Abstract
Background:
This meta-analysis aimed to provide critically estimated evidence for the advantages and disadvantages of Chinese herbal medicines used for premature ovarian failure (POF), which could provide suggestions for rational treatments.
Materials and Methods:
The databases searched included MEDLINE, EMBASE, CNKI, VIP, China Dissertation Database, China Important Conference Papers Database, and online clinical trial registry websites. Published and unpublished randomized controlled trials of traditional Chinese medicine (TCM) combined with hormone therapy (HT) and HT alone for POF were assessed up to December 30, 2015. Two authors extracted data and assessed trial quality independently using Cochrane systematic review methods. Meta-analysis was used to quantitatively describe serum hormone levels and Kupperman scores associated with perimenopause symptoms.
Results:
Seventeen randomized controlled trials involving 1352 participants were selected. Compared with HT alone, although no significant effects were observed in the levels of luteinizing hormone, therapy with TCM combined with HT compared to HT alone effectively altered serum hormone levels of follicle stimulating hormone (P<0.01) and estradiol (P < 0.01), and improved Kupperman index scores (P< 0.01).
Conclusions:
The reported favorable effects of TCM combined with HT for treating POF patients are better than HT alone.However, the beneficial effects derived from this combination therapy cannot be viewed conclusive. In order to better support the clinical use, more rigorously designed trials are required to provide.
Keywords: Traditional Chinese medicine, Hormone therapy, Premature ovarian failure, Meta-analysis
Introduction
Premature ovarian failure (POF) is characterized by the cessation of the menstrual cycle, sustained amenorrhea, atrophy of sex organs, and accompanied by increased levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) and decreased levels of estrogen (E2) before the age of 40 years in women whose secondary sexual characteristics have developed normally and regular menstruation had been established (Kalantaridou et al., 1998).
There is currently no definitive and effective therapy for treating POF. Symptomatic treatment is primarily used, such as hormone therapy (HT), ovulation induction, immunosuppressive therapy, and oocyte donation (Altuntas et al., 2006). Estrogen-progestin replacement therapy can rapidly improve premenopausal symptoms that are caused by low estrogen status. Additionally, estrogen therapy (ET) can achieve short-term therapeutic effects by promoting the development of the uterus, hypertrophy and hyperplasia in uterine smooth muscle cells, the uterine muscular layer thickness, thickness of the endometrium, and increasing the uterine blood flow. Thus, it can effectively prevent uterine atrophy; however, it cannot improve ovarian function or promote follicular development and ovulation rates. Therefore, long-term ET is required due to a failure to restore normal ovarian endocrine function. Surprisingly, these long-term ET have also increased the risk of endometrial cancer and breast cancer, especially a higher occurrence of breast cancer (Woad et a1., 2013). Another study showed that HT for < 4 years incurred a relatively low risk, whereas this risk may increase with therapy of over 4 years. Thus, individual assessments must be done at least once each year to decide whether to continue this treatment (Van and Schoemaker, 1999). As a result, this strategy results in a low clinical compliance.
Traditional Chinese medicine (TCM) have been used for thousands of years, and are still commonly used to treat people who suffer from different diseases in Southeast Asian countries. According to the TCM theory, there is a unique herbal formulation for each syndrome or symptom, thus, formulations or prescriptions must be adapted by adding or subtracting herbs. For complex diseases in China, the diagnostic paradigms of Western medicine are often used for herbal medicines without applying TCM theory (Poll, 2005). An essential feature of integrated traditional Chinese and Western medicine for treating diseases involves fasting cures, changes in dietary habits, and so on (Dobos and Tao,2011). However, the levels of evidence are lower (Rakel, 2012).
The current literature indicates that herbal medicines are being used for POF in China. To increase the therapeutic efficacy, shorten recovery time, and reduce any side effects, combination treatments with Western medicines plus herbal medicines are selected to treat POF (Chao et al., 2003). Current research suggests that a combination of a TCM with HT, if used correctly, can not only shorten the treatment time, improve efficacy, and reduce the side effects of long-term use of hormones, but can also promote the recovery of ovarian function and menstruation.
As described above, this method is safe, economic, effective, and worthy of clinical application. However, the related clinical research studies on these combination therapies were done independently and only involved small sample sizes. Therefore, in this study, we conducted a meta-analysis for the purpose of providing rational treatment evidence for POF based on a TCM combined with HT.
