Abstract
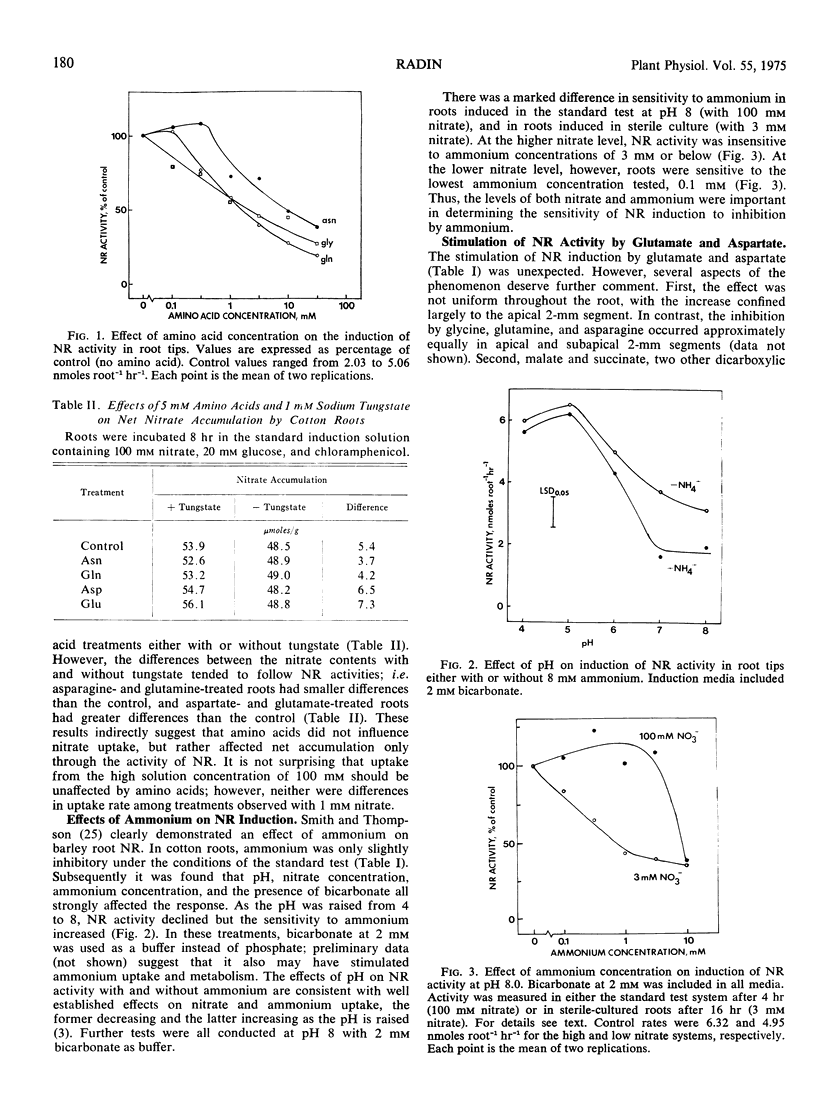
The induction of nitrate reductase activity in root tips of cotton (Gossypium hirsutum L.) was regulated by several amino acids and by ammonium. Glycine, glutamine, and asparagine strongly inhibited induction of activity by nitrate and also decreased growth of sterile-cultured roots on a nitrate medium. Methionine, serine, and alanine weakly inhibited induction, and 11 other amino acids had little or no effect. Ammonium also decreased induction in root tips, but was most effective only at pH 7 or higher. The optimum conditions for ammonium regulation of induction were identical to those for growth of sterile-cultured roots on ammonium as the sole nitrogen source. Aspartate and glutamate strongly stimulated induction, but several lines of evidence indicated that the mechanism of this response was different from that elicited by the other amino acids. The effects of amino acids on induction appeared to be independent of nitrate uptake.
In green shoot tissues, all attempts to demonstrate regulation of induction by amino acids failed. The great difference in observed responses of root and shoot to amino acids suggests that their nitrate reductase activities are regulated differently. Differential regulation of this enzyme is consistent with the responses of root and shoot nitrate reductase activity to nitrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferrari T. E., Varner J. E. Control of nitrate reductase activity in barley aleurone layers. Proc Natl Acad Sci U S A. 1970 Mar;65(3):729–736. doi: 10.1073/pnas.65.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway of cultured tobacco cells. II. Properties of a variant cell line. Biochim Biophys Acta. 1970 Jul 21;215(1):152–165. doi: 10.1016/0304-4165(70)90398-3. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Wray J. L., Filner P. The effect of tungstate on nitrate assimilation in higher plant tissues. Plant Physiol. 1969 Aug;44(8):1197–1199. doi: 10.1104/pp.44.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J. The regulation of activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem J. 1966 Sep;100(3):577–588. doi: 10.1042/bj1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Shen T. C. Nitrate reductase in Agrostemma githago. Comparison of the inductive effects of nitrate and cytokinin. Biochim Biophys Acta. 1972 Nov 24;286(1):118–125. doi: 10.1016/0304-4165(72)90097-9. [DOI] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of Organic Acids in Corn Roots II. The Cytoplasmic Pool of Malic Acid. Plant Physiol. 1966 Apr;41(4):713–717. doi: 10.1104/pp.41.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada M., Paneque A., Aparicio P. J., Vega J. M., Cárdenas J., Herrera J. Inactivation and repression by ammonium of the nitrate reducing system in chlorella. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1009–1015. doi: 10.1016/0006-291x(70)90340-2. [DOI] [PubMed] [Google Scholar]
- Radin J. W. Distribution and development of nitrate reductase activity in germinating cotton seedlings. Plant Physiol. 1974 Mar;53(3):458–463. doi: 10.1104/pp.53.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. In vivo assay of nitrate reductase in cotton leaf discs: effect of oxygen and ammonium. Plant Physiol. 1973 Feb;51(2):332–336. doi: 10.1104/pp.51.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Hageman R. H. Regulation of Nitrate Reductase Activity in Corn (Zea mays L.) Seedlings by Endogenous Metabolites. Plant Physiol. 1967 Dec;42(12):1750–1756. doi: 10.1104/pp.42.12.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


