Abstract
The leukocyte adhesion cascade involves multiple events that efficiently localize circulating leukocytes into the injured sites to mediate inflammatory responses. From rolling to firm adhesion, the interactions between adhesion molecules have pivotal roles in increasing the avidity of leukocytes to endothelial cells. Thrombomodulin (TM), an essential anticoagulant protein in the vasculature, is also expressed on leukocytes. We previously demonstrated that Lewisy (Ley), a specific ligand of TM, is upregulated in inflamed endothelium and is involved in leukocyte adhesion. The current study aimed to investigate whether leukocyte-expressed TM promotes cell adhesion by interacting with Ley. Using human monocytic THP-1 cells as an in vitro cell model, we showed that TM increases THP-1 cell adhesion to inflamed endothelium as well as to Ley-immobilized surface. When THP-1 adhered to activated endothelium and Ley-immobilized surface, the TM distribution became polarized. Addition of soluble Ley to a suspension of THP-1 cells with TM expression triggered an increase in the level of phosphorylated p38 mitogen-activated protein kinase (MAPK), which enabled THP-1 to adhere firmly to intercellular adhesion molecule (ICAM)-1 by activating β2 integrins. In vivo, macrophage infiltration and neointima formation following arterial ligation-induced vascular injury were higher in wild-type TM (TMflox/flox) than in myeloid-specific TM-deficient (LysMcre/TMflox/flox) mice. Taken together, these results suggest a novel function for TM as an adhesion molecule in monocytes, where it enhances cell adhesion by binding Ley, leading to β2 integrin activation via p38 MAPK.
During inflammation, large numbers of macrophages accumulate in the injured tissue to exert the immune functions and defend against infectious pathogens. These infiltrated macrophages are derived from circulating monocytes recruited from the circulation through the sequential steps of leukocyte trafficking, including rolling, arrest and firm adhesion, and transmigration.1 Various leukocyte and endothelial adhesion molecules are differentially regulated during each phase of leukocyte recruitment.2 Selectins mediate the initial tethering and rolling allowing leukocytes to roll along the endothelial surface to prepare for firm adhesion.3 Selectin ligand- and chemokine receptor-triggered signaling pathways are then required to activate leukocyte integrins, which interact with members of the immunoglobulin superfamily to facilitate firm adhesion.2, 4, 5, 6 Finally, leukocytes transmigrate across the endothelium to invade the injured tissue.
Thrombomodulin (TM) was first discovered on the vascular endothelium and was identified as an essential activator of protein C,7 which inactivates coagulation pathways when it is transformed to activated protein C by the complex of TM/thrombin. TM has been shown to be expressed in various cell types to mediate multiple cellular functions. TM is expressed not only in the endothelium, but also by circulating leukocytes, such as monocytes and neutrophils,8, 9 and the expression level of TM can be altered by different types of stimulation.10, 11, 12, 13, 14, 15 These previous studies have generally focused on the effects of TM on activated protein C-related functions in leukocytes; however, little is known about other specific functions of TM in leukocytes.
We previously demonstrated that the extracellular N-terminal domain of TM (domain 1, D1), a C-type lectin-like domain, has specific affinity for Lewisy (Ley; Fucα1–2Galβ1–4(Fucα1–3)GlcNAcβ1-R),16 a carbohydrate moiety that decorates glycoproteins to regulate cellular functions. Most studies on Ley have shown that its expression is correlated with inflammation. For example, Ley expressed on intercellular adhesion molecule (ICAM)-2 functions as an essential carbohydrate ligand of dendritic cell-specific adhesion molecule (DC-SIGN), a C-type lectin, to mediate dendritic cell rolling and adhesion on the vascular endothelium.17, 18 Further, Ley has been reported to be induced in the synovial endothelium of patients with rheumatoid arthritis, where it is involved in monocyte recruitment.19, 20 Therefore, we hypothesized that TM may serve as a receptor of Ley to promote monocyte adhesion to endothelial cells. Herein, we used a laminar shear flow system to test the adhesive ability of TM in human monocytic THP-1 cells in vitro. In vivo, carotid artery ligation was performed in myeloid-specific TM-deficient mice to observe whether TM was required for monocyte recruitment following vascular injury.
RESULTS
TM is required for THP-1 cell adhesion to the inflamed endothelium
To explore the potential role of TM in monocyte adhesion, we knocked down TM expression in human monocytic THP-1 cells by transducing lentiviral vector expressing TM-specific short hairpin RNA (shTM). We examined the effects of TM on THP-1 cell adhesion to the endothelium by adopting a microfluidic perfusion system. TM expression was successfully repressed in shTM-treated cells as compared with control (luciferase-specific shRNA (shLuc)) THP-1 cells (Figure 1a). The adhesion of control and TM-knockdown THP-1 cells to endothelial cells was significantly increased following stimulation with tumor necrosis factor (TNF)-α. Notably, the number of adherent cells was lower in TM-knockdown than in control cells (Figure 1b), indicating that TM participates in THP-1 adhesion to endothelial cells on inflammation.
Figure 1.