A PRISMA checklist is available as supporting information for study selection (Figure 1).
Figure 1.

Flow diagram of activity
Materials and Methods
Search Methods and Study Selection
The medical literature up to December 30, 2015 was searched in the following databases: MEDLINE, EMBASE, Chinese National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), China Dissertation Database, China Important Conference Papers Database, and online clinical trial registry websites and the Cochrane library. The search terms used included “premature ovarian failure,” “traditional Chinese herbal medicine,” “hormone therapy,” “clinical trial,” and “randomized controlled trials.”
Two authors independently performed literature searches, study selection, and data extraction. Data extracted from selected papers included authors, paper title, publication year, study size, age of subjects, information on methodology used, name of a Chinese herb, course of treatment, treatment measures for a control group, and outcomes and adverse events. Any disagreement was resolved by a discussion and reached a consensus with the third party (JXC).
Inclusion and Exclusion Criteria
Studies were included if they met all the following criteria:(i) The purpose of article is to study on clinical effect of TCM combined with HT compared with HT alone for POF patients;(ii) The primary efficacy variable including serum hormone and some clinical symptoms such as hectic fever, night sweats, emotional depression, vaginal dryness and decreased libido; and (iii) Included studies must be clearly record the mean and standard deviation which related to studies. The studies with non-RCTs were excluded; multiple journals that reported on the same groups of subjects were also excluded. Literature searches were conducted for two languages: English and Chinese.
Trial Quality Assessment
Two authors (MJK, YL) assessed the quality of all the included studies. The quality of the included RCTs qualities was assessed based on Jadad scores(Jadad and Moore.1996). The methods used were: randomization (appropriate: 2 points; unclear: 1 point; inappropriate: 0 points); concealment of allocation (appropriate: 2 points; unclear: 1 point; inappropriate: 0 points); double blinding (double blinding and description: 2 points; double blinding and description was unclear: 1 point; no blinding or no description: 0 points); and withdrawals and dropouts (description: 1 point; no description: 0 points). The scores ranged from 1 to 7 points, with scores of 1-3 categorized as poor-quality studies and 4-7 as high-quality studies.
Data Analysis
Data were analyzed using RevMan 5.0 software. Data were summarized using the standardized mean difference (SMD) along with the corresponding 95% CI for continuous outcomes. Meta-analysis was used if trials had a good homogeneity with regard to study design, control, participants, interventions, and outcome measures; this was evaluated by determining the I2 value. The quality component of the methodology was used, including generation of an allocation sequence, double blinding, and concealment of allocation. For publication bias, funnel plot analysis was explored.
Results
Included Studies
Figure 1 is a flowchart for our search procedures and trial selection. After a thorough literature search using six databases, a total of 364 publications were identified, 289 of which were excluded after screening their titles and abstracts. A total of 75 full-text papers were retrieved, and finally, a total of 17 studies were included in our meta-analysis (Feng and Li, 2013; Gao and Bi,2008; Han et al.,2008;Jin et al.,2013; Li and Peng, 2009; Liu et al., 2009; Li, 2009; Lu, 2009; Qi et al., 2008; Qin,2006; Yi et al., 2008; Zhang, 2008; Zhu and Xie, 2012; Yang and Shen, 2015; Liu,2015; Lu,2014; Jin, 2015). The details for these included studies are listed in Table 1.
Table 1.
