Abstract
Using yeast two-hybrid screen, we previously isolated HELZ2 (helicase with zinc finger 2, transcriptional coactivator) that functions as a coregulator of peroxisome proliferator-activated receptorγ (PPARγ). To further delineate its molecular function, we here identified thyroid hormone receptor-associated protein3 (THRAP3), a putative component of the Mediator complex, as a protein stably associating with HELZ2 using immunoprecipitation coupled with mass spectrometry analyses. In immunoprecipitation assays, Thrap3 could associate with endogenous Helz2 as well as Pparg in differentiated 3T3-L1 cells. HELZ2 interacts with the serine/arginine-rich domain and Bcl2 associated transcription factor1-homologous region in THRAP3, whereas THRAP3 directly binds 2 helicase motifs in HELZ2. HELZ2 and THRAP3 synergistically augment transcriptional activation mediated by PPARγ, whereas knockdown of endogenous THRAP3 abolished the enhancement by HELZ2 in reporter assays. Thrap3, similar to Helz2, is evenly expressed in the process of adipogenic differentiation in 3T3-L1 cells. Knockdown of Thrap3 in 3T3-L1 preadipocytes using short-interfering RNA did not influence the expression of Krox20, Klf5, Cebpb, or Cebpd during early stages of adipocyte differentiation, but significantly attenuated the expression of Pparg, Cebpa, and Fabp4/aP2 and accumulation of lipid droplets. Pharmacologic activation of Pparg by troglitazone could not fully restore the differentiation of Thrap3-knockdown adipocytes. In chromatin immunoprecipitation assays, endogenous Helz2 and Thrap3 could be co-recruited, in a ligand-dependent manner, to the PPARγ-response elements in Fabp4/aP2 and Adipoq gene enhancers in differentiated 3T3-L1 cells. These findings collectively suggest that Thrap3 could play indispensable roles in terminal differentiation of adipocytes by enhancing PPARγ-mediated gene activation cooperatively with Helz2.
Nuclear hormone receptors (NRs) are transcription factors that regulate target gene transcription in ligand-dependent and -independent manners and profoundly participate in multiple aspects of development, differentiation, and homeostasis in eukaryotes. NRs are therefore implicated in pathogenesis and treatments of a wide variety of human disorders (1, 2). Emerging evidence established that NRs require diverse classes of transcriptional coregulators (TCRs) to fully activate or repress target gene transcription (3–5). TCRs are basically categorized to a class of proteins that interact directly or in close association with partner transcription factors to modulate recruitments of RNA polymerase II and basal transcription factors by utilizing their intrinsic and associated enzymatic activities such as ATP-dependent remodeling of chromatin structures, epigenetic modifications of histone tails with acetylation, methylation, phosphorylation, or ubiquitination, thereby orchestrating to stimulate or repress target gene transcription (3–5). NRs are well known to possess a characteristic modular structure, and TCRs are recruited to specific domains of NRs in a ligand-independent or -dependent manner (3–5). Although the DNA-binding domain (DBD) of NRs is necessary for binding to their cognate hormone-responsive elements located near proximal promoters as well as in enhancer elements far distant from transcription start sites, many previous studies indicate that the DBD could also function as an interaction surface with other proteins (6–10). Using yeast two-hybrid (YTH) screen with the DBD and a part of the hinge region of thyroid hormone receptor (TR) β as bait, we previously isolated PSMC3 (also known as Tat-binding protein1), one of the 6 ATPase subunits consisting of 19S regulatory subunit of 26S proteasome and showed that Tat-binding protein1 functions as a coactivator of TR as well as androgen receptor (11–13).