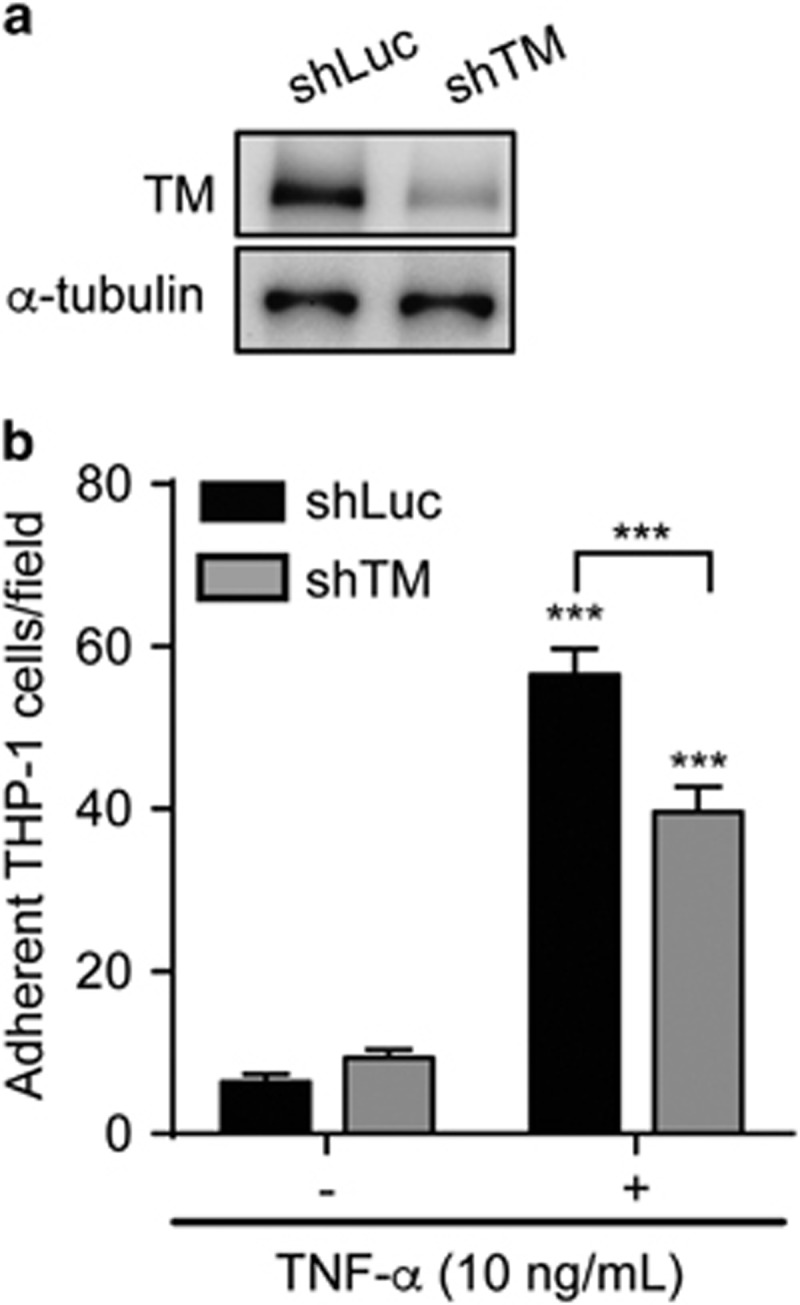
TM promotes leukocyte adhesion to TNF-α-activated endothelium. (a) Representative western blotting of TM and α-tubulin in THP-1 cells transduced with TM (shTM) or control (shLuc) shRNA. (b) Adhesion of control (shLuc) and TM-knockdown (shTM) THP-1 cells to resting (TNF-α (−)) or activated (TNF-α (+)) human umbilical vein endothelial cells. Quantitative data show the number of adherent cells per field from three independent experiments. ***P<0.001; ***P<0.001 versus TNF-α (−).
TM mediates THP-1 cell adhesion by binding Ley
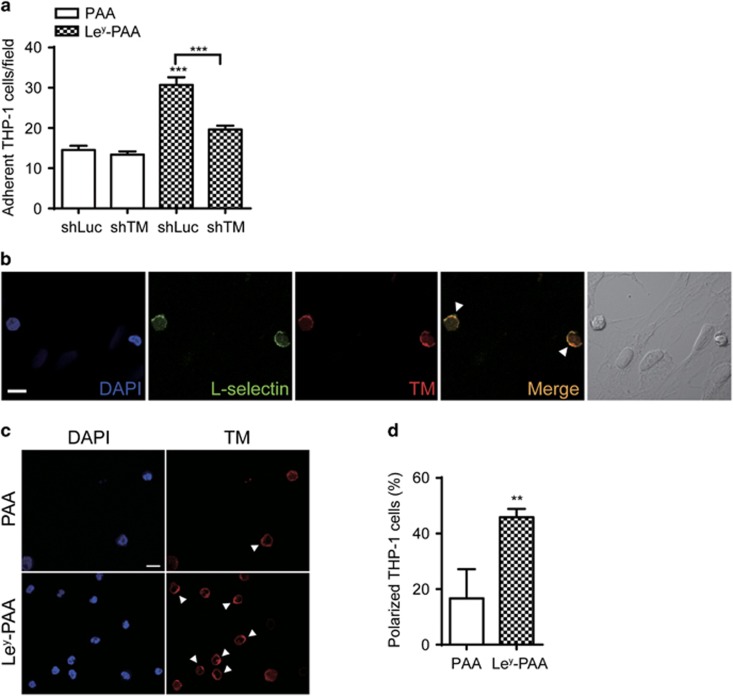
As we previously demonstrated that Ley, a ligand of recombinant TM protein, is induced in activated endothelial cells and injured vessels where it facilitates leukocyte arrest,21 we next tested whether Ley may function as a ligand in TM-mediated THP-1 adhesion. Multivalent biotinylated polymers of polyacrylamide (PAA), as a backbone control, or Ley (Ley-PAA) were immobilized on the flow chamber and a shear force was applied. The adhesion of control (shLuc) but not TM-knockdown (shTM) THP-1 cells was significantly induced in the presence of Ley (Figure 2a). We further characterized the membrane distribution of TM on adherent cells in the flow chamber by using confocal microscopy. L-selectin, an adhesion molecule essential to monocyte adhesion regulation, was examined concomitantly. After adhesion to TNF-α-stimulated human umbilical vein endothelial cells the THP-1 cells were double-stained for L-selectin and TM. Interestingly, TM had a polarized distribution (Figure 2b; Supplementary Figure 1) and colocalized with L-selectin (Figure 2b, arrowheads). To determine whether this polarization was triggered by Ley, we determined the distribution of TM in cells arrested on PAA- or Ley-PAA-coated flow chamber. In this assay too, TM expression was observed in clusters (Figure 2c, arrowheads), and a higher percentage of cells exhibited TM polarization in the presence than in the absence of Ley (Figure 2d). Thus, Ley not only serves as a ligand for TM-mediated THP-1 cell adhesion, but also leads to TM clustering.