Study characteristics of the included randomized controlled trials
| Time (months) | N (TCHM/HT) | Hormones/Drugs | Traditional Chinese medicines | |
|---|---|---|---|---|
| Feng and Li 2013 | 5 | 33/32 | estradiol valerate, dydrogesterone | Peikun pills |
| Gao and B i 2008 | 3 | 32/31 | conjugated estrogen, medroxyprogesterone acetate | Ziyinshugan decoction |
| Han et al. 2008 | 3 | 58/58 | Diethylstilbestrol, medroxyprogesterone acetate | kangluanshuai granules |
| Jin et al. 2013 | 6 | 91/88 | conjugated estrogen, medroxyprogesterone acetate | Bushenhuoxue decoction |
| Li and Peng 2009 | 3 | 36/32 | estradiol valerate, medroxyprogesterone acetate | Yijingdecoction |
| Liu et al. 2009 | 3 | 24/22 | estradiol valerate, estradiol cyproterone | Yishenkangshuai decoction |
| Li and Wu 2009 | 3 | 18/18 | estradiol valerate, medroxyprogesterone acetate | Yishen decoction |
| Lu 2009 | 3 | 37/30 | conjugated estrogen, medroxyprogesterone acetate | Modified erxian decoction |
| Qi et al. 2008 | 3 | 26/26 | estradiol valerate, medroxyprogesterone acetate | Yishenfuchao decoction |
| Qin 2006 | 3 | 35/37 | conjugated estrogen, medroxyprogesterone acetate | Yishen decoction |
| Yi et al. 2008 | 3 | 21/20 | conjugated estrogen, medroxyprogesterone acetate | Tiaojingkangshuai decoction |
| Zhang 2008 | 6 | 30/28 | conjugated estrogen, medroxyprogesterone acetate | Qingrehuoxue decoction |
| Zhu and Xie 2012 | 3 | 21/21 | estradiol valerate, estradiol cyproterone | Bushentiaochong decoction |
| Yang and Shen 2015 | 3 | 92/95 | estradiol valerate, medroxyprogesterone acetate | Bushentiaochong decoction |
| Liu2015 | 6 | 30/30 | Estradiol and progesterone tablets | Kuntai decoction |
| Lu2014 | 3 | 30/30 | estradiol valerate, medroxyprogesterone acetate | Bushenyangxue decoction |
| Jin2015 | 3 | 70/70 | estradiol valerate, medroxyprogesterone acetate | Gushentiaochong decoction |
All included studies were evaluated using a graded scale, and the methodological quality of most included papers was assessed as poor. Randomized allocation of individuals was described in all studies; however, only six trials described the methods used for sequence generation, including a random number table (Gao and Bi, 2008;Jin et al.,2013; Liu et al.,2009; Zhang,2008; Zhu and Xie,2012; Yang and Shen,2015). Because of insufficient information provided for randomization methods, we could not accurately judge whether or not studies were conducted properly. Both allocation concealment and double-blinding were not used for all trials. Only three trials reported follow-ups, drop-outs, or withdrawals (Gao and Bi, 2008; Li and Peng, 2009; Yi et al., 2008).
General Characteristics of the Included Trials
Six of the 13 trials used medroxyprogesterone acetate (MPA) and conjugated equine estrogens (CEE) for hormone control therapy, three used MPA and estradiol valerate (EV), and one used MPA and diethylstilbestrol (DES); three used EV and ethinyl estradiol-cyproterone acetate (EE-CA). The general characteristics of the included studies are shown in Table 1 (Sackett et al.,1996; Sackett and Wennberg,1997; Rakel D, 2012; Chao et al., 2003; Jadad and Moore. 1996; Feng and Li, 2013; Gao and Bi, 2008; Han et al., 2008;Jin et al., 2013; Li and Peng, 2009; Liu et al., 2009; Li, 2009; Lu, 2009; Qi et al., 2008; Qin,2006; Yi et al., 2008; Zhang, 2008; Zhu and Xie, 2012; Yang and Shen, 2015; Liu, 2015; Lu, 2014; Jin, 2015).
Methodological Quality of the Included Trials
Eight of the 17 RCTs did not describe the randomization method used, and six used a random number table, although random allocation was unconcealed and it was not specified whether a blinding method was used. Three RCTs described follow-ups and drop-outs. Overall, 14 RCTs were assessed as being of poor quality. The results of the quality assessments for the included studies are shown in Table 2 (Feng and Li, 2013; Gao and Bi,2008; Han et al.,2008;Jin et al.,2013; Li and Peng,2009; Liu et al.,2009; Li,2009; Lu,2009; Qi et al., 2008; Qin,2006; Yi et al.,2008;Zhang,2008; Zhu and Xie,2012; Yang and Shen,2015; Liu,2015; Lu,2014; Jin,2015).
Table 2.
