Abstract
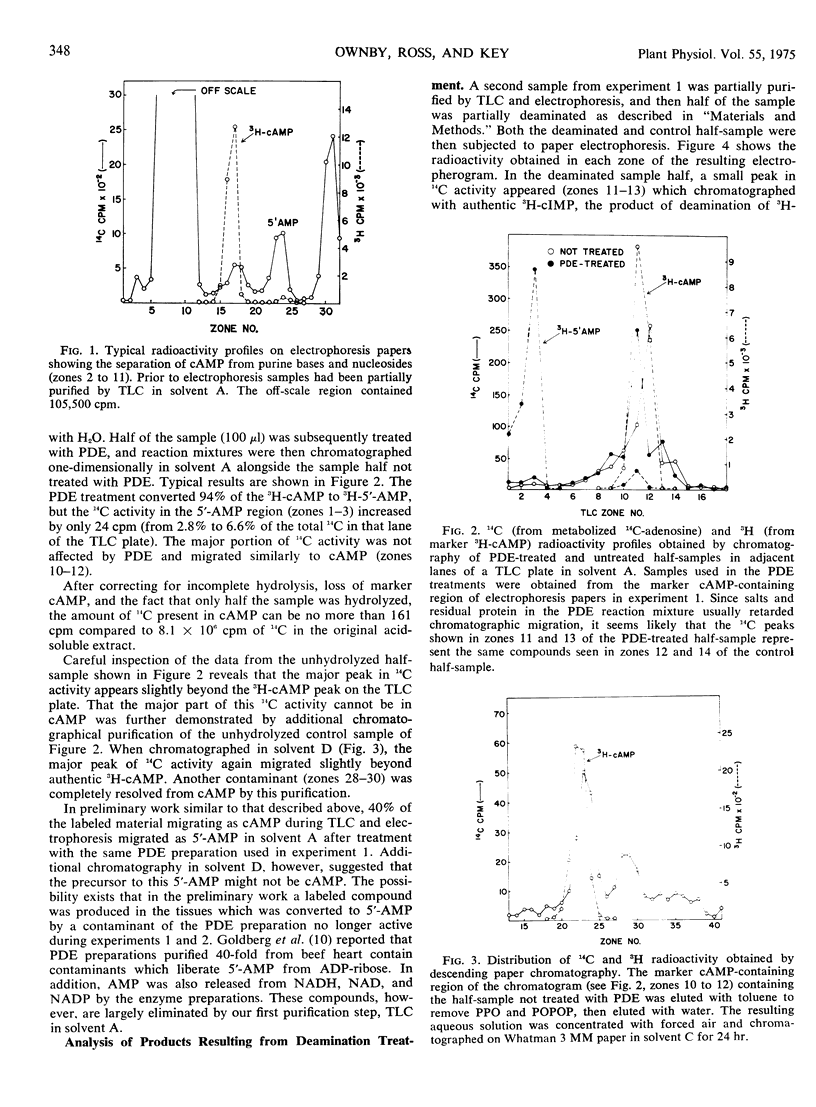
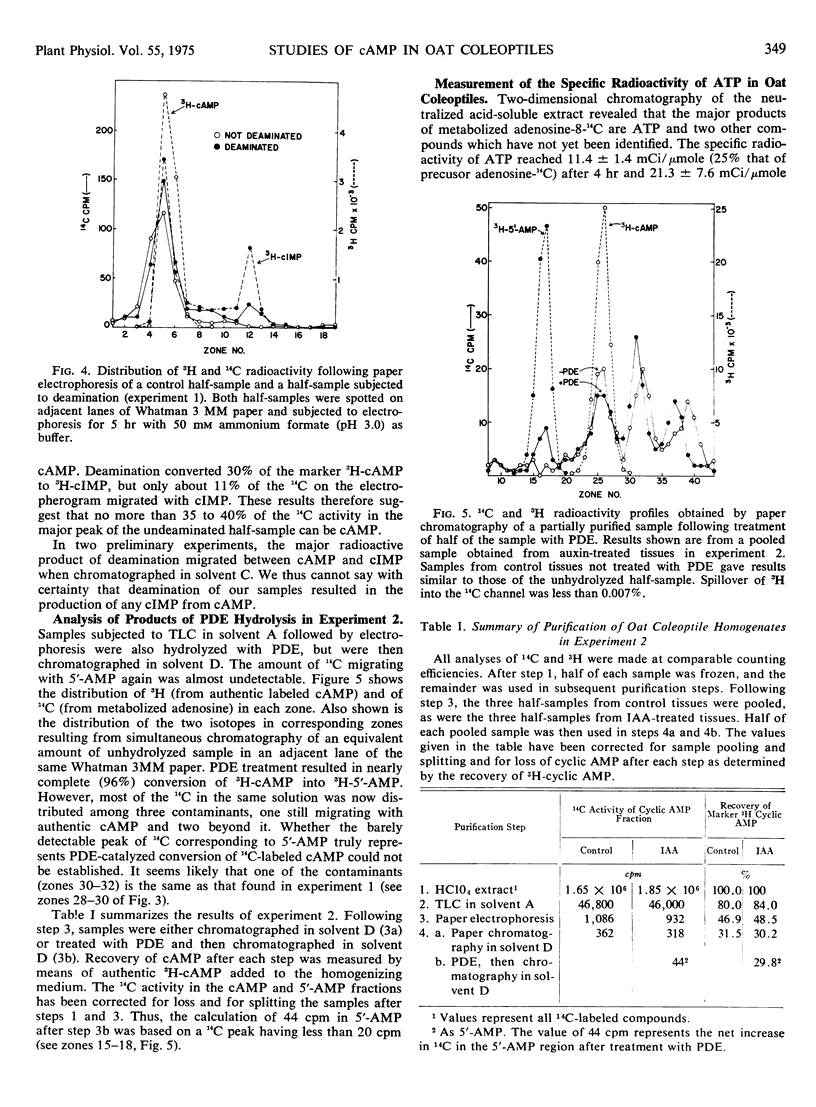
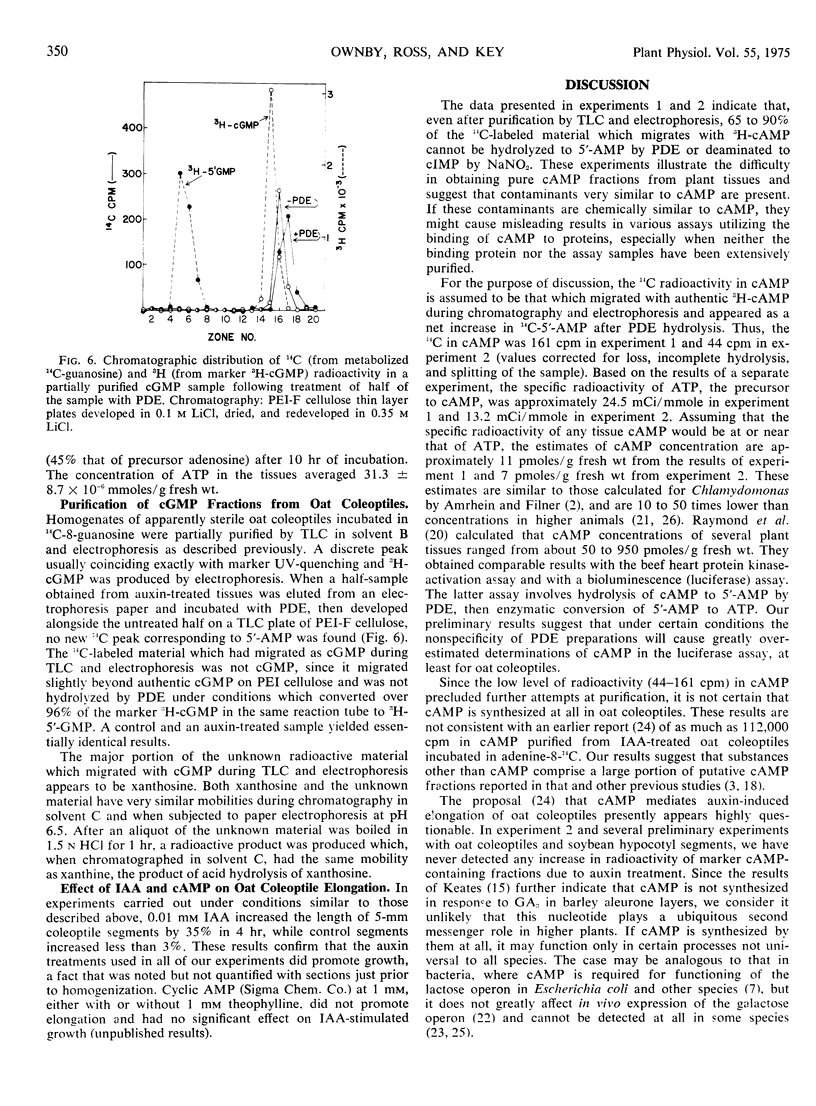
The incorporation of adenosine-8-14C into adenosine cyclic 3′:5′-monophosphate in coleoptile-first leaf segments of Avena sativa L. was investigated. Homogenates of segments incubated in adenosine-8-14C for either 4 or 10 hours were partially purified by thin layer chromatography followed by paper electrophoresis. A radioactive fraction, less than 0.06% of the 14C present in the original homogenate, migrated as adenosine cyclic 3′:5′-monophosphate during electrophoresis. Upon treatment with cyclic nucleotide phosphodiesterase, however, less than 10% of this radioactive fraction appeared as 5′-AMP. Deamination with NaNO2 as well as further chromatographical purification also suggested that only a small fraction of the 14C in the partially purified samples could be in adenosine cyclic 3′:5′-monophosphate. The data suggest that levels of this nucleotide can probably be no greater than 7 to 11 picomoles per gram of fresh weight in oat coleoptiles. Treatment of such coleoptiles with physiologically active concentrations of indoleacetic acid, furthermore, had no significant effect on the 14C radioactivity in marker adenosine cyclic 3′:5′-monophosphate-containing fractions at any stage of purification during several experiments.