Peroxisome proliferator-activated receptor (PPAR)γ, a member of the NR family, is well-recognized to function as a master regulator of adipocyte differentiation and thereby believed to play fundamental roles in lipid and glucose metabolism in the body (14–16). A number of TCRs are shown to directly interact with different domains of PPARγ including DBD and involved in adipogenesis in vivo and in vitro (17–19). In an additional attempt to identify proteins that interact with the DBD and a part of the hinge region of human PPARγ using YTH screen, we isolated a partial cDNA of KIAA1769, a human expression sequence tag with unknown function at the time of experiments, and termed PPARγ-DBD-interacting protein 1 (PDIP1) (20). Surapureddi et al (21) independently identified the rat orthlogue of KIAA1769 in biochemically purified liver proteins that associates with liganded full-length PPARα and designated as PPARα-interacting cofactor complex 285 (PRIC285). In several human cell lines, we identified the expression of 2 PDIP1 isoforms that differ in their N-terminal amino acid (aa) alignment due to alternative splicing (the short isoform named as PDIP1α that corresponds to KIAA1769 [PRIC285 isoform2] and the long isoform as PDIP1β [PRIC285 isoform1]) (20). Cotransfection of PRIC285/PDIP1α or PDIP1β could enhance PPARγ and PPARα-mediated transcriptional activation and also augment transcription mediated by several other NRs in cell culture systems (20, 21). Recently, Hugo Gene Nomenclature Committee has approved HELZ2 (helicase with zinc finger 2, transcriptional coactivator) as the symbol name for PRIC285/PDIP1, and here we designated PDIP1β as HELZ2β and PDIP1α/PRIC285 as HELZ2α. However, the molecular mechanism whereby HELZ2 enhances PPARγ-mediated gene transcription remains to be elucidated.
To gain further insights into the molecular function of HELZ2, we attempted to isolate HELZ2-associating proteins in HeLa cells by employing immunoprecipitation (IP) with epitope-tagged HELZ2β followed by mass spectrometry analyses in the present study. We here isolated the TR-associated protein 3 (THRAP3) (also known as TR-associated protein, 150-kDa, TRAP150) (22) as a protein stably associating with HELZ2β. We characterized the interaction domains and functional cooperation in PPARγ-mediated gene transcription between HELZ2β and THRAP3 and identified novel physiologic roles of Thrap3 in adipocyte differentiation.
Materials and Methods
Cell cultures
HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum (Biowest, Rue de la Caille, France) and ampicillin/streptomysin (GIBCO, Life Technologies Corporation Japan, Tokyo, Japan) at 37°C under 5% CO2 atmosphere. 3T3-L1 cells were obtained from Health Science Research Resources Bank (Osaka, Japan) and cultured in DMEM containing 10% fetal bovine serum and antibiotics. Adipogenic differentiation was induced using insulin, dexamethasone (Sigma-Aldrich Japan, Osaka, Japan), and isobutylmethylxanthine (Enzo Life Science, Ann Arbor, Michigan) as previously described (20).
Plasmids
The expression vectors for human HELZ2β (pSVSPORT-HELZ2β) and mouse PPARγ2 (pSVSPORT-PPARγ2) were described previously (20). The HELZ2β cDNA was excised by EcoRI and XbaI from pSVSPORT-HELZ2β (20) and ligated to the EcoRI/XbaI site of p3×FLAG-CMV10 (pCMV10-HELZ2β) (Sigma-Aldrich Japan). The full-length cDNA encoding human THRAP3 was amplified by RT-PCR using HeLa cell RNA and ligated to pCR2.1 vector (Invitrogen, Life Technologies Corp., Gaithersburg, Maryland) (pCR2.1-THRAP3). THRAP3 cDNA was then excised by EcoRI from pCR2.1-THRAP3 and inserted to EcoRI site of pcDNA3.1 vector (Invitrogen, Life Technologies Corp) (pcDNA3.1-THRAP3). Partial THRAP3 cDNAs (aa 168–995 [ΔN], aa 1–591 [ΔC], and aa 168–591 [ΔNC]) were excised from pCR2.1-THRAP3 by BamHI/SalI, EcoRI/MluI, and BamHI/MluI, respectively, and ligated in flame into pVP16 vector (Takara Bio Inc., Otsu, Japan). Partial cDNAs encoding THRAP3 ΔN, ΔC, and ΔNC were also amplified by PCR and subcloned in flame into pcDNA4/HisMax vector (Invitrogen, Life Technologies Corp.). The full-length HELZ2β cDNA was amplified by PCR and ligated in flame into pAcGFP1-N1 and -C1 vectors (CLONTECH Laboratories, Inc, Palo Alto, California). PCR-amplified THRAP3 cDNA was ligated into pDsRed-Monomer-C1 vector (CLONTECH). The nucleotide sequences of all constructs were verified by sequencing.