Quality assessments of the included randomized controlled trials
| Study ID | Sequence generation | Allocation concealment | Blinding | Attrition and Dropout | score |
|---|---|---|---|---|---|
| Feng and Li 2013 | Unclear | Unclear | No | Yes | 1 |
| Gao and Bi 2008 | Appropriate | Unclear | No | No | 2 |
| Han et al. 2008 | Unclear | Unclear | No | No | 1 |
| Jin et al. 2013 | Appropriate | Unclear | No | Yes | 3 |
| Li and Peng 2009 | Unclear | Unclear | No | No | 1 |
| Liu et al. 2009 | Unclear | Unclear | No | No | 1 |
| Li and Wu 2009 | Unclear | Unclear | No | No | 1 |
| Lu 2009 | Unclear | Unclear | No | No | 1 |
| Qi et al. 2008 | Unclear | Unclear | No | No | 1 |
| Qin 2006 | Appropriate | Unclear | No | No | 2 |
| Yi et al. 2008 | Appropriate | Unclear | No | Yes | 3 |
| Zhang 2008 | Appropriate | Unclear | No | No | 2 |
| hu and Xie 2012 | Unclear | Unclear | No | No | 1 |
| Yang and Shen 2015 | Appropriate | Unclear | No | No | 2 |
| Liu2015 | Unclear | Unclear | No | No | 1 |
| Lu2014 | Unclear | Unclear | No | No | 1 |
| Jin2015 | Unclear | Unclear | No | No | 1 |
Publication Bias
Funnel plots analysis of the 13 trials on FSH, LH, E2 were conducted to investigate the publication bias. It demonstrated symmetry, suggesting no potential publication bias (Figure 2).

Effect Estimates
Comparisons of Serum Hormones Levels (FSH, LH, and E2)
FSH: All 17 studies included comparisons of the effects on serum FSH levels in patients with POF after treatment with a TCM combined with HT and HT alone. Our meta-analysis results are shown in Figure 3 (Feng and Li, 2013; Gao and Bi,2008; Han et al.2008;Jin et al.,2013; Li and Peng,2009; Liu et al.,2009; Li, 2009; Lu,2009; Qi et al.,2008; Qin,2006; Yi et al.2008;Zhang,2008;Zhu and Xie,2012; Yang and Shen,2015; Liu,2015; Lu,2014; Jin,2015). Treatment with a TCM combined with HT was significantly superior compared with that of HT alone for reducing FSH levels (SMD: -7.08, 95% CI: -9.80,-4.37, P<0.00001).
Figure 3.

Comparisons of treatment efficacy between traditional Chinese medicine combined with hormone therapy and hormone therapy alone on FSH levels.
LH: Fifteen trials compared the effects on serum LH levels in patients with POF after treatment with a TCM combined with HT and HT alone (Gao and Bi,2008;Han et al.,2008;Jin et al.,2013; Li and Peng,2009; Liu et al.,2009; Li, 2009; Lu,2009; Qi et al., 2008; Qin,2006; Yi et al.,2008;Zhang,2008; Yang and Shen,2015; Liu,2015; Lu,2014; Jin,2015). However, our meta-analysis results showed that there was no significance in reducing LH levels between treatment with a TCM combined with HT and HT alone (Figure 4).
Figure 4.
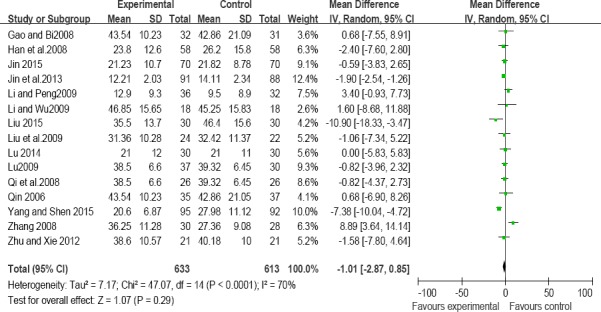
Comparisons of treatment efficacy between traditional Chinese medicine combined with hormone therapy and hormone therapy alone on LH levels.
E2: All 17 trials included comparisons of the effects on serum E2 levels in patients with POF after using treatment with a TCM combined with HT and HT alone(Feng and Li, 2013; Gao and Bi,2008; Han et al.,2008;Jin et al.,2013; Li and Peng,2009; Liu et al.,2009; Li, 2009; Lu,2009; Qi et al.,2008; Qin,2006; Yi et al.,2008;Zhang,2008; Zhu and Xie,2012; Yang and Shen,2015; Liu,2015; Lu,2014; Jin,2015). The meta-analysis results are shown in Figure 5 (Feng and Li, 2013; Gao and Bi,2008; Han et al.,2008;Jin et al.,2013; Li and Peng,2009; Liu et al.,2009; Li, 2009; Lu,2009; Qi et al.,2008; Qin,2006; Yi et al.,2008;Zhang,2008; Zhu and Xie,2012; Yang and Shen,2015; Liu,2015; Lu,2014; Jin,2015). Treatment with a TCM combined with HT was significantly superior compared with that with HT alone for increasing E2 levels (SMD: 3.45, 95% CI: 2.11, 4.79, P < 0.00001).