Figure 2.
TM mediates THP-1 cell adhesion to Ley and exhibits a polarized distribution on adherent cells. (a) Control (shLuc) and TM-knockdown (shTM) THP-1 cells were perfused through flow chambers coated with 1 μm biotinylated PAA (PAA) or biotinylated Ley-PAA (Ley-PAA). Quantitative data show the number of adherent cells per field from three independent experiments. ***P<0.001 versus control (shLuc) THP-1 cells adhesion on biotinylated PAA. (b) THP-1 cells were perfused through a monolayer of activated human umbilical vein endothelial cells, fixed and stained with anti-L-selectin and anti-TM mAb. Nuclei were counterstained with 4,6 diamidino-2-phenylindole (DAPI). Fluorescence images (DAPI in blue; L-selectin in green; TM in red) show two adherent cells with L-selectin and TM polarization and colocalization (arrowheads) on the endothelium. The right panel shows the image of bright-field image for visualization of adherent THP-1 cells on the endothelium. Scale bar, 10 μm. (c) THP-1 cells were perfused through flow chambers coated with 1 μM buitinylated PAA (PAA) or biotinylated Ley-PAA (Ley-PAA), fixed and stained with anti-TM mAb. Nuclei were counterstained with DAPI. Fluorescence images (DAPI in blue; TM in red) show adherent cells on biotinylated PAA (PAA) or biotinylated Ley-PAA (Ley-PAA). Arrowheads indicate polarized TM on adherent cells. Scale bar, 10 μm. (d) Quantitative data show the percentage of TM polarized THP-1 cells from three independent experiments. **P<0.01.
Ley/TM interaction triggers THP-1 cell adhesion by activation of p38 mitogen-activated protein kinase (MAPK) and β2 integrins
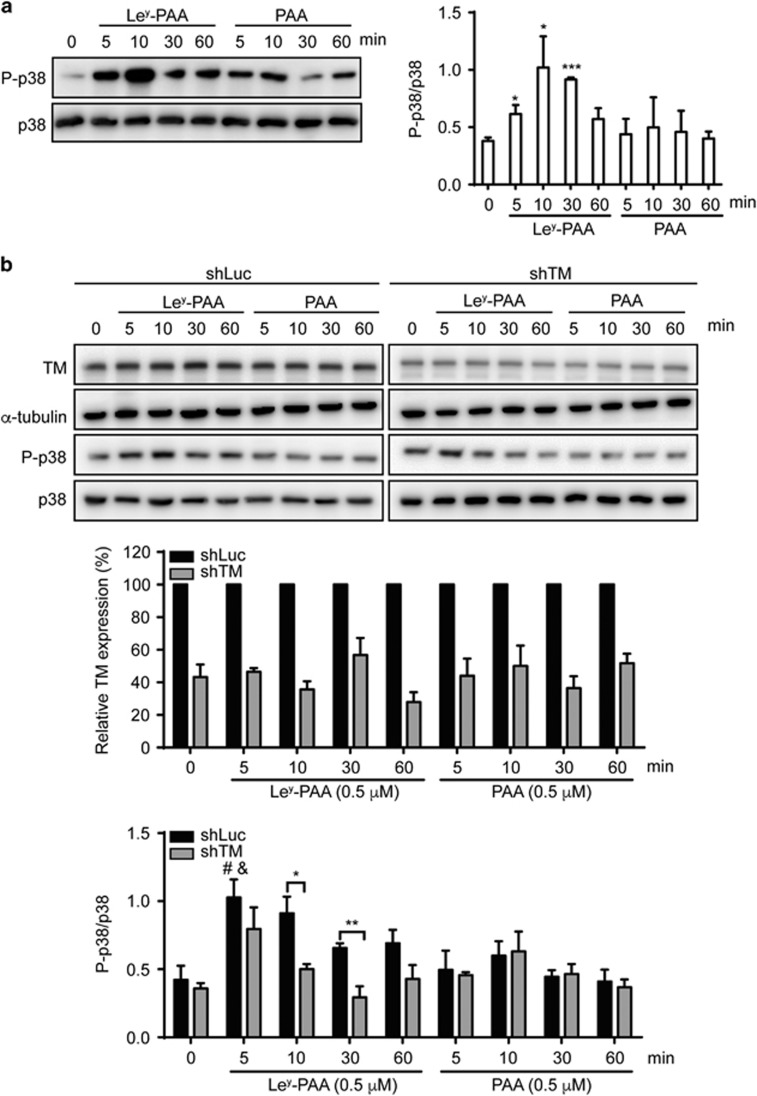
Previous studies have shown that ligation of selectins with their ligands can induce p38 MAPK activation in leukocytes, leading to the redistribution of adhesion molecules and leukocyte arrest.4, 5, 22, 23 Therefore, we assessed whether Ley/TM interaction-mediated THP-1 cell adhesion involved p38 MAPK activation. Addition of Ley-PAA to a THP-1 suspension induced p38 phosphorylation in a time-dependent manner, with peak phosphorylation observed at 10 min; this effect was not observed following the addition of PAA (Figure 3a). To determine whether TM was required for Ley-induced p38 phosphorylation, control and TM-knockdown THP-1 cells were treated with PAA or Ley-PAA. As shown in Figure 3b, 5 min of Ley stimulation significantly induced p38 phosphorylation in control cells when compared with untreated cells or cells treated with PAA for 5 min. The phosphorylation levels were ~2 times higher in control cells than in TM-knockdown cells after 10 or 30 min of Ley stimulation (0.91±0.12 versus 0.50±0.04 at 10 min, P<0.05; 0.66±0.03 versus 0.29±0.08 at 30 min, P<0.01), and there were no significant differences between both cell lines following treatment with PAA.