Preparation of nuclear extract (NE), immunoprecipitation (IP), and liquid chromatography-tandem mass spectrometry (LC-MS/MS)
pCMV10-HELZ2β or p3×FLAG-CMV7-bacterial alkaline phosphatase (FLAG-BAP) (Sigma-Aldrich Japan) was transfected to HeLa cells in a 10-cm dish using Lipofectamine 2000 (Invitrogen, Life Technologies Corp), and NE was prepared after 24 hours according to the method as described previously (23). One milligram of NE was subjected to immunoprecipitation with anti-FLAG M2 antibody (Ab)-conjugated agarose (Sigma-Aldrich Japan) overnight at 4°C, and washed, and bound proteins were eluted using excess amounts of FLAG peptide (Sigma-Aldrich Japan). The released proteins were then resolved in SDS-PAGE, and the gel was stained with silver staining kit (Life Technologies Corp.). The specific bands not observed in the eluent from FLAG-BAP-transfected cells were cut out and subjected to aa analysis by nano LC-MS/MS on a ThermoFisher LTQ Orbitrap XL (Nextgensciences, Ann Arbor, Michigan). In other experiments, preparation of NE and IP were carried out using Universal magnetic Co-IP kit (Active Motif Japan, Tokyo, Japan). The protein concentration was determined by Bradford's method using albumin as standard.
IP and Western blot analysis
IP and immunoblotting were performed as we described previously (11–13, 20) using anti-FLAGM2 Ab (Sigma-Aldrich Japan), anti-THRAP3 Ab (H-300, Santa Cruz Biotechnology, Inc, Santa Cruz, California), anti-PPARγ Ab (catalog no. 81B8, Cell Signaling Technology Japan, Tokyo, Japan; and catalog no.sc-7273, Santa Cruz Biotechnology), antirabbit polyclonal Helz2 Ab (no. 2332–2) generated by immunizing a polypeptide derived from mouse Helz2 (Immuno-Biological Laboratories Co., Ltd., Fujioka, Japan), and anti-Xpress Ab (Invitrogen, Life Technologies Corp). Anticyclophilin A Ab (Upstate Biotechnology Inc., Lake Placid, New York), anti-β actin Ab (Sigma-Aldrich Japan), Living Colors GFP (green fluorescent protein) and DsRed monoclonal Abs (CLONTECH) were used for Western blotting.
Mammalian two-hybrid assay
Expression vectors for GAL4DBD-fused HELZ2β polypeptides (pMHELZ2) were previously constructed (20). Transient transfection with 3×upstream activating sequence-thymidine kinase-luciferase vector (3×UAS-TKLuc) (Takara Bio, Inc.) in the absence or presence of pMHELZ2 and/or pVP16THRAP3 was carried out using the calcium phosphate precipitation method in HeLa cells. The total amounts of transfected plasmids were adjusted by adding empty pM or pVP16 vector. Luciferase activity was measured after 24 hours of transfection and normalized by protein concentration as described previously (11–13, 20).
Glutathione S-transferase (GST) pull-down assay
pGEX4T1 vectors expressing GST-fused partial HELZ2β polypeptides were constructed previously, and GST-fused polypeptides were prepared in Escherichia coli as described elsewhere (20). Proper synthesis of fusion proteins was verified by SDS-PAGE analysis. 35S-labeled full-length THRAP3 protein was synthesized using in vitro transcription/translation system (Promega Corp, Madison, Wisconsin) and pcDNA3.1-THRAP3. Equivalent amounts of GST alone or GST-fused proteins were incubated with 35S-labeled THRAP3 for 1 hour at 4°C and, after extensive washing, subjected to SDS-PAGE as described previously (20). The binding was quantitated using an image analyzer (BAS-2000, Fujifilm Corp, Tokyo, Japan).
Fluorescence microscopy analyses
HeLa cells were grown on chamber slides and AcGFP1-tagged HELZ2β and/or DsRed-tagged THRAP3 expression vector was transfected in the presence of pSVSPORT-PPARγ2 using Lipofectamine 2000 (Invitrogen, Life Technologies Corp.). In other experiments, pCMV10HELZ2β was transfected to HeLa cells with DsRedTHRAP3 and pSVSPORT-PPARγ2. After fixation, cells were incubated with anti-FLAG M2Ab for 2 hours at 37°C. After washing, cells were incubated with antimouse IgG-FITC (Sigma-Aldrich Japan) for 45 minutes at 37°C. Direct and indirect fluorescence microscopy analysis was performed 24 hours after transfection. The microscopic images at 520–550 nm and 460–490 nm excitation wavelengths were merged using Adobe Photoshop Elements 8 (Adobe Systems, Inc., San Jose, California).
