Abstract
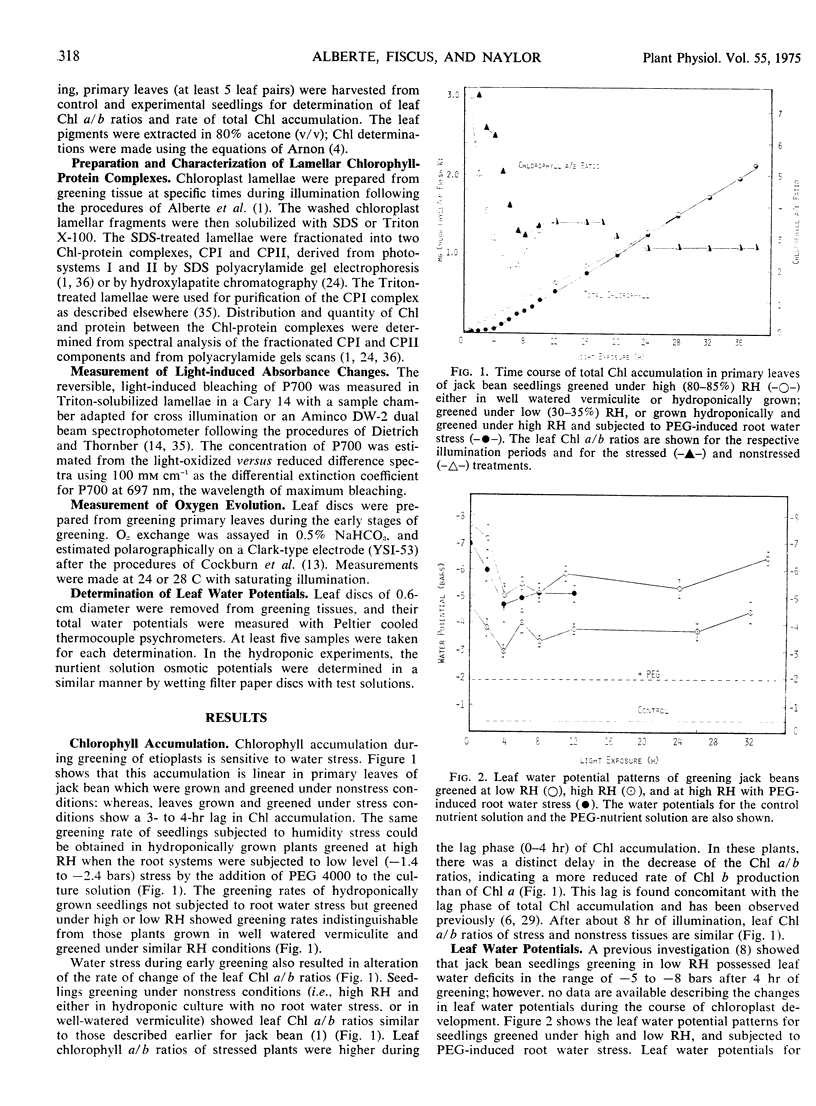
The effects of low and high relative humidity and of polyethylene glycol-induced root water stress on chlorophyll accumulation, on formation of the lamellar chlorophyll-protein complexes, and on the development of photosynthetic activity during chloroplast differentiation were examined. Low relative humidity or polyethylene glycol-induced root water stress (stress conditions) resulted in a 3 to 4 hour lag in chlorophyll accumulation, retarded the rate of chlorophyll b accumulation, and reduced the rate of formation of the light-harvesting chlorophyll a/b protein. All of these effects could be overcome by high relative humidity (nonstress) conditions. Concomitant measurement of leaf water potential showed that under stress conditions greening leaves were subjected to initial water deficits of −8 bars which decreased to −5 bars after 3 to 4 hours of illumination corresponding to the end of the lag phase. Leaves greening under nonstress conditions did not experience leaf water deficits greater than about −5 bars. It seems that the attainment of a minimum leaf water potential of −5 bars may be critical in the control of early chloroplast development. These results demonstrate that the lag phase is not indicative of a programmed event in chloroplast development, but rather is attributable to environmental conditions prevailing during leaf development and greening.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Thornber J. P., Naylor A. W. Biosynthesis of the photosystem I chlorophyll-protein complex in greening leaves of higher plants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):134–137. doi: 10.1073/pnas.70.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque D. P., Naylor A. W. Large Effects of Small Water Deficits on Chlorophyll Accumulation and Ribonucleic Acid Synthesis in Etiolated Leaves of Jack Bean (Canavalia ensiformis [L.] DC.). Plant Physiol. 1971 Apr;47(4):591–594. doi: 10.1104/pp.47.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol. 1970 Aug;46(2):236–239. doi: 10.1104/pp.46.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Nonstomatal inhibition of photosynthesis in sunflower at low leaf water potentials and high light intensities. Plant Physiol. 1971 Nov;48(5):532–536. doi: 10.1104/pp.48.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Potter J. R. Chloroplast response to low leaf water potentials: I. Role of turgor. Plant Physiol. 1973 Jun;51(6):989–992. doi: 10.1104/pp.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. E., Jr, Thornber J. P. The P700-chlorophyll -protein of a blue-green alga. Biochim Biophys Acta. 1971 Sep 7;245(2):482–493. doi: 10.1016/0005-2728(71)90164-2. [DOI] [PubMed] [Google Scholar]
- Fry K. E. Some factors affecting the Hill reaction activity in cotton chloroplasts. Plant Physiol. 1970 Apr;45(4):465–469. doi: 10.1104/pp.45.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble G. D., Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971 Oct;48(4):447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P. Photosystem I and II chlorophyll-protein complexes of higher plant chloroplasts. Biochim Biophys Acta. 1971 Nov 2;253(1):285–289. doi: 10.1016/0005-2728(71)90255-6. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Zalik S. Effect of Light Quality, Light Intensity and Temperature on Pigment Accumulation in Barley Seedlings. Plant Physiol. 1965 May;40(3):569–574. doi: 10.1104/pp.40.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E. Photophosphorylation Can Provide Sufficient Adenosine 5'-Triphosphate to Drive K Movements during Stomatal Opening. Plant Physiol. 1972 Apr;49(4):649–650. doi: 10.1104/pp.49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]