Figure 3.
Ley induces p38 MAPK phosphorylation via TM. THP-1 cells (a) and which were transduced with TM (shTM) or control (shLuc) shRNA (b) were stimulated with 0.5 μm Ley-PAA or PAA for the indicated duration, and total cell lysates were used for western blotting with the indicated antibodies. Quantitative data show the relative density as indicated from three independent experiments. *P<0.05 and ***P<0.001 versus parental THP-1 cells at 0 min. #P<0.05 versus control (shLuc) THP-1 cells at 0 min; &P<0.05 versus control (shLuc) THP-1 cells treated with PAA at 5 min; **P<0.01.
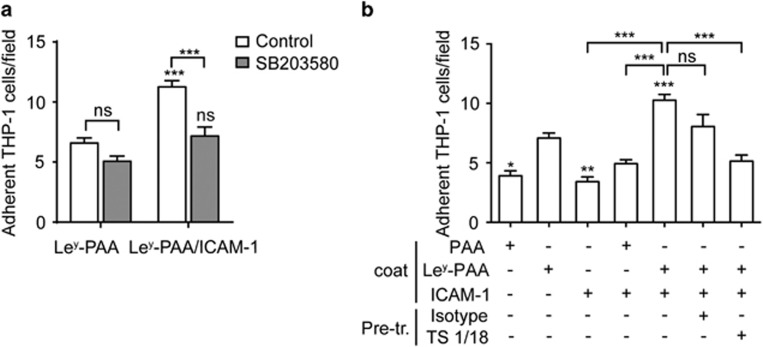
Next, we tested whether p38 MAPK activation triggered by Ley was involved in leukocyte adhesion. The flow chambers were coated with Ley alone or in combination with ICAM-1, which allowed for firm arrest of leukocytes. The data showed that co-immobilization of Ley and ICAM-1 on the flow chamber increased the adhesion of THP-1 cells as compared with that induced by Ley alone. Pretreatment with SB203580, an inhibitor of p38, obviously reduced leukocyte adhesion on Ley and ICAM-1 co-immobilized surfaces, but had no effect on adhesion to Ley-immobilized surfaces (Figure 4a), suggesting that Ley-induced p38 signaling pathway is required for the binding of THP-1 cells to ICAM-1.
Figure 4.
Adhesion on Ley induces p38 MAPK and β2 integrin activation. (a) THP-1 cells were pretreated with control (dimethyl sulfoxide) or p38 MAPK inhibitor SB203580 (10 μm for 30 min at room temperature), then perfused through flow chambers coated with Ley-PAA-biotin alone or in combination with ICAM-1-Fc. NS, no significant and ***P<0.001 versus the control. (b) THP-1 cells were pretreated (Pre-tr.) with or without 10 μg ml−1 of isotype antibody or TS1/18, then perfused through flow chambers coated with PAA-biotin, Ley-PAA-biotin or ICAM-1-Fc as indicated. The number of adherent cells per field was counted after perfusion. Adhesion is shown as the number of adherent cells per field from three independent experiments. NS, no significant and ***P<0.001; *P<0.05, **P<0.01 and ***P<0.001 versus Ley-PAA-biotin alone.
Firm adhesion of leukocytes to ICAM-1 requires β2 integrin activation, which is rapidly transduced by p38.4 Therefore, we examined whether Ley-triggered p38 phosphorylation would in turn activate β2 integrins to initiate firm leukocyte adhesion. We examined THP-1 cell adhesion in the presence of ICAM-1 and TS1/18, an inhibitory anti-β2 integrin monoclonal antibody (mAb), to test the status of integrin activation.24, 25 As shown in Figure 4b, Ley-coated surface captured significantly higher amount of THP-1 cells than PAA or ICAM-1, and the adherent cells were further increased on the surface co-immobilized with Ley and ICAM-1. When cells were treated with the anti-β2 integrin mAb TS1/18, adhesion was strongly reduced. Collectively, these results demonstrated that when TM binds its ligand, Ley, p38 MAPK signaling is triggered, leading to β2 integrin activation, which supports firm adhesion of THP-1 cells.