Figure 5.

Comparisons of treatment efficacy between traditional Chinese medicine combined with hormone therapy and hormone therapy alone on E2 levels.
Perimenopausal Syndrome and Symptom Scores
Three trials compared Kupperman index scores associated with perimenopausal syndrome and symptoms after treatment with a TCM combined with HT and HT alone (Feng and Li, 2013; Liu et al., 2009; Yi et al., 2008). Heterogeneity test results showed that there was a significant difference between these two treatments (I2=63%, P=0.07); therefore, the effective data were combined in a random effects model. The meta-analysis results are shown in Figure 6. The total efficacy showed that therapies with a TCM combined with HT were better than those with HT alone for improvements in perimenopausal syndrome and symptoms (-1.19, 95% CI: -1.77, -0.61, p<0.0001).
Figure 6.

Comparisons of treatment efficacy between traditional Chinese medicine combined with hormone therapy and hormone therapy alone on Kupperman scores.
Discussion
POF adversely affects women’s reproductive capacity and the physical health of offspring. POF recently became the focus of research(Multidisciplinary Working Group convened by the British Fertility Society,2003; Hudson,2010; Jolande et al.,2008), due to the possibility of preserving the fertility of young women who suffered from cancer during childhood, and reproductive technologies in this area are rapidly developing(Wallace et al.,2005). This could have a profound effect on those patients for whom premature menopause is suspected (Wallace, 2005).
Until recently, the most commonly used therapies in Western medicine were difficult to be accepted by patients because of the significant side effects and the uncertain efficacy with their long-term use (Liu and Ouyang, 2010). In fact, there are several different treatment goals for women with POF, and not all of these apply to all women. The first is fertility for women with POF who desire to become pregnant; in this case, “treatment” generally refers to some means to restore ovulatory function and allow for conception. For women who do not desire additional childbearing, treatment usually refers to therapies that address a second issue: managing their symptoms of estrogen depletion, such as vasomotor symptoms (e.g., hot flashes, sweating, and facial flushing), reduced libido, decreased vaginal lubrication, and others. A third issue that applies to all women with POF, but which may receive the least amount of attention, is the increased risk of conditions like osteopenia/bone fracture and cardiovascular disease(O’Donnell, et al., 2012). Consequently, it is necessary to offer rational strategies for clinical therapies by using alternative methods.
In TCM theories, although there is no designation “premature ovarian failure,” this can be classified into hypomenorrhea, delayed menorrhea, amenorrhea, infertility, early menopause, and others. Dialectical thought and TCM approaches indicate that a Chinese herbal medicine can not only maintain homeostasis but also improve the circulation and microcirculation in the body to further regulate neuroendocrine function and improve hormone levels based on Chinese dialectical therapy (Yang and Han, 2003). Therefore, this therapeutic regime is superior to Western medicine in regulating the neuroendocrine system.
However, numerous TCM physicians treated diseases based on their experiences; they paid less attention to rigorous design clinical researches. Consequently, results in the majority of meta-analysis could not provide for conclusive evidence as well as this review. There were several reasons for these.
Firstly, all studies mentioned that they used randomized allocation; however, only six trials described the methods for random sequence generation by using random number tables (Jin et al., 2013; Liu et al., 2009; Yi et al., 2008; Zhang, 2008; Zhu and Xie, 2012; Yang and Shen, 2015). Only four RCTs described their randomization procedures, although most of these provided only incomplete information for which it was difficult to judge whether or not randomization procedures were carried out properly comparing with a CONSORT checklist for RCTs. In the other nine trials, “the subjects were randomly distributed into two groups,” but without providing any details for how randomization was accomplished. Six of the 17 RCTs used random number tables; however, the random allocation was unconcealed, and it was not mentioned whether or not a blinding method was used. We can only speculate that it was very difficult to use a blinding method, because of some the characteristics of Chinese medicines, such as aroma, color, and appearance, make it easy for patients and doctors to determine which kinds of Chinese herbal medicine they were allocated. Only three trials reported on follow-ups, drop-outs, or withdrawals. Consequently, insufficient information was provided to judge whether or not these trials were carried out properly. If the methodological approaches were not conducted rigorously, it may result in distinct differences between the study and control groups compared with the results of the trials that were performed rigorously (Schulz et al., 1995; Moher et al., 2010; Kjaergard et al., 2001; Chand et al., 2010; Beck and Persani, 2006; Chen and Hu, 2006). This means that the investigators could not appropriately evaluate any therapeutic efficacy. Poor methodology might result in biased or invalid results.
