Abstract
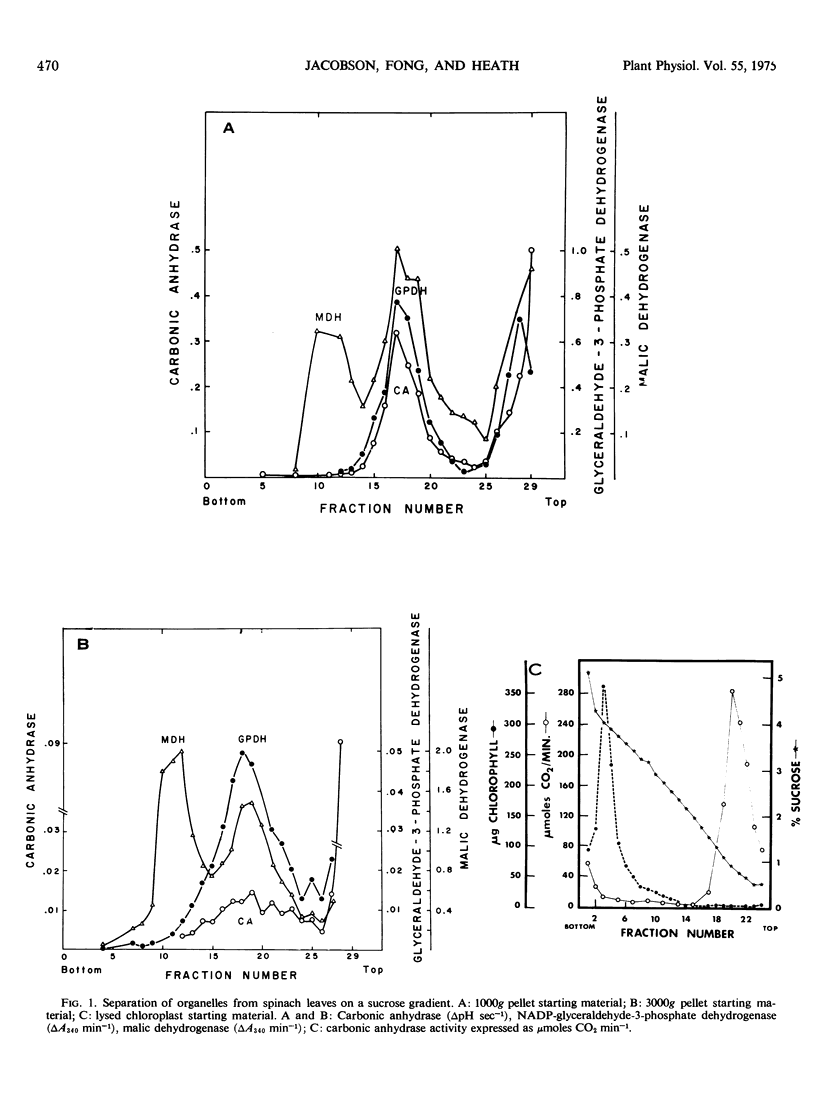
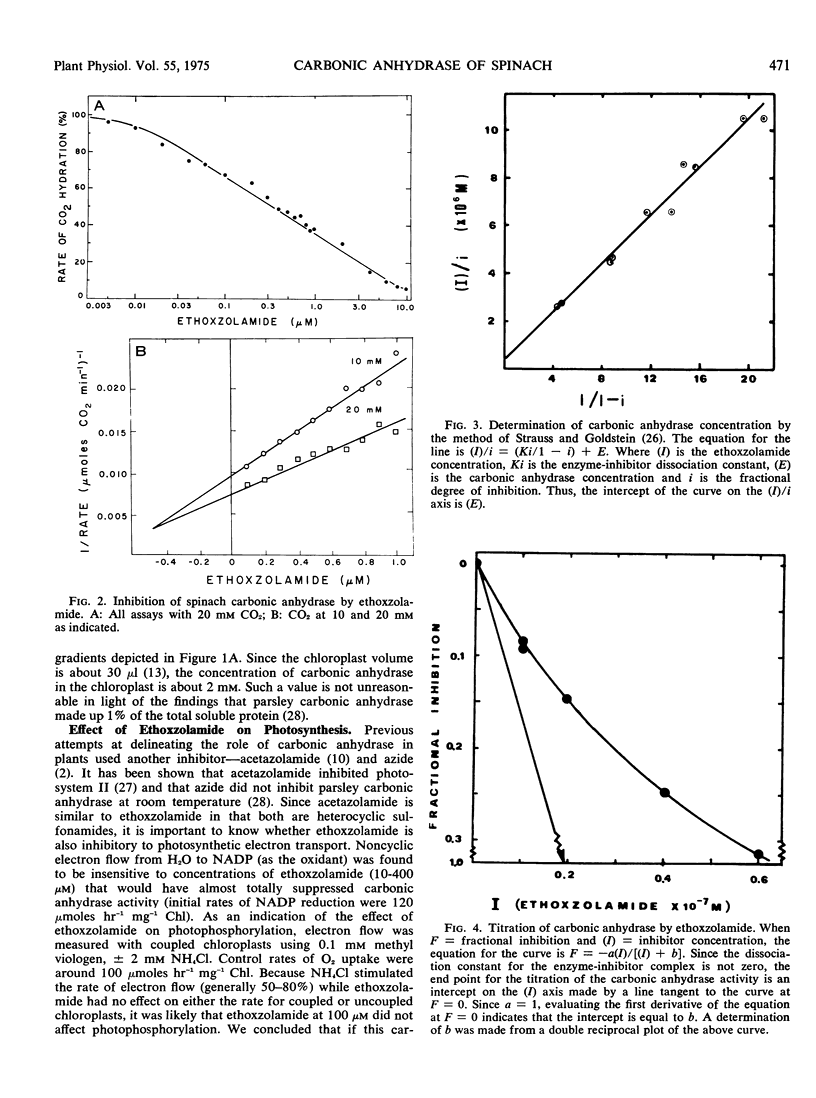
Carbonic anhydrase activity was determined in spinach (Spinacia oleracea) leaf organelles isolated on sucrose density gradients and was found to be predominantly in the intact chloroplast fraction. The small amount of activity associated with the mitochondrial fractions was probably due to intact chloroplast contamination. No activity could be associated with the broken chloroplast or microbody fractions. Based upon inhibitor studies, carbonic anhydrase was found to be around 2 mm in the chloroplast. Ethoxzolamide, an inhibitor of carbonic anhydrase, reduced CO2 fixation in intact chloroplasts. The concentration required to inhibit CO2 fixation 20 to 40% was in excess of that required to inhibit the purified enzyme. The inhibition was partially reversed by CO2. Ethoxzolamide had no effect on photosynthetic NADP reduction or photophosphorylation measured by methyl viologen reduction. The physiological role of carbonic anhydrase was shown not to be associated with CO2 diffusion or CO2 concentration. It is proposed that other functions of carbonic anhydrase could be the protection against denaturation by transient localized changes in pH or the hydration of compounds other than CO2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun G., Selegny E., Minh C. T., Thomas D. Facilitated transport of CO(2) across a membrane bearing carbonic anhydrase. FEBS Lett. 1970 Apr 16;7(3):223–226. doi: 10.1016/0014-5793(70)80166-1. [DOI] [PubMed] [Google Scholar]
- Enns T. Facilitation by carbonic anhydrase of carbon dioxide transport. Science. 1967 Jan 6;155(3758):44–47. doi: 10.1126/science.155.3758.44. [DOI] [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Kannangara C. G., Stumpf P. K. Biosynthesis of -linolenic acid by disrupted spinach chloroplasts. Biochem Biophys Res Commun. 