Macrophage infiltration and neointima formation are reduced after carotid artery ligation in myeloid-specific TM-deficient mice
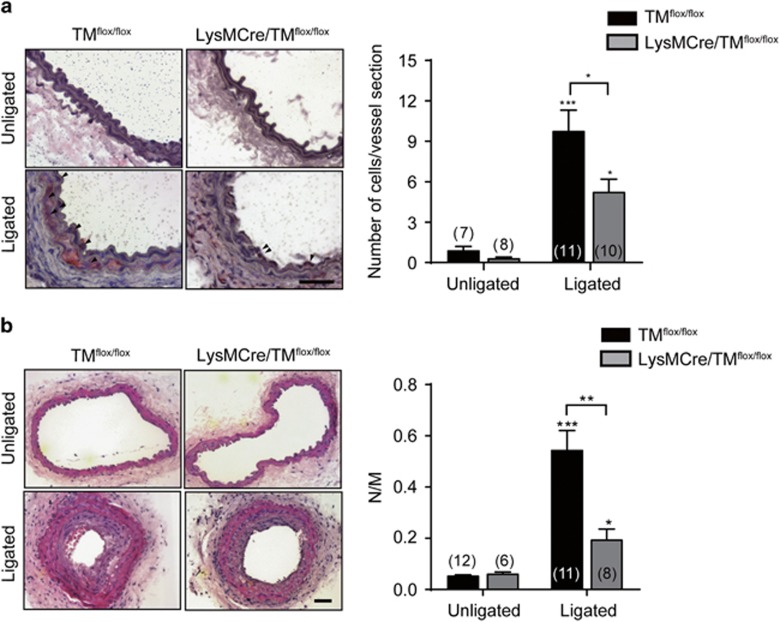
Monocyte/macrophage infiltration is an early response in the vessel lesion after carotid ligation. These inflammatory cells release cytokines and growth factors to augment smooth muscle cell proliferation, leading to neointima formation and narrowing of the lumen.26, 27 To confirm the effect of TM on monocyte adhesion in response to vascular injury, carotid artery ligation was performed in TMflox/flox and LysMcre/TMflox/flox mice, in which TM was knocked out in myeloid cells by using the Cre/loxP recombination system.28 MOMA-2 staining for monocyte/macrophage identification revealed a significant increase in monocyte/macrophage accumulation in ligated arteries in both TMflox/flox and LysMcre/TMflox/flox mice 7 days after ligation. The number of MOMA-2-positive monocytes/macrophages in ligated arteries was lower in LysMcre/TMflox/flox than in TMflox/flox mice (5.2±1.0 versus 9.7±1.6 cells per vessel section; P<0.05; Figure 5a). Furthermore, neointima development in ligated arteries was significantly induced at 4 weeks after ligation in both groups of mice; however, the increase in LysMcre/TMflox/flox mice was smaller than that in TMflox/flox mice (0.19±0.04 versus 0.54±0.08; P<0.01; Figure 5b). In unligated arteries, there were no significant differences in macrophage accumulation or neointima formation between both mouse groups. These findings indicated that myeloid TM expression is associated with vascular inflammation by promoting monocyte/macrophage infiltration into the vessel wall after injury.
Figure 5.
Decreased leukocyte recruitment and neointima formation in TM conditional knockout mice. Carotid ligation was performed in wild-type (TMflox/flox) and myeloid-specific TM-deficient mice (LysMcre/TMflox/flox). The carotid arteries were harvested at 7 (a) and 28 days (b) after ligation. (a) Representative sections of carotid arteries from mice stained with anti-MOMA-2 mAb for macrophages. Arrowheads indicate MOMA-2-positive macrophages. Scale bar, 50 μm. Quantitative data show the number of macrophages infiltrated into the ligated vessel. The number of mice in each group is indicated. *P<0.05 and ***P<0.001 versus unligated mice. (b) Representative hematoxylin–eosin-stained sections of carotid arteries from the mice. Scale bar, 50 μm. Quantitative data show the ratios of neointima (N) and media (M). The number of mice in each group is indicated. **P<0.01; *P<0.05 and ***P<0.001 versus unligated mice.
DISCUSSION
Although TM expression in leukocytes has been identified for decades, its function is still largely unknown. The present study revealed that monocyte-expressed TM plays a crucial role in mediating cell adhesion. We propose a model (Figure 6) wherein through interaction between TM and its ligand, Ley, THP-1 cells exhibit redistribution of TM, phosphorylation of p38 MAPK and activation of β2 integrins, consequently resulting in firm adhesion between THP-1 and ICAM-1. When TM expression was suppressed specifically in myeloid-lineage cells, macrophage infiltration was reduced, thus lowering vascular pathogenesis. These results demonstrated that TM functions as an adhesion molecule in monocytes and TM/Ley interaction-mediated cell adhesion to the endothelium, suggesting a novel mechanism in monocyte recruitment.
Figure 6.
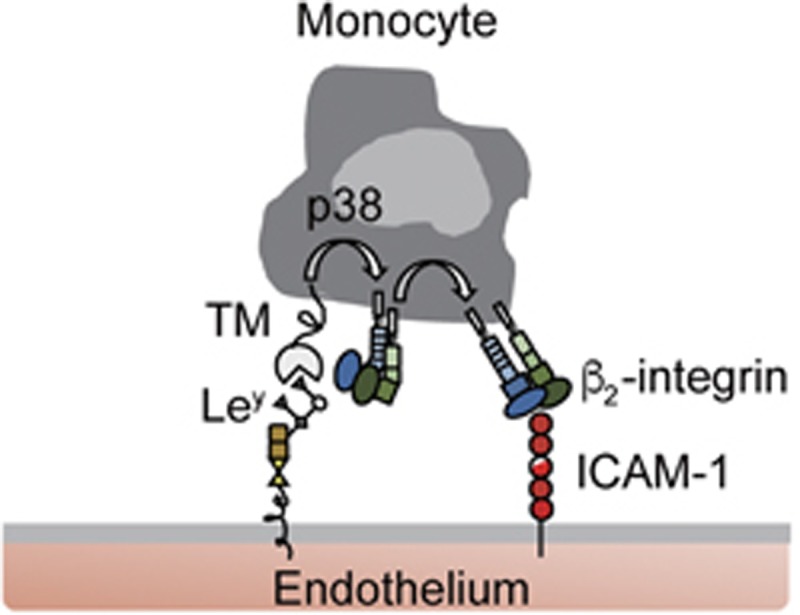
Model of TM-mediated leukocyte adhesion via binding to Ley. Our results support a model in which TM interacts with Ley-conjugated adhesion molecules to initiate p38 MAPK signaling pathway within monocytes as they roll over the endothelial surface. This signal consequently activates β2 integrins, which are able to bind ICAM-1, leading to firm adhesion.