Secondly, syndrome differentiation was applied in the majority of the trials cited. The features and advantage of TCM is using a therapy that is based on an individual’s syndrome pattern. In clinical practice with TCM, an herbal prescription must be well-matched with the symptom type; i.e., “Bianzhenglunzhi,” which is a specific diagnostic approach used in TCM. This method is also viewed as a therapy based on an individual’s characteristics, and is considered to be one of the advantages of Chinese medicine. However, surprisingly, only two trials provided detailed information on patients’ syndrome differentiation. Almost all practitioners of Chinese medicine believe that ineffective treatments may result from failing to perform syndrome differentiation. Ultimately, they cannot achieve the desired therapeutic effect or may worsen the disease condition. Thus, authors should be encouraged to explain individual syndrome differentiation (Bianzhenglunzhi) using common medical terms so that it is very easily understood or accepted by physicians and readers. In addition, unfortunately, because of a lack of placebo controls, the explanation of meaningful results using TCM combined with HT therapy requires more rigorous evaluations.
Thirdly, there was significant heterogeneity in the combined therapies and control interventions between the trials cited, which was the major obstacle to estimate their general effects. Because the sources of included studies, the characteristics of subjects, the study sites, study designs, diagnostic criteria, interventions, controls, and outcome evaluations were different, there was considerable variation among these studies. It also made it difficult to explore specific factors by using subgroup analyses, and this could certainly influence how to judge the effects of these treatments.
Finally, clinical efficacy was evaluated using four outcome measures, including FSH, LH, and E2 levels and Kupperman scores for premenopausal symptoms. These outcome measures were selected because they had good homogeneity, a small publication bias, and greater credibility. The patients were diagnosed based on elevated serum FSH levels, which is one criterion to diagnose POF, i.e., serum FSH levels > 40 IU/L and amenorrhea >6 months (Chand et al., 2010). Furthermore, POF is associated with decreased E2 levels and elevated LH levels (Beck and Persani,2006)
Kupperman index scores are the most commonly used for evaluating menopausal syndrome. In this paper, the results of our meta-analysis showed that therapies with combined traditional Chinese and Western medicine were better than those with HRT for modulating FSH and E2 levels and for improvements in premenopausal syndrome and symptoms, although the LH levels showed that there were no significant differences between these two groups. These findings provide preliminary support for the use of combined traditional Chinese and Western medicine for treating POF patients.
Recently, the World Health Organization advocated evidence-based practices in the field of TCM, because medical decision-making should follow clinical research methodology (Woad et a1., 2013). A lack of rigorous research evidence contributes to TCM facing huge challenges. The results of the present review suggest that the therapeutic efficacy of combined traditional Chinese and Western medicine should be strictly evaluated in accordance with the basic criteria for evidence-based medicine, even though numerous physicians’ experiences, especially clinical case reporting, have been repeatedly demonstrated for decades (Chen and Hu 2006). Both an intention-to-treat analysis and appropriate methods, including dropouts, are important for the design of trials and data analysis procedures. Considering that there were insufficient high-quality trials on TCM and HT based on syndrome differentiation for treating POF, the proof of the effectiveness of this therapy pattern will need to be confirmed and reevaluated, including adequate high-quality trials, large sample sizes, randomized trials, more rigorous placebo-controlled trials, and more rigorously designed RCTs.
Conclusions
Although treatment with a combination of TCM and HT was superior to that of HT alone in treating patients with POF, the beneficial effects derived from this combination therapy cannot be viewed conclusive. Due to a lack of high-quality trials for treating patients with POF to prove the therapeutic effects of the combination therapy, more rigorous trials that conform to the CONSORT statement are warranted. A randomization strategy should be used as a standard and described completely and clearly.