Luciferase assay and short interfering RNA (siRNA)
HeLa cells were cultured in 6-well plates and transfected with a firefly luciferase reporter vector driven by 3 copies of direct repeat with 1-bp spacer (DR1-TKLuc) (20) in the absence or presence of pSVSPORT-PPARγ2, pSVSPORT-HELZ2β, or pcDNA3.1-THRAP3 using the calcium phosphate precipitation method. The total amounts of transfected plasmids were adjusted by adding empty pSVSPORT vector. Luciferase activity was measured after 24 hours of incubation with 10 μM troglitazone (Daiichi Sankyo Co, Tokyo, Japan) or dimethylsulfoxide as described elsewhere (11–13, 20). siGENOME SMRTpool siRNA for human and murine THRAP3 and control siRNA were obtained from Thermo Fisher Scientific (Lafayette, Colorado). siRNA targeting murine Helz2 was obtained from Sigma-Aldrich Japan. The knockdown efficiency of siRNA introduced into HeLa cells was evaluated by Western blotting of whole cell lysate (WCL) and/or qRT-PCR using total RNA prepared from siRNA-transfected cells. Twenty-four hours after transfection of siRNA, DR1-TKLuc was transfected in the presence or absence of expression vectors for PPARγ and/or HELZ2β by calcium phosphate precipitation method, and a luciferase assay was performed as described above.
Electroporation of siRNA and quantitative RT-PCR (qRT-PCR)
Electroporation of siRNA into 3T3-L1 preadipocytes was carried out using Gene Pulser II (Bio-Rad Laboratories, Inc., Hercules, California) at 100 V and 950 μF as described (24). At the indicated time, WCL was prepared as described and total RNA was isolated using Isogen (Nippon Gene Co, Ltd, Tokyo, Japan). qRT-PCR was preformed as described previously (25). The TaqMan probes and PCR primers used were summarized in Supplemental Table 1 published on the Endocrine Society's Journals Online web site at http://mend.endojournals.org. Oil Red O staining was performed using a kit according to the manufacturer's protocol (Cayman Chemical, Ann Arbor, Michigan).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using a kit (EMD Millipore Corp., MA) according to the protocol as we described previously (12, 13). Antibodies used in ChIP assays were as described above. Primers for qRT-PCR to amplify the genomic region containing PPARγ-response elements (PPREs) in mouse Fabp2/aP2 and Adipoq gene enhancers were as previously described (26, 27).
Statistical analysis
Statistical analysis between 2 samples was performed using unpaired t test, and multiple comparison was carried out using ANOVA, followed by the Turkey's multiple comparison tests. Significance was set at P < .05.
Results
Identification of THRAP3 as a factor associating with HELZ2β using coimmunoprecipitation coupled with LC-MS/MS
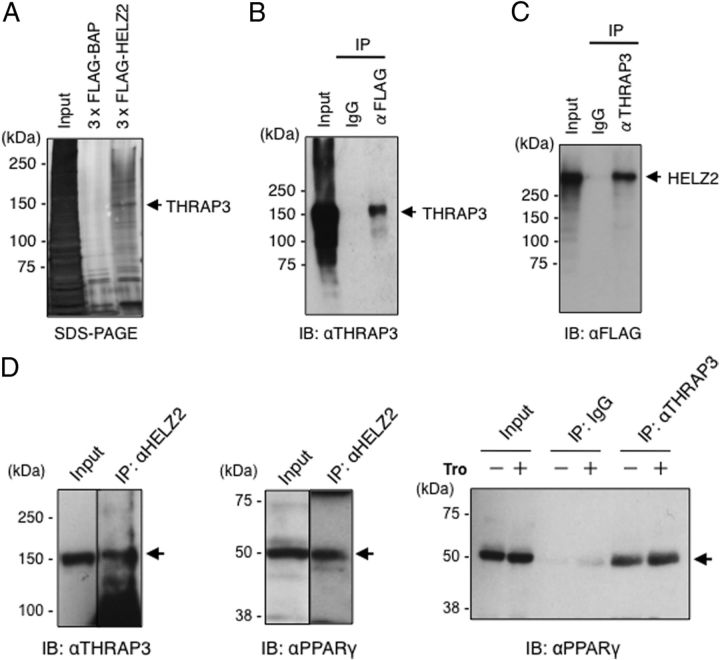