Monocyte-expressed L-selectin is known to function as an essential adhesion molecule for monocyte adhesion and migration by binding to endothelial glycoprotein ligands such as CD34, glycosylation-dependent cell adhesion molecule-1 and mucosal addressin cell adhesion molecule-1.29 In neutrophils, L-selectin is redistributed and polarized when binding to its ligand, to increase the avidity of leukocytes for endothelial cells.22 Although this effect of L-selectin on monocyte adhesion has not been reported, the polarization and colocalization of TM and L-selectin on adherent THP-1 cells reported here may imply that TM and L-selectin, which commonly facilitate cell adhesion via the property of carbohydrate binding, can coordinately mediate monocyte adhesion through exhibiting clustered expression that augment the binding avidity of monocytes to endothelial cells.
We demonstrated that Ley treatment triggered signals in THP-1 cells via TM. Ley-induced signals have been previously reported in other cell types. One research group investigated the effects of increased Ley expression on tissues affected by rheumatoid arthritis, showing that H-2g, a glucose analog structurally related to Ley,19 mediates human monocyte migration by phosphorylation of PKCα1/β2, Akt and nuclear factor κB in monocytes.20 In addition, H-2g can induce basic fibroblast growth factor and vascular endothelial growth factor expression and signal through Janus kinase 2, phosphoinositide-3 kinase and nuclear factor κB pathways to mediate angiogenesis in human endothelial cells.30 Thus, the inflammatory responses that occur in patients with rheumatoid arthritis may be caused by stimulation of Ley. Therefore, we speculate that the phosphorylation of p38 as observed in THP-1 cells may represent either the crosstalk of the signaling molecules or another independent pathway induced by Ley. However, the mechanism by which TM transduces signals via Ley in leukocytes should require further investigation. The phosphorylation of cytoplasmic tail of L-selectin has been reported for the binding of PKC isoenzymes, leading to subsequent transduction of signals for recruitment.31, 32 Although evidence of TM phosphorylation has not been shown, several predicted phosphorylation motifs, including those for PKC-mediated phosphorylation, have been identified within TM cytoplasmic domain (http://www.hprd.org/PhosphoMotif_finder). Thus, TM may be phosphorylated on stimulation, providing a platform for signaling events.
Previous studies investigating the effects of monocyte TM expression on the outcomes of coronary artery bypass graft surgery showed that TM congregates in these cells following stimulation with interleukin-6 and that this event is associated with actin-polymerization and monocyte chemotaxis.33 Although our experimental conditions were somewhat different, clustering of TM was also observed. In addition, we previously demonstrated the binding of the cytoplasmic domain of TM (TMD5) and ezrin, a member of the ezrin/radixin/moesin (ERM) proteins, controls epithelial morphology and promotes collective cell migration.34 It strongly suggests that TM polarization may affect actin cytoskeleton rearrangement, further mediating cell adhesion and migration.
TMD1 is considered an essential anti-inflammatory component of TM. Previous studies have shown that whole-body TMD1 knockout (TMLeD/LeD) transgenic mice exhibit increased immune responses and decreased survival rates in inflammatory disease models. Moreover, inflammatory cell accumulation was found to be higher in TMLeD/LeD than in wild-type mice.35 We found that macrophage infiltration in LysMcre/TMflox/flox mice was lower than that in TMflox/flox mice. We previously showed that LysMcre/TMflox/flox mice have reduced macrophage accumulation and inflammatory cytokine production in a mouse abdominal aortic aneurysm model.36 Thus, myeloid-specific expressed TM may have distinct effects on inflammation. In summary, we reported a novel function for TM as an adhesion molecule in monocytes, enhancing their adhesion to the endothelium by binding to Ley, leading to β2 integrin activation via p38 MAPK. Our findings suggest TM as a potential drug target in vascular inflammatory diseases.
METHODS
Materials
The multivalent polymers PAA [HOCH2(HOCH)4CH2NH-PAA] and Ley-PAA, and the multivalent biotinylated polymers PAA-biotin and Ley-PAA-biotin were purchased from GlycoTech Corporation (Gaithersburg, MD, USA). Recombinant human ICAM-1-Fc and TNF-α were purchased from R&D Systems (Minneapolis, MN, USA). The p38 MAPK inhibitor SB203580 and anti-phosphor-p38 MAPK (Thr180/Tyr182) (D3F9) mAb were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-p38 MAPK polyclonal Ab (C-20), anti-TM (D-3), anti-L-selectin (lam1-116) and anti-α-tubulin (B-7) mAbs were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-TS1/18 mAb and Alexa Fluor 488- and 546-conjugated secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Anti-MOMA-2 mAb was purchased from Abcam (Cambridge, UK).
Lentiviral delivery of short hairpin RNA
All lentiviral vectors and the viral delivery system were established by and obtained from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan). To knockdown TM expression, shTM with the targeting sequence of 5′-GCCGATGTCATTTCCTTGCTA-3′ was used. shLuc with the targeting sequence of 5′-TCACAGAATCGTCGTATGCAG-3′ was used as a negative control. For infection, THP-1 cells were seeded in culture medium containing recombinant virus and polybrene (8 μg ml−1) and incubated at 37 °C overnight. Infected cells were selected using puromycin (2 μg ml−1),28 and the expression of TM was determined by using western blotting.
Cell adhesion under shear flow condition
For investigation of THP-1 cell adhesion to the endothelium, human umbilical vein endothelial cells were grown to confluence on laminar flow chamber slides (μ-slide I 0.8luer; ibidi, Munich, Germany) and activated by TNF-α (10 ng ml−1) for 24 h. After washing of the human umbilical vein endothelial cells with serum-free medium, THP-1 cells were perfused into the flow chamber.