Authors’ Contribution
Jia-Xu Chen conceived and designed the study. Mei-Jing Kou and Xiu-Fang Ding analyzed the data. Mei-Jing Kou, Jia-Xu Chen and Xiu-Fang Ding wrote the paper; Yan Liu and Yue-Yun Liu extracted data.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81473597), Beijing Municipal Natural Science Foundation (Grant No. 7152093), China National Funds for Distinguished Young Scientists (Grant No. 30825046), Chang Jiang Scholars Program) and the 111 Project (No. B07007).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Altuntas C.Z, Johnson J.M, Tuohy V.K. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. The Journal of Immunology. 2006;177(7):1998–1996. doi: 10.4049/jimmunol.177.3.1988. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet Journal Of Rare Diseases. 2006;1(1):9. doi: 10.1186/1750-1172-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao S.L, Huang L.W, Yen H.R. Pregnancy in premature ovarian failure after therapy using Chinese herbal medicine. Chang Gung Medical Journal. 2003;26:449–452. [PubMed] [Google Scholar]
- 4.Chand A.L, Harrison C.A, Shelling A.N. Inhibin and premature ovarian failure. Human Reproduction Update. 2010;16(1):39–50. doi: 10.1093/humupd/dmp031. [DOI] [PubMed] [Google Scholar]
- 5.Chen J.X, Hu L.S. Traditional Chinese medicine on the treatment of chronic prostatitis in China: a systematic review and meta-analysis. Journal of Alternative & Complementary Medicine. 2006;12(8):763–769. doi: 10.1089/acm.2006.12.763. [DOI] [PubMed] [Google Scholar]
- 6.Dobos G, Tao I. “The model of Western integrative medicine: the role of Chinese medicine”. Chinese Journal of Integrative Medicine. 2011;17(1):1. doi: 10.1007/s11655-011-0601-x. [DOI] [PubMed] [Google Scholar]
- 7.Feng P, Li Q.K. Clinical observation on artificial cycle with Peikun Pills for treatment of Premature Ovarian Failure. Journal of New Chinese Medicine. 2013;45(3):82–84. [Google Scholar]
- 8.Gao X.J, Bi H. Chinese Medicine with HRT for treatment of idiopathic premature ovarian failure. Shandong Journal Of Traditional Chinese Medicine. 2008;27(1):814–815. [Google Scholar]
- 9.Han L.Y, Ye Y.Z, Zhu P.Q. Kangluanshuai granules with HRT affecting quality of sexual life in patients with premature ovarian failure. Liaoning Journal of Traditional Chinese Medicine. 2008;35(4):242–243. [Google Scholar]
- 10.Hudson M.M. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116(5):1171–1183. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadad R, Moore R. A. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.Jin Z.C, Huang X.T, Yang Y.Q. Clinical study of premature ovarian failure using Bushen Huoxue recipe combined with estrogen and progesterone. Integrated Traditional Chinese Medicine And Western Medicine. 2013;33(5):586–589. [PubMed] [Google Scholar]
- 13.Jolande G, van der Stege Henk G, van Zadelhoff Saskia JN, LC B, BDD M, van Kasteren Yvonne M, van Santbrink Evert J P, AMJ A, WSWC M, Annemieke H. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15(1):23–31. doi: 10.1097/gme.0b013e3180f6108c. [DOI] [PubMed] [Google Scholar]
- 14.Kalantaridou S.N, Davis S. R, Nelson L.M. Premature ovarian failure, Endocrinology And Metabolism Clinics Of North America. 1998;27(4):989–1006. doi: 10.1016/s0889-8529(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 15.Kjaergard L.L, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine. 2001;135(11):982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 16.Li K.Y, Peng Q.H. Treatment of 36 cases with premature ovarian failure. Journal of Practice Of Integrated Traditional Chinese Medicine and Western Medicine. 2009;25(11):749–750. [Google Scholar]
- 17.Liu N, Wang Y.H, Xiong L. Clinical observation of kidney premature ovarian failure using Yishen Kangshuai decoction combined with kelingmen treatment. Journal of Hunan University of Chinese Medicine. 2009;29(3):49–51. [Google Scholar]
- 18.Li X.L. 36 cases with premature ovarian failure using treatment of Integrated Traditional Chinese medicine and western medicine, modern traditional. Chinese medicine. 2009;29(1):24–25. [Google Scholar]
- 19.Lu Li. Curative effect of 67 cases with premature ovarian failure in plateau area using integrated traditional Chinese and Western Medicine. Journal Of High Altitude Medicine. 2009;19(3):57–59. [Google Scholar]
- 20.Liu M.R, Ouyang H.Q. Practical Gynecology of traditional Chinese medicine, second edition. Shanghai Science And Technology Press. 2010:242–245. [Google Scholar]
