and extramitochondrial adenine nucleotides as related to nucleotide translocation. J Biol Chem. 1969 Jul 25;244(14):3932–3940. [PubMed] [Google Scholar]
- Forti G., Rosa L., Garlaschi F. Synthesis of ADP by isolated "coupling factor" from chloroplasts. FEBS Lett. 1972 Oct 15;27(1):23–26. doi: 10.1016/0014-5793(72)80400-9. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Ballard F. J. Regulation of phosphoenolpyruvate metabolism in mitochondria from guinea pig liver. J Biol Chem. 1970 May 10;245(9):2229–2240. [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. W., de la Roche I., Pomeroy M. K. Structural and functional responses of wheat mitochondrial membranes to growth at low temperatures. Plant Physiol. 1974 Mar;53(3):426–433. doi: 10.1104/pp.53.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T. Adenosine monophosphate as the first phosphoryl acceptor in oxidative phosphorylation. Arch Biochem Biophys. 1966 Nov;117(2):201–223. doi: 10.1016/0003-9861(66)90405-x. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., REIF A. E. Inhibition of an electron transport component by antimycin A. J Biol Chem. 1952 Jan;194(1):287–297. [PubMed] [Google Scholar]
- Pomeroy M. K. Studies on the respiratory properties of mitochondria isolated from developing winter wheat seedlings. Plant Physiol. 1974 Apr;53(4):653–657. doi: 10.1104/pp.53.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H., Moudrianakis E. N. Interactions between ADP and the coupling factor of photophosphorylation. Proc Natl Acad Sci U S A. 1971 Feb;68(2):464–468. doi: 10.1073/pnas.68.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusness D. G., Still G. G. Simultaneous measurement of oxidative phosphorylation and adenylate kinase in plant mitochondria. Arch Biochem Biophys. 1973 Nov;159(1):279–291. doi: 10.1016/0003-9861(73)90454-2. [DOI] [PubMed] [Google Scholar]
- Sarkissian I. V., Srivastava H. K. High efficiency of oxidative phosphorylation in mitochondria of wheat. Can J Biochem. 1970 Jun;48(6):692–698. doi: 10.1139/o70-109. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. 13. Redox state changes of cytochrome b 562 in mung bean seedling mitochondria treated with antimycin A. Biochim Biophys Acta. 1972 Apr 20;267(1):48–64. doi: 10.1016/0005-2728(72)90137-5. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B. The effect of respiratory inhibitors on NADH, succinate and malate oxidation in corn mitochondria. Plant Physiol. 1969 Sep;44(9):1335–1341. doi: 10.1104/pp.44.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Young R. E., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. VI. Controlled Oxidations and Coupled Phosphorylations. Plant Physiol. 1964 May;39(3):312–322. doi: 10.1104/pp.39.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]