For investigation of THP-1 cell adhesion to the PAA-biotin or Ley-PAA-biotin immobilized surface, the laminar flow chamber slides were filled with streptavidin (100 μg ml−1; Sigma-Aldrich, St. Louis, MO, USA) overnight. After washing with PBS, the slides were filled with PAA-biotin or Ley-PAA-biotin (1 μm) for 2 h and blocked with 1% BSA at room temperature for 1 h. After washing the slides with serum-free medium, THP-1 cells were perfused into the flow chamber. For immobilization of ICAM-1, the laminar flow chamber slides were filled with Protein G (200 μg ml−1; Invitrogen, Waltham, MA, USA) overnight. After washing with PBS, the slides were filled with recombinant ICAM-1-Fc (5 μg ml−1) for 2 h, followed by the same steps as mentioned above. For p38 MAPK inhibition, THP-1 cells were pretreated with SB203580 (10 μm) for 1 h at 37 °C before perfusion. To inhibit integrin activation, THP-1 cells were pretreated with the integrin mAb (10 μg ml−1) at room temperature for 30 min before perfusion.
In these experiments, the shear flow was generated using a perfusion loop system and an air pressure pump (ibidi). THP-1 cells (1 × 106) were perfused into the flow chamber at a constant flow rate of 0.5 dynes cm−2. The number of adherent THP-1 cells was determined after 30 min of perfusion. In each experiment, 10–15 random fields were observed; statistics were calculated using 3–5 experiments.
Immunofluorescence for confocal microscopy analysis
After shear flow adhesion, adherent cells were incubated with primary antibodies at 4 °C overnight. Secondary antibodies conjugated with Alexa Fluor 488 or 546 were used for detection. Images were taken using an FV-1000 confocal microscope (Olympus) and analyzed with the ImageJ 1.45 s software (National Institute of Health, Bethesda, MD, USA). Polarized cells were defined as those having fluorescent signal within a discrete region in which the pixel intensity was at least twofold greater than that in the rest of the cell body (Supplementary Figure 2).5 The percentage of polarized cells was obtained by counting cells in 5–10 random fields in a single experiment.
Assay of p38 MAPK phosphorylation
THP-1 cells (5 × 105 cells ml−1) were left untreated or were treated with PAA or Ley-PAA (0.5 μm) for indicated times at 37 °C, after which the reaction mixtures were immediately centrifuged to remove the supernatant; the cell pellet was rapidly suspended in ice-cold cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing protease inhibitor cocktail (Sigma-Aldrich). The lysates were centrifuged at 15 000g for 10 min and the supernatants were prepared for western blotting to detect phosphorylated p38 MAPK (Thr180/Tyr182).
Mice
Myeloid-specific TM-deficient mice (LysMcre/TMflox/flox) were generated using a Cre/loxP system was described previously and the conditionally expression of TM was tested.28 TMflox/flox and LysMcre/TMflox/flox littermates were used for experiments at 8–12 weeks of age. Animals were randomized into different groups. Each group contained 6–12 animals. All the mice were maintained at the National Cheng Kung University Laboratory Animal Center. All animal experiments were approved by the Institutional Animal Care and Use Committee of National Cheng Kung University.
Carotid artery ligation model
Carotid artery ligation was performed as previously described.37 On day 0, the left carotid artery was completely ligated just proximal to the carotid bifurcation to disrupt blood flow. The right contralateral artery was used as a control. The animals were killed on days 7 or 28 after ligation, and the segments of the right and the left common carotid arteries just proximal to the ligation were excised for histological analysis. Immunohistochemistry for anti-mouse MOMA-2 mAb was used to determine macrophage infiltration at 7 days after ligation. The average number of cells on the luminal surface of the five sections in each artery was counted. Neointima formation at 28 days after ligation was measured as previously described.37
Statistics
Data from three separate experiments are expressed as the mean±s.e.m. Statistical significance between two groups was analyzed using an unpaired Student's t-test. Comparisons of three or more were carried out using one-way analysis of variance with a post hoc Tukey correction. A P-value<0.05 was considered statistically significant.
Acknowledgments
This work was supported by Ministry of Science and Technology (Taipei, Taiwan, MOST-102-2320-B-006-039-MY2) and a grant from the ‘Top-Notch Project' of the National Cheng Kung University (Taiwan).
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
The authors declare no conflict of interest.
