Abstract
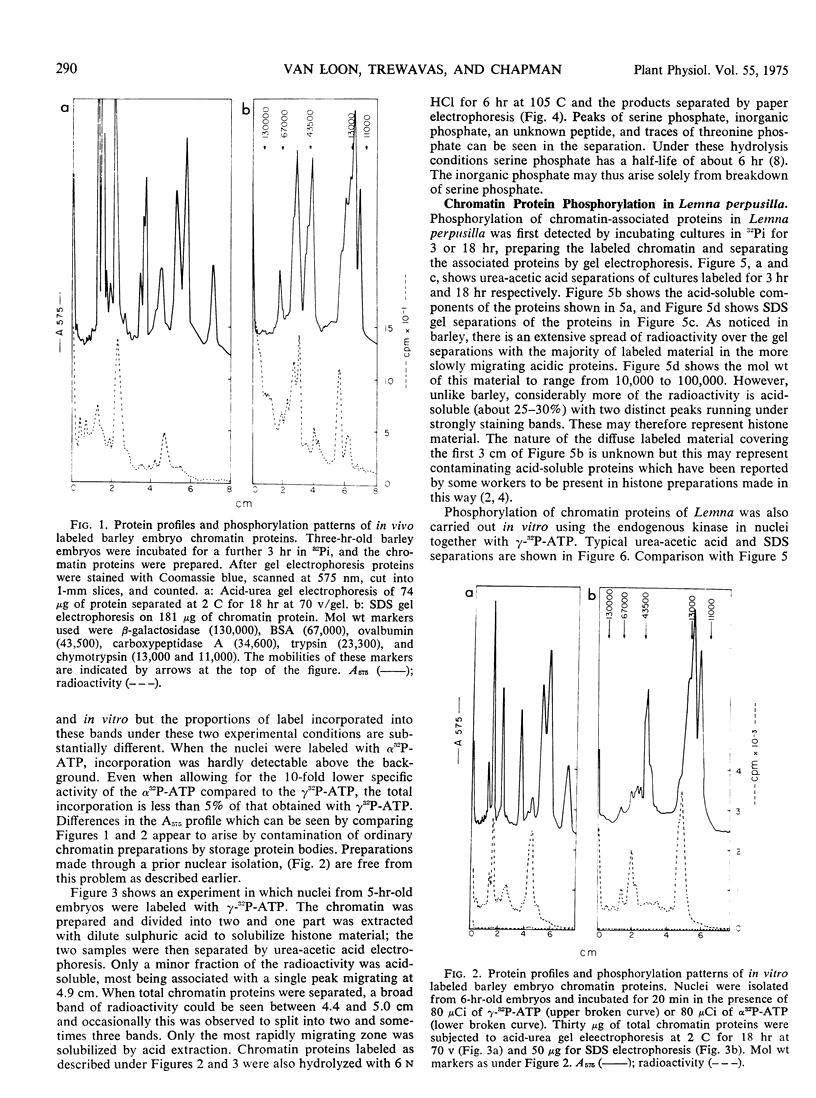
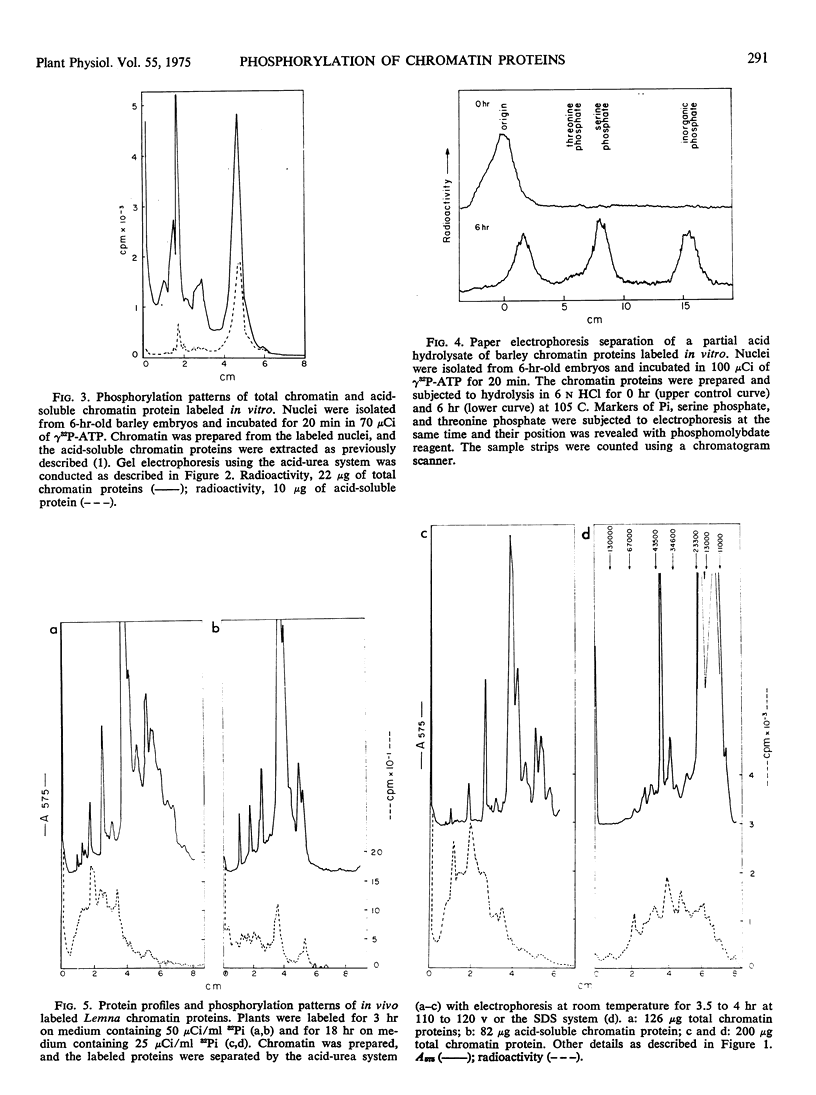
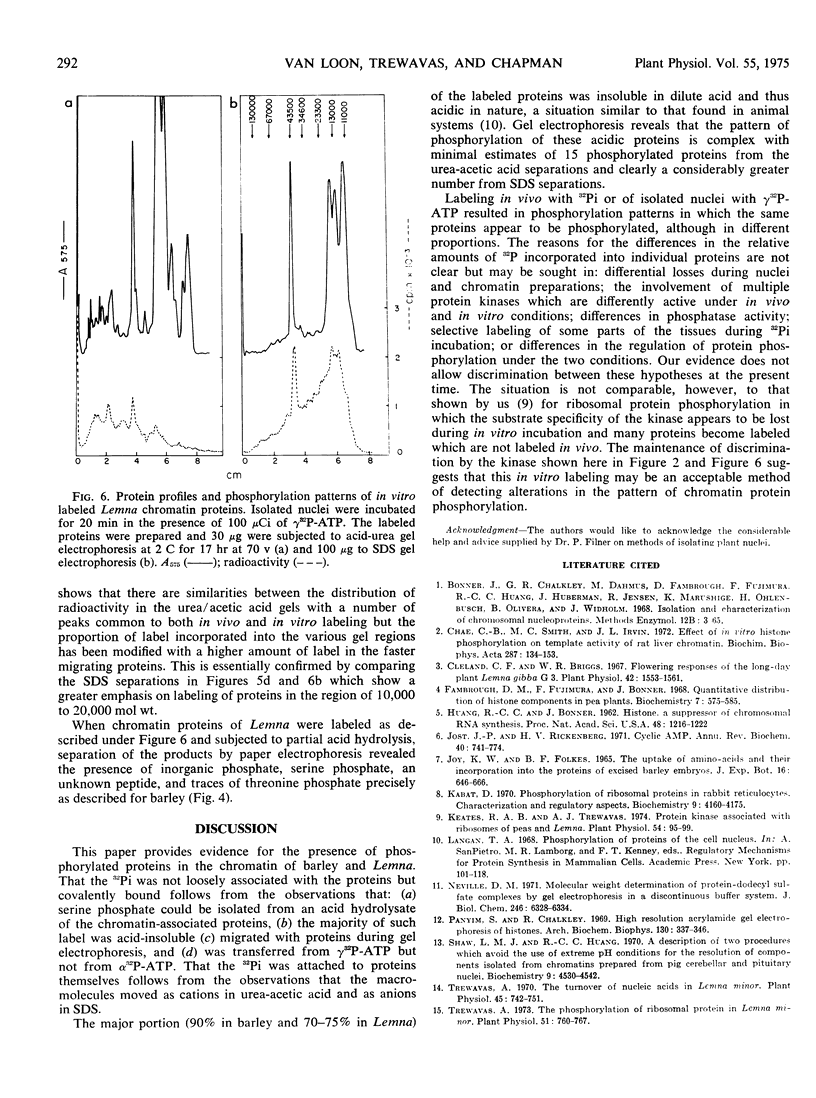
Sterile embryos of barley (Hordeum vulgare) and cultures of Lemna perpusilla have been labeled with 32Pi and the chromatin proteins prepared and separated by acid-urea and sodium dodecyl sulfate gel electrophoresis. Under these conditions chromatin proteins became labeled and the gel radioactivity profiles which were complex indicated a probable minimum of 15 to 20 proteins phosphorylated with molecular weights ranging from 104 to 105. The majority of the radioactivity, 80 to 90% of the total, is found in the acidic protein fraction and this can be recovered as serine phosphate after partial acid hydrolysis.
Nuclei have been isolated from Lemna and barley and found to possess endogenous kinase activity. In vitro labeling of these nuclei with 32P-adenosine triphosphate indicated that similar proteins appear to become labeled as in vivo labeling with 32Pi but the proportions of label in each protein were different.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae C. B., Smith M. C., Irvin J. L. Effect of in vitro histone phosphorylation on template activity of rat liver chromatin. Biochim Biophys Acta. 1972 Nov 16;287(1):134–153. doi: 10.1016/0005-2787(72)90337-1. [DOI] [PubMed] [Google Scholar]
- Cleland C. F., Briggs W. R. Flowering Responses of the Long-day Plant Lemna gibba G3. Plant Physiol. 1967 Nov;42(11):1553–1561. doi: 10.1104/pp.42.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmbrough D. M., Fujimura F., Bonner J. Quantitative distribution of histone components in the pea plant. Biochemistry. 1968 Feb;7(2):575–585. doi: 10.1021/bi00842a010. [DOI] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Trewavas A. J. Protein kinase activity associated with isolated ribosomes from peas and lemna. Plant Physiol. 1974 Jul;54(1):95–99. doi: 10.1104/pp.54.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Shaw L. M., Huang R. C. A description of two procedures which avoid the use of extreme pH conditions for the resolution of components isolated from chromatins prepared from pig cerebellar and pituitary nuclei. Biochemistry. 1970 Nov 10;9(23):4530–4542. doi: 10.1021/bi00825a012. [DOI] [PubMed] [Google Scholar]
- Trewavas A. The Phosphorylation of Ribosomal Protein in Lemna minor. Plant Physiol. 1973 Apr;51(4):760–767. doi: 10.1104/pp.51.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970 Jun;45(6):742–751. doi: 10.1104/pp.45.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]