1973 Mar 17;51(2):487–493. doi: 10.1016/0006-291x(73)91283-7. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Stumpf P. K. Fat metabolism in higher plants. LV. Acetate uptake and accumulation by class I and II chloroplasts from Spinacia oleracea. Arch Biochem Biophys. 1972 Dec;153(2):656–663. doi: 10.1016/0003-9861(72)90384-0. [DOI] [PubMed] [Google Scholar]
- KARLER R., WOODBURY D. M. Intracellular distribution of carbonic anhydrase. Biochem J. 1960 Jun;75:538–543. doi: 10.1042/bj0750538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Meany J. E. The catalytic versatility of erythrocyte carbonic anhydrase. II. Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry. 1967 Jan;6(1):239–246. doi: 10.1021/bi00853a037. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Ng S. Y. Plant carbonic anhydrase. Properties and carbon dioxide hydration kinetics. Biochemistry. 1973 Dec 4;12(25):5127–5134. doi: 10.1021/bi00749a016. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Intracellular distribution of carbonic anhydrase in spinach leaves. Biochim Biophys Acta. 1972 Feb 28;258(2):637–642. doi: 10.1016/0005-2744(72)90255-0. [DOI] [PubMed] [Google Scholar]
- Randall P. J., Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973 Sep;52(3):229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. J., Yates D. W., Pogson C. I. Dihydroxyacetone phosphate. Its structure and reactivity with -glycerophosphate dehydrogenase, aldolase and triose phosphate isomerase and some possible metabolic implications. Biochem J. 1971 Apr;122(3):285–297. doi: 10.1042/bj1220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- Tobin A. J. Carbonic anhydrase from parsley leaves. J Biol Chem. 1970 May 25;245(10):2656–2666. [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


