Abstract
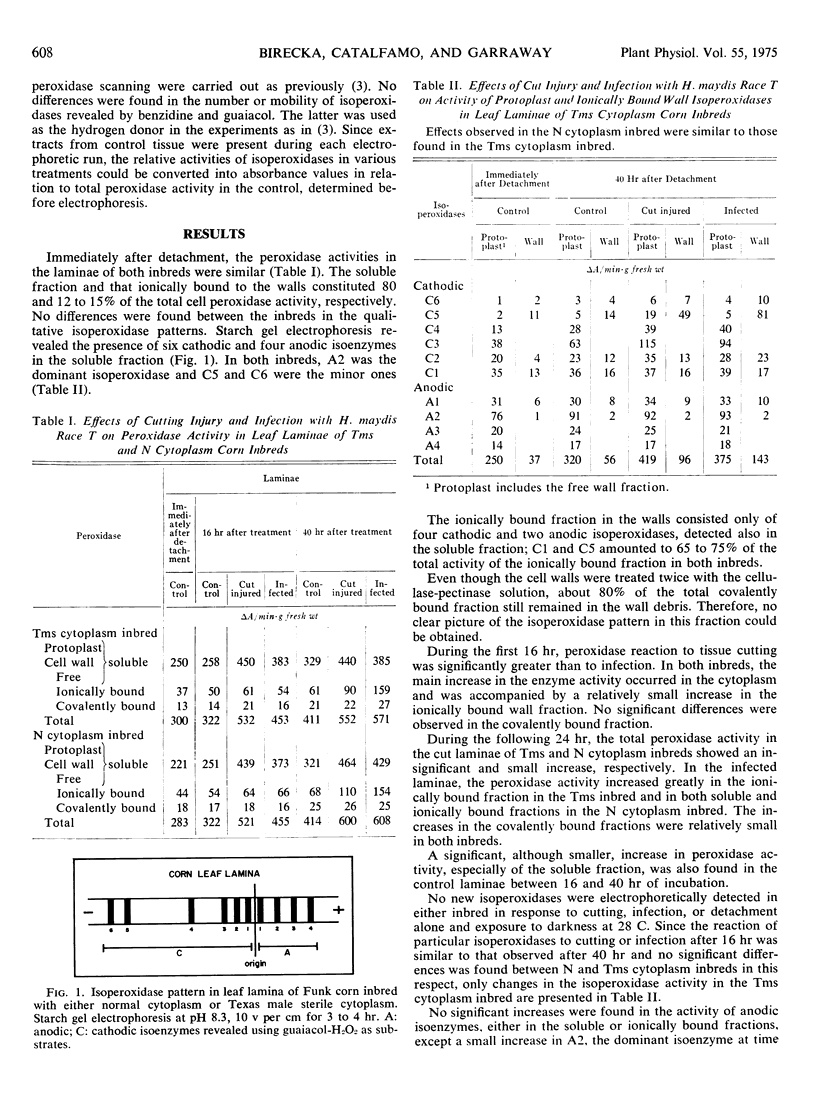
Young leaves of two corn (Zea mays) inbreds with normal and Texas male-sterile cytoplasm, which differed in their susceptibility to Helminthosporium maydis Nisikado and Miyake race T, showed no significant qualitative or quantitative differences in their isoperoxidase patterns. Of six cathodic and four anodic isoenzymes present in the soluble fraction, four and two, respectively, comprised the fraction ionically bound to the cell wall. Peroxidase fractions ionically and covalently bound to the wall constituted about 20% of the total peroxidase activity. No new isoperoxidases were detected in either inbred line in response to cutting, infection, or detachment only and exposure to darkness for 40 hours. Three isoperoxidases, all cathodic, mainly reacted to cutting injury as well as fungal infection. One of the isoperoxidases appeared responsible for the increase in the peroxidase activity of the soluble fraction while the other two were responsible for the increase in that of the fraction ionically bound to the walls. The relative increase in the latter fraction was greater for infected leaves than for mechanically injured ones. No significant differences were found between the two inbreds in their peroxidase reactions to cutting injury or infection. Thus, the corn leaf isoperoxidases were distinctive in their distribution in the cell and in their reaction to injury. Changes in their activity induced by infection may result from a nonspecific response to injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belding M. E., Klebanoff S. J., Ray C. G. Peroxidase-mediated virucidal systems. Science. 1970 Jan 9;167(3915):195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Birecka H., Catalfamo J. L. Cell wall and protoplast isoperoxidases in tobacco plants in relation to mechanical injury and infection with tobacco mosaic virus. Plant Physiol. 1975 Apr;55(4):611–619. doi: 10.1104/pp.55.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Miller A. Cell wall and protoplast isoperoxidases in relation to injury, indoleacetic Acid, and ethylene effects. Plant Physiol. 1974 Apr;53(4):569–574. doi: 10.1104/pp.53.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare B., Weber D. J., Stahmann M. A. Peroxidase and resistance to ceratocystis in sweet potato increased by volatile materials. Science. 1966 Jul 1;153(3731):62–63. doi: 10.1126/science.153.3731.62. [DOI] [PubMed] [Google Scholar]
- Jennings P. H., Brannaman B. L., Zscheile F. P., Jr Peroxidase and polyphenol oxidase activity associated with Helminthosporium leaf spot of maize. Phytopathology. 1969 Jul;59(7):963–967. [PubMed] [Google Scholar]
- Klebanoff S. J., Smith D. C. Peroxidase-mediated antimicrobial activity of rat uterine fluid. Gynecol Invest. 1970;1(1):21–30. doi: 10.1159/000301903. [DOI] [PubMed] [Google Scholar]
- Lovrekovich L., Lovrekovich H., Stahmann M. A. Tobacco mosaic virus-induced resistance to Pseudomonas tabaci in tobacco. Phytopathology. 1968 Jul;58(7):1034–1035. [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Seevers P. M., Daly J. M., Catedral F. F. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 1971 Sep;48(3):353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]


