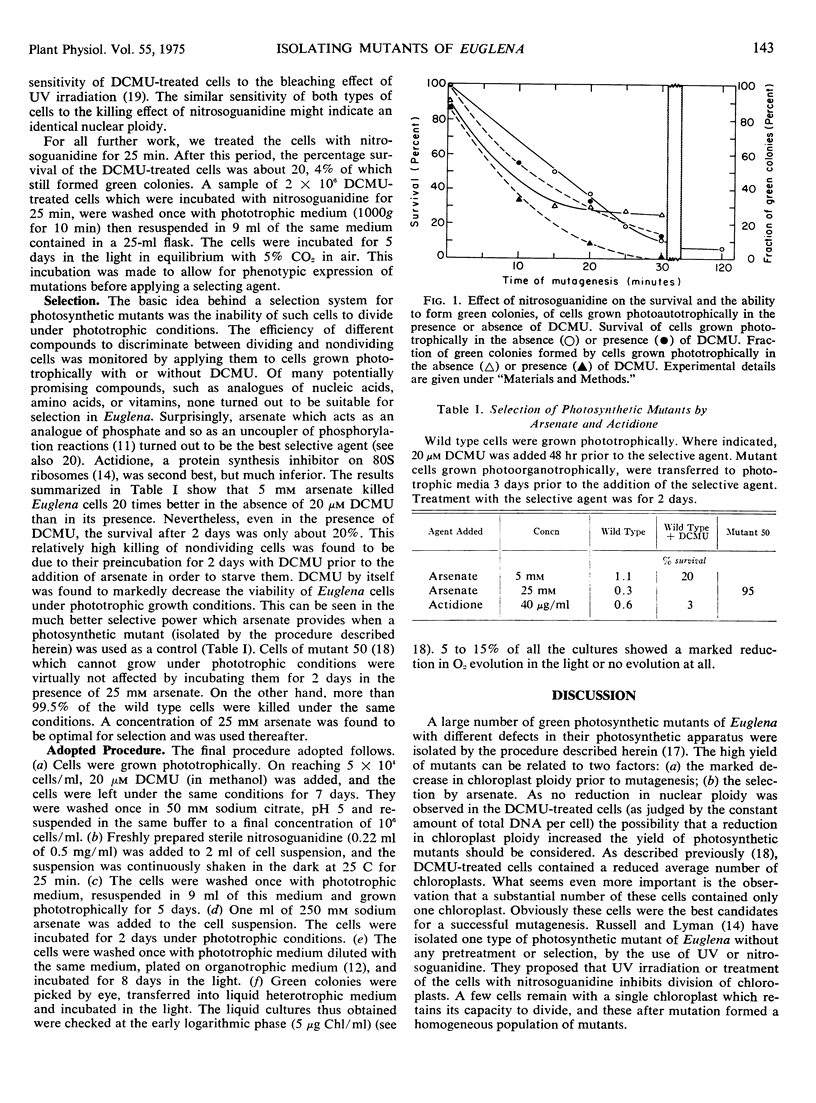
Abstract
A method was developed for the isolation of photosynthetic mutants of Euglena gracilis. It consists of the following steps. (a) Incubation of the cells under phototrophic conditions in the presence of 3 (3,4-dichlorophenyl)-1, 1-dimethylurea for 1 week. This step caused a drastic reduction in the number of chloroplasts per cell; (b) mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine; (c) phototrophic growth for a few days to allow for phenotype expression; (d) selection by incubation in the presence of arsenate under phototrophic conditions for 2 days; (e) plating and growth under photoorganotrophic conditions; (f) assay of green colonies for ability to evolve oxygen. About 10% of the green colonies were found to be deficient in their ability to evolve oxygen. In principle the method may prove suitable for the isolation of other types of mutants of Euglena.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EPSTEIN H. T., BOY DE LA TOUR E., SCHIFF J. A. Fluorescence studies of chloroplast development in Euglena. Nature. 1960 Mar 19;185:825–826. doi: 10.1038/185825a0. [DOI] [PubMed] [Google Scholar]
- Hill H. Z., Schiff J. A., Epstein H. T. Studies of chloroplast development in Euglena. 13. Variation of ultraviolet sensitivity with extent of chloroplast development. Biophys J. 1966 Mar;6(2):125–133. doi: 10.1016/s0006-3495(66)86644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEDALE G. F. The evidence for a meiotic process in the Euglenineae. Arch Mikrobiol. 1962;42:237–245. doi: 10.1007/BF00422042. [DOI] [PubMed] [Google Scholar]
- LYMAN H., EPSTEIN H. T., SCHIFF J. A. Studies of chloroplast development in Euglena. I. Inactivation of green colony formation by u.v. light. Biochim Biophys Acta. 1961 Jun 24;50:301–309. doi: 10.1016/0006-3002(61)90328-6. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Goodenough U. W. The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardi. Annu Rev Genet. 1970;4:397–408. doi: 10.1146/annurev.ge.04.120170.002145. [DOI] [PubMed] [Google Scholar]
- Powls R., Wong J., Bishop N. I. Electron transfer components of wild-type and photosynthetic mutant strains of Scenedesmus obliquus D3. Biochim Biophys Acta. 1969 Aug 5;180(3):490–499. doi: 10.1016/0005-2728(69)90027-9. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Lyman H. Isolation of mutants of Euglena gracilis with impaired photosynthesis. Plant Physiol. 1968 Aug;43(8):1284–1290. doi: 10.1104/pp.43.8.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneyour A., Avron M. Properties of Photosynthetic Mutants Isolated from Euglena gracilis. Plant Physiol. 1975 Jan;55(1):137–141. doi: 10.1104/pp.55.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneyour A., Ben-Shaul Y., Avron M. Structural changes in Euglena gracilis grown autotrophically in the light with 3,(3,4-dichlorophenyl)-1, 1-dimethyl urea (DCMU). Exp Cell Res. 1969 Nov;58(1):1–9. doi: 10.1016/0014-4827(69)90110-4. [DOI] [PubMed] [Google Scholar]