In a single experiment, no labeled guanosine cyclic 3′:5′-monophosphate could be detected in oat coleoptile-first leaf segments incubated in guanosine-8-14C either with or without indoleacetic acid. These results do not support the hypothesis that a cyclic nucleotide mediates the action of indoleacetic acid on oat coleoptile extension.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez R. Formation of adenosine 5'-phosphorofluoridate and the assay of adenyl cyclase in barley seeds. Plant Physiol. 1974 Feb;53(2):144–148. doi: 10.1104/pp.53.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein N., Filner P. Adenosine 3':5'-Cyclic Monophosphate in Chlamydomonas reinhardtii: Isolation and Characterization. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1099–1103. doi: 10.1073/pnas.70.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S., Murti C. R. Effect of indole-3-acetic acid on the synthesis of cyclic 3'-5' adenosine phosphate by Bengal gram seeds. Biochem Biophys Res Commun. 1971 Apr 2;43(1):58–64. doi: 10.1016/s0006-291x(71)80085-2. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Ebadi M. S., Weiss B., Costa E. Microassay of adenosine-3',5'-monophosphate (cyclic AMP) in brain and other tissues by the luciferin-luciferase system. J Neurochem. 1971 Feb;18(2):183–192. doi: 10.1111/j.1471-4159.1971.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Dietz S. B., O'Toole A. G. Cyclic guanosine 3',5'-monophosphate in mammalian tissues and urine. J Biol Chem. 1969 Aug 25;244(16):4458–4466. [PubMed] [Google Scholar]
- Goldberg N. D., Larner J., Sasko H., O'Toole A. G. Enzymic analysis of cyclic 3', 5'-AMP in mammalian tissues and urine. Anal Biochem. 1969 Apr 4;28(1):523–544. doi: 10.1016/0003-2697(69)90208-5. [DOI] [PubMed] [Google Scholar]
- Janistyn B. Indol-3-essigsäure-induzierte Nukleotidabgabe bei gleichzeitig erhöhter adenosin-3':5'-monophosphorsäure (c-AMP)-synthese in Maiskoleoptilzylindern. Z Naturforsch B. 1972 Mar;27(3):273–276. doi: 10.1515/znb-1972-0310. [DOI] [PubMed] [Google Scholar]
- Kessler B., Levinstein R. Adenosine 3',5'-cyclic monophosphate in higher plants: assay, distribution and age-dependency. Biochim Biophys Acta. 1974 Mar 20;343(1):156–166. doi: 10.1016/0304-4165(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Pollard C. J. Influence of gibberellic acid on the incorporation of 8-14C adenine into adenosine 3',5'-cyclic phosphate in barley aleurone layers. Biochim Biophys Acta. 1970 Mar 24;201(3):511–512. doi: 10.1016/0304-4165(70)90176-5. [DOI] [PubMed] [Google Scholar]
- Pradet A., Raymond P., Narayanan A. Confirmation de la présence de l'AMP cyclique dans les semences de laitue, var. reine de mai. C R Acad Sci Hebd Seances Acad Sci D. 1972 Oct 30;275(18):1987–1988. [PubMed] [Google Scholar]
- Raymond P., Narayanan A., Pradet A. Evidence for the presence of 3', 5'-cyclic AMP in plant tissues. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1115–1121. doi: 10.1016/0006-291x(73)90580-9. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Hesse J. E., Epstein W. Role of cyclic adenosine 3',5'-monophosphate in the in vivo expression of the galactose operon of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1040–1044. doi: 10.1128/jb.114.3.1040-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., Durr I. F. Evidence against the presence of 3',5'-cyclic adenosine monophosphate and relevant enzymes in Lactobacillus plantarum. J Bacteriol. 1972 Oct;112(1):421–426. doi: 10.1128/jb.112.1.421-426.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D., Mascarenhas J. P. Auxin-induced synthesis of cyclic 3', 5'-adenosine monophosphate in Avena coleoptiles. Life Sci II. 1971 Aug;10(15):879–885. doi: 10.1016/0024-3205(71)90200-1. [DOI] [PubMed] [Google Scholar]
- Setlow P. Inability of detect cyclic AMP in vegetative or sporulating cells or dormant spores of Bacillus megaterium. Biochem Biophys Res Commun. 1973 May 15;52(2):365–372. doi: 10.1016/0006-291x(73)90720-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Ashby J. P., Wellburn F. A. Occurrence and biosynthesis of adenosine 3',5'-cyclic monophosphate in isolated Avena etioplasts. Biochim Biophys Acta. 1973 Sep 14;320(2):363–371. doi: 10.1016/0304-4165(73)90317-6. [DOI] [PubMed] [Google Scholar]