- 21.Multidisciplinary Working Group convened by the British Fertility Society. A strategy for fertility services for survivors of childhood Cancer, Human Fertility (Camb) 2003;6(2):A1–A39. doi: 10.1080/1464770312331369133. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz K.F, Montori V, G0tzsche P.C, Devereaux P.J, Elbourne D, Egger M, Altman D.G. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trial. Journal Of Clinical Epidemiology. 2010;63(8):e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell R.L, Warner P, Lee R.J, Walker J, Bath L. E, Kelnar C. J, Wallace W. H. B, Critchley H. O. D. Physiological sex steroid replacement in premature ovarian failure: randomized crossover trial of effect on uterine volume, endometrial thickness and blood flow, compared with a standard regimen. Human Reproduction. 2012;34(4):1130–1138. doi: 10.1093/humrep/des004. [DOI] [PubMed] [Google Scholar]
- 24.Qi H.Q, Hu H.J, Xie Y.H. Clinical observation of 26 cases with premature ovarian failure by using integrated traditional Chinese and Western Medicine. Shandong Journal of Traditional Chinese Medicine. 2008;27(6):398–399. [Google Scholar]
- 25.Qin J.J. The clinical observation of 72 cases with premature ovarian failure using treatment of integrated traditional Chinese and Western Medicine. Sichuan Journal Of Traditional Chinese Medicine. 2006;24(8):86–87. [Google Scholar]
- 26.Rakel D. Integrative medicine. Elsevier Health Sciences; 2012. [Google Scholar]
- 27.Schulz K. F, Chalmers I, Hayes R. J, Altman D. G. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 28.Van Kasteren Y.M, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and athieve pregnancy. Human Reproduction Update. 1999;5(5):483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 29.Woad Kathryn J, Watkins Wendy J, Prendergast Deborah Shelling, Andrew N. The genetic basis of premature ovarian failure, Australian And New Zealand. Journal of Obstetrics and Gynaecology. 2013;46(3):242–244. doi: 10.1111/j.1479-828X.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 30.Wallace W.H, Anderson R.A, Irvine D.S. Fertility preservation for young patients with cancer: who is at risk and what can be offered? The lancet oncology. 2005;6(4):209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 31.Wallace W.H, Thomson A.B, Saran F, Tom W, Kelsey Predicting age of ovarian failure after radiation to a field that includes the ovaries. International Journal of Radiation Oncology Biology Physics. 2005;62(3):738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 32.Yang YZ, Han Z.Y. Principle of prevention and treatment of premature ovarian failure. Journal of Practical Gynecology And Obstetrics. 2003;4(19):201–203. [Google Scholar]
- 33.Yi W.Y, Goo X.L, Liu C.Q. The observation of the clinical curative effect of premature ovarian failure using hormone replacement therapy combined with traditional Chinese medicine in the treatment of premature ovarian failure. Maternal And Child Health Care Of China. 2008;23(32):4563–4565. [Google Scholar]
- 34.Zhang Y. Journal of Ji Ning Medical University. 2008 The comparison study of Heat clearing and blood activating method combined with western medicine in the treatment of autoimmune premature ovarian failure;31(2):155–156. [Google Scholar]
- 35.Zhu Y, Xie Q. The observation of curative effect of Bushentiaochong decoction combined with western medicine to treat premature ovarian failure. Hubei Journal of Traditional Chinese Medicine. 2012;34(12):15–16. [Google Scholar]
- 36.Yang X.H, Shen L. The observation of clinical curative effect of Bushentiaochong decoction combined with western medicine to treat premature ovarian failure. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2015;25(12):1131–1133. [Google Scholar]
- 37.Liu Q. Clinical study of premature ovarian failure using Kuntai recipe combined with estrogen and progesterone. Shenzhen Journal of Integrated Traditional Chinese And Western Medicine. 2015;25(9):81–82. [PubMed] [Google Scholar]
- 38.Lu D.F, Gao M. The observation of curative effect of Integrated Traditional Chinese and Western Medicine in the treatment of premature ovarian failure. Hebei Medical Journal. 2014;36(8):1210–1211. [Google Scholar]
- 39.Jin H.B. The observation of the clinical curative effect of premature ovarian failure using Integrated Traditional Chinese and Western Medicine. Journal of Medical Forum. 2015;36(8):114–115. [Google Scholar]