Supplementary Material
References
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 1994; 8: 504–512. [PubMed] [Google Scholar]
- Crockett-Torabi E. Selectins and mechanisms of signal transduction. J Leukoc Biol 1998; 63: 1–14. [PubMed] [Google Scholar]
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol 2000; 164: 4348–4358. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 2007; 26: 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 2010; 116: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA 1981; 78: 2249–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Nowakowski B, Steiner-Mosonyi M. Human neutrophils synthesize thrombomodulin that does not promote thrombin-dependent protein C activation. Blood 1992; 80: 1254–1263. [PubMed] [Google Scholar]
- McCachren SS, Diggs J, Weinberg JB, Dittman WA. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood 1991; 78: 3128–3132. [PubMed] [Google Scholar]
- Grey ST, Hancock WW. A physiologic anti-inflammatory pathway based on thrombomodulin expression and generation of activated protein C by human mononuclear phagocytes. J Immunol 1996; 156: 2256–2263. [PubMed] [Google Scholar]
- Kanehara H, Tohda G, Oida K, Suzuki J, Ishii H, Miyamori I. Thrombomodulin expression by THP-1 but not by vascular endothelial cells is upregulated by pioglitazone. Thromb Res 2002; 108: 227–234. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JE, Chung J, Kim YT, Kang SH, Han KS et al. Lipopolysaccharide down-regulates the thrombomodulin expression of peripheral blood monocytes: effect of serum on thrombomodulin expression in the THP-1 monocytic cell line. Blood Coagul Fibrinolysis 2007; 18: 157–164. [DOI] [PubMed] [Google Scholar]
- Kizaki K, Ishii H, Horie S, Kazama M. Thrombomodulin induction by all-trans retinoic acid is independent of HL-60 cells differentiation to neutrophilic cells. Thromb Haemost 1994; 72: 573–577. [PubMed] [Google Scholar]
- Kizaki K, Naito S, Horie S, Ishii H, Kazama M. Different thrombomodulin induction in monocytic, macrophagic and neutrophilic cells differentiated from HL-60 cells. Biochem Biophys Res Commun 1993; 193: 175–181. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1α,25-dihydroxyvitamin d(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 2000; 102: 2867–2872. [DOI] [PubMed] [Google Scholar]
- Shi CS, Shi GY, Hsiao HM, Kao YC, Kuo KL, Ma CY et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood 2008; 112: 3661–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol 2000; 1: 353–357. [DOI] [PubMed] [Google Scholar]
- Garcia-Vallejo JJ, van Liempt E, da Costa Martins P, Beckers C, van het Hof B, Gringhuis SI et al. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through Lewisy antigen expressed on icam-2. Mol Immunol 2008; 45: 2359–2369. [DOI] [PubMed] [Google Scholar]
- Halloran MM, Carley WW, Polverini PJ, Haskell CJ, Phan S, Anderson BJ et al. Ley/h: an endothelial-selective, cytokine-inducible, angiogenic mediator. J Immunol 2000; 164: 4868–4877. [DOI] [PubMed] [Google Scholar]
- Amin MA, Ruth JH, Haas CS, Pakozdi A, Mansfield PJ, Haghshenas J et al. H-2g, a glucose analog of blood group h antigen, mediates mononuclear cell recruitment via src and phosphatidylinositol 3-kinase pathways. Arthritis Rheum 2008; 58: 689–695. [DOI] [PubMed] [Google Scholar]
- Lin WL, Chang CF, Shi CS, Shi GY, Wu HL. Recombinant lectin-like domain of thrombomodulin suppresses vascular inflammation by reducing leukocyte recruitment via interacting with Lewis y on endothelial cells. Arterioscler Thromb Vasc Biol 2013; 33: 2366–2373. [DOI] [PubMed] [Google Scholar]
- Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity β2-integrin on neutrophils. J Immunol 2004; 172: 7780–7790. [DOI] [PubMed] [Google Scholar]
- Smolen JE, Petersen TK, Koch C, O'Keefe SJ, Hanlon WA, Seo S et al. L-selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinase. J Biol Chem 2000; 275: 15876–15884. [DOI] [PubMed] [Google Scholar]
- Wang XG, Cheng YP, Ba XQ. Engagement of PSGL-1 enhances β(2)-integrin-involved adhesion of neutrophils to recombinant ICAM-1. Acta Pharmacol Sin 2006; 27: 617–622. [DOI] [PubMed] [Google Scholar]
- Zwartz GJ, Chigaev A, Dwyer DC, Foutz TD, Edwards BS, Sklar LA. Real-time analysis of very late antigen-4 affinity modulation by shear. J Biol Chem 2004; 279: 38277–38286. [DOI] [PubMed] [Google Scholar]
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 2000; 190: 300–309. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 1997; 17: 2238–2244. [DOI] [PubMed] [Google Scholar]
- Ma CY, Shi GY, Shi CS, Kao YC, Lin SW, Wu HL. Monocytic thrombomodulin triggers LPS- and gram-negative bacteria-induced inflammatory response. J Immunol 2012; 188: 6328–6337. [DOI] [PubMed] [Google Scholar]
- Leon B, Ardavin C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood 2008; 111: 3126–3130. [DOI] [PubMed] [Google Scholar]
- Zhu K, Amin MA, Zha Y, Harlow LA, Koch AE. Mechanism by which H-2g, a glucose analog of blood group H antigen, mediates angiogenesis. Blood 2005; 105: 2343–2349. [DOI] [PubMed] [Google Scholar]
- Ivetic A. Signals regulating L-selectin-dependent leucocyte adhesion and transmigration. Int J Biochem Cell Biol 2013; 45: 550–555. [DOI] [PubMed] [Google Scholar]
- Kilian K, Dernedde J, Mueller EC, Bahr I, Tauber R. The interaction of protein kinase C isozymes α, ι, and θ with the cytoplasmic domain of L-selectin is modulated by phosphorylation of the receptor. J Biol Chem 2004; 279: 34472–34480. [DOI] [PubMed] [Google Scholar]
- Tsai CS, Tsai YT, Lin CY, Lin TC, Huang GS, Hong GJ et al. Expression of thrombomodulin on monocytes is associated with early outcomes in patients with coronary artery bypass graft surgery. Shock 2010; 34: 31–39. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Shi GY, Kuo CH, Liu SL, Wu CM, Ma CY et al. Thrombomodulin is an ezrin-interacting protein that controls epithelial morphology and promotes collective cell migration. FASEB J 2012; 26: 3440–3452. [DOI] [PubMed] [Google Scholar]
- Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappab and mitogen-activated protein kinase pathways. J Exp Med 2002; 196: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Li YH, Shi GY, Tsai HW, Luo CY, Cheng MH et al. Membrane-bound thrombomodulin regulates macrophage inflammation in abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2015; 35: 2412–2422. [DOI] [PubMed] [Google Scholar]
- Liu SL, Li YH, Shi GY, Chen YH, Huang CW, Hong JS et al. A novel inhibitory effect of naloxone on macrophage activation and atherosclerosis formation in mice. J Am Coll Cardiol 2006; 48: 1871–1879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.