Abstract
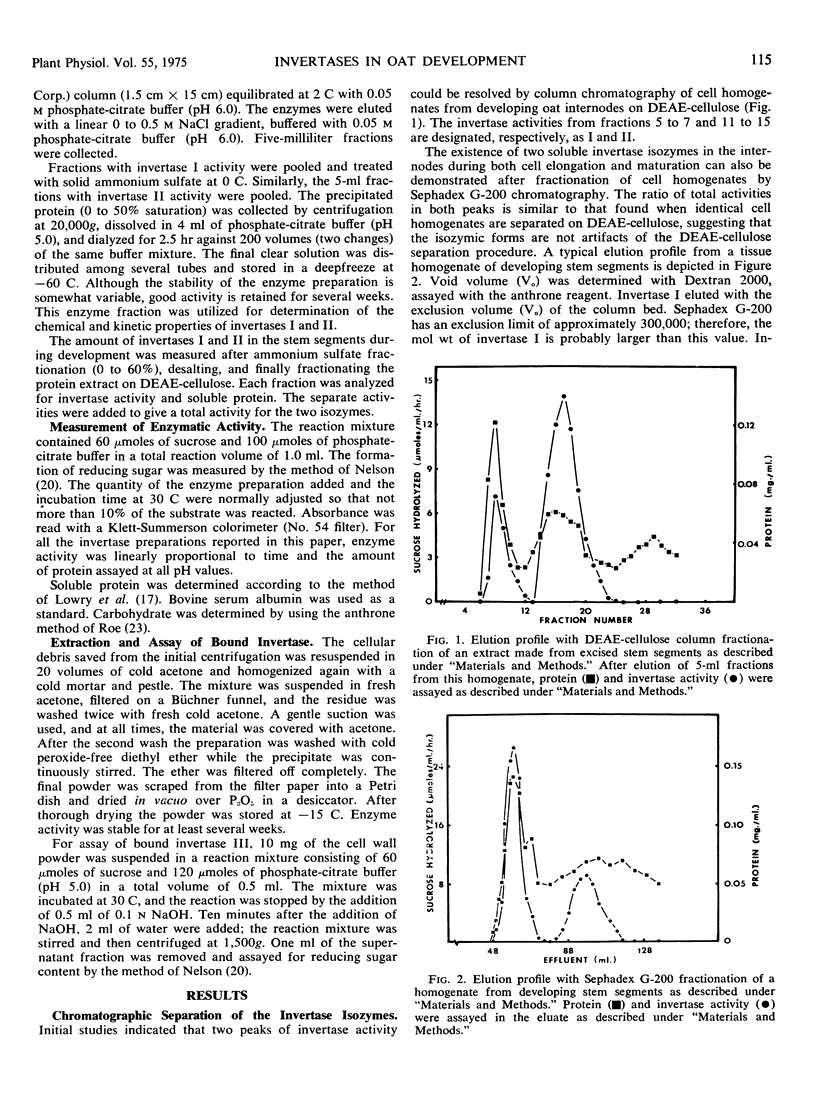
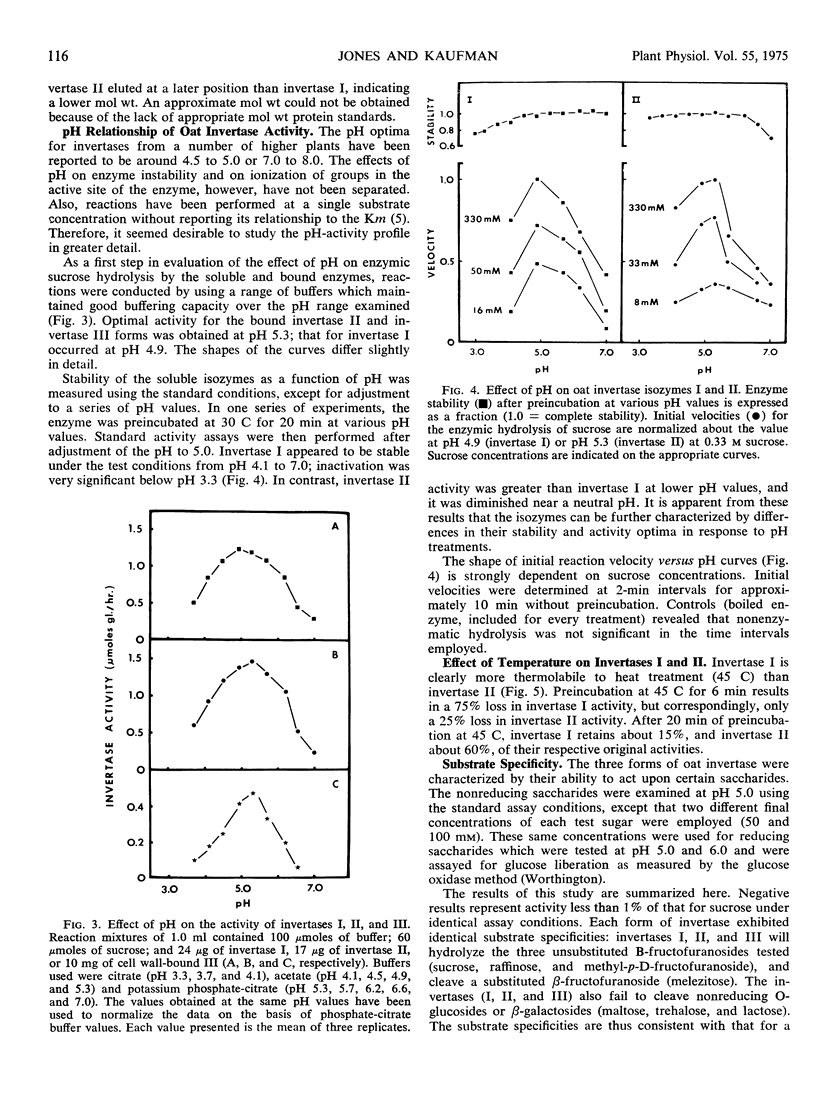
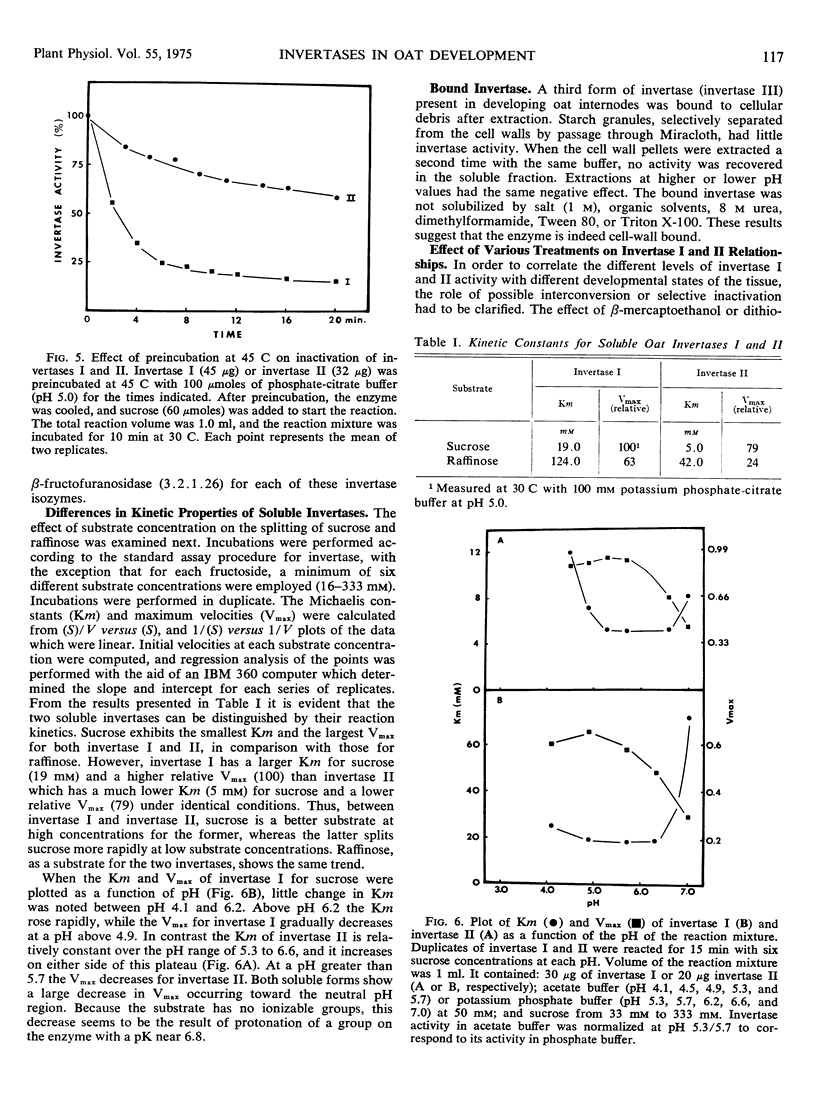
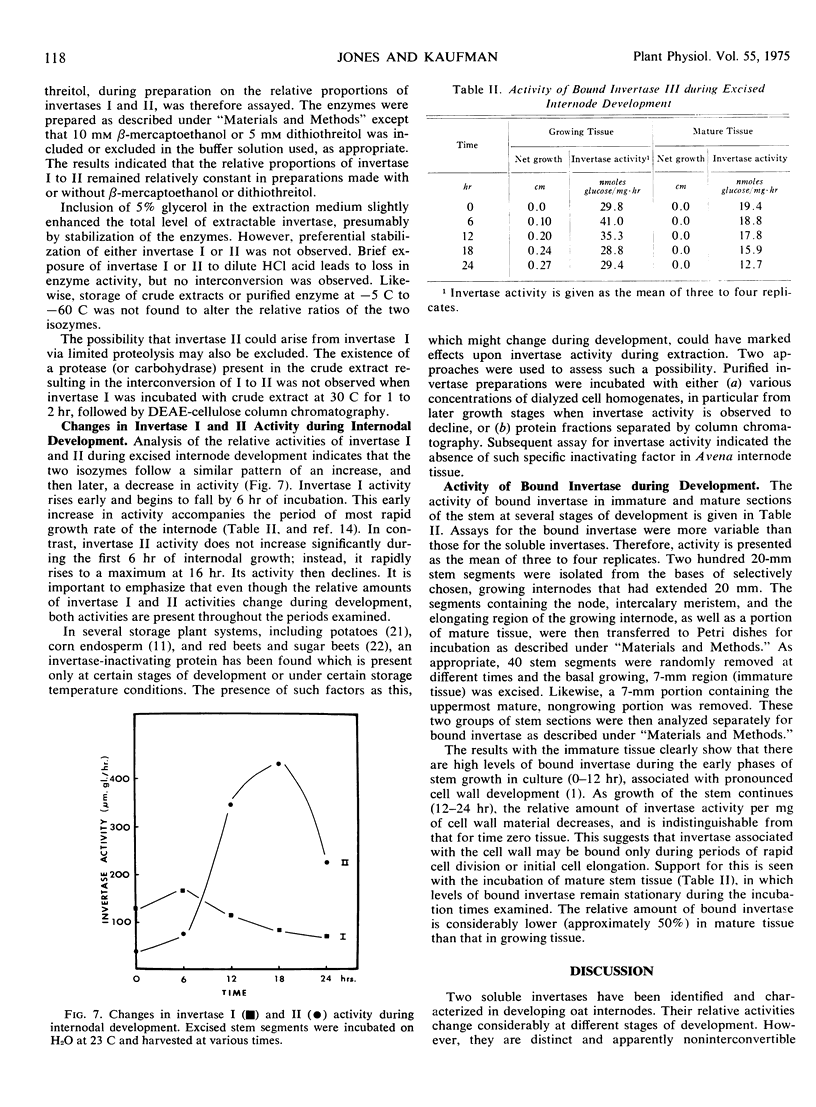
Three different invertases are found in the developing internodes of oat (Avena sativa cv. Victory). Two soluble invertases (I and II) are separable on diethylaminoethylcellulose and Sephadex columns. They are further distinguished by their kinetic constants, heat stability, and differences in stability and apparent activity optima in response to pH treatments. Relative activities of the two soluble isozymes change considerably during the developmental stages examined. Invertase I activity rises early and begins to fall after maximal activity is reached at 6 hours of incubation. This early increase in activity accompanies the period of most rapid growth rate of the internode. Invertase II activity does not increase significantly during the first 6 hours of internode extension, but rapidly rises to a maximum activity at 16 hours, then declines. The third form of invertase, bound invertase (III), is present in both immature and mature stem tissue. Its activity increases (by 6 hours) during immature growth stages, decreases considerably with maturation, and remains relatively constant in mature tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beteta P., Gascon S. Localization of invertase in yeast vacuoles. FEBS Lett. 1971 Mar 22;13(5):297–300. doi: 10.1016/0014-5793(71)80245-4. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Greenshields R. N. The partial purification and some properties of two sucrases of Phaseolus vulgaris. Biochem J. 1964 Aug;92(2):357–364. doi: 10.1042/bj0920357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. EFFECT OF GROWTH HORMONES ON THE DEVELOPMENT OF INVERTASE ASSOCIATED WITH CELL WALLS. Nature. 1964 Jan 18;201:296–297. doi: 10.1038/201296b0. [DOI] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. An invertase inactivator in maize endosperm and factors affecting inactivation. Plant Physiol. 1971 May;47(5):629–634. doi: 10.1104/pp.47.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. Invertase Activity in Normal and Mutant Maize Endosperms during Development. Plant Physiol. 1971 May;47(5):623–628. doi: 10.1104/pp.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N. S., Lacroix J. D., Soni S. L., Ikuma H. Regulation of invertase levels in Avena stem segments by gibberellic Acid, sucrose, glucose, and fructose. Plant Physiol. 1973 Sep;52(3):221–228. doi: 10.1104/pp.52.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Invertase inhibitors from red beet, sugar beet, and sweet potato roots. Plant Physiol. 1968 Sep;43(9):1430–1434. doi: 10.1104/pp.43.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. The regulation of sugar uptake and accumulation in bean pod tissue. Plant Physiol. 1966 Jan;41(1):181–189. doi: 10.1104/pp.41.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus J. Invertase in Cell Walls of Plant Tissue Cultures. Plant Physiol. 1962 May;37(3):342–348. doi: 10.1104/pp.37.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]