To isolate HELZ2β-associating proteins, we transiently transfected FLAG-tagged HELZ2β into HeLa cells, and IP of NE was performed using anti-FLAG Ab. A silver-stained band at 150 kDa, not observed in control IP from FLAG-BAP-transfected cells, was excised and subjected to LC-MS/MS analysis. Six different polypeptides matching to the aa alignments in THRAP3 (22) were identified by database search (Figure 1A). To verify the association between HELZ2β and THRAP3, IP was carried out using anti-FLAG Ab with HeLa cell NE transfected with FLAG-HELZ2β, and subjected to immunoblotting using anti-THRAP3 Ab. As shown in Figure 1B, endogenous THRAP3 was precipitated with anti-FLAG Ab, but not with preimmune IgG. In a reciprocal manner, FLAG-tagged HELZ2β was precipitated with anti-THRAP3 Ab (Figure 1C). These findings clearly illustrate that HELZ2β and THRAP3 stably associate in the HeLa cell nuclei.
Figure 1.
A, Identification of THRAP3 as a HELZ2β-interacting protein in HeLa cells using LC-MS/MS. Transfection, preparation of NE, and IP were performed as described in Materials and Methods. Silver-stained SDS-PAGE gel is shown. The positions of molecular size markers are indicated in kilodaltons (kDa) and “Input” represents 3% of input NE. A band around 150 kDa in FLAG-HELZ2β-transfected cells, not observed in FLAG-BAP-transfected cells, was cut out and subjected to aa analysis using LC-MS/MS. B, Endogenous THRAP3 was coprecipitated with FLAG-HELZ2β. HeLa cells were transfected with 3× FLAG-HELZ2β, and NEs were prepared after 24 hours. IP of 500 μg NE was carried out using anti-FLAG Ab or preimmune IgG, and IP samples were subjected to SDS-PAGE. Immunoblotting (IB) was performed using anti-THRAP3 Ab (αTHRAP3). “Input” represents 4% of input NE. C, HELZ2β was coprecipitated with endogenous THRAP3. IP of 500 μg of 3×FLAG-HELZ2β-transfected NE was carried out using αTHRAP3 or IgG. IB was performed using anti-FLAG Ab (αFLAG). “Input” represents 4% of input NE. D, Association of endogenous Thrap3 with Helz2 and Pparg in differentiated 3T3–L1 cells. Five hundred micrograms of NE prepared from troglitazone (Tro)-treated differentiated 3T3–L1 cells were subjected to IP using indicated Abs followed by IB employing indicated Abs. “Input” represents 4% of input NE.
To next evaluate whether endogenous Thrap3 protein could associate with endogenous Helz2 or Pparg, IP coupled with immunoblotting was carried out using NE prepared from differentiated 3T3-L1 cells. As shown in Figure 1D, endogenous Thrap3 protein could be immunoprecipitated with anti-Helz2 Ab (left panel). Endogenous Pparγ could also be immunoprecipitated with anti-Helz2 Ab (middle panel). Moreover, endogenous Pparγ could be coprecipitated with anti-Thrap3 Ab (right box). The association among endogenous Pparg, Helz2, or Thrap3 in 3T3-L1 cells did not apparently increase by 24-hour treatment with troglitazone, a synthetic ligand for PPARγ, in our experimental condition (Figure 1D, right panel and data not shown). We also studied the cellular colocalization of GFP-tagged HELZ2β with DsRed-tagged THRAP3 using direct and indirect fluorescence microscopy analyses and found that HELZ2β and THRAP3 could colocalize in the HeLa cell nucleus (data not shown). These findings taken together indicated that endogenous Pparg, Thrap3, and Helz2 could stably associate each other in the nucleus of differentiated 3T3-L1 cells.
HELZ2β interacts with the serine (S)/arginine (R)-rich domain and the Bcl2-associated transcription factor (BCLAF) 1-homologous region in THRAP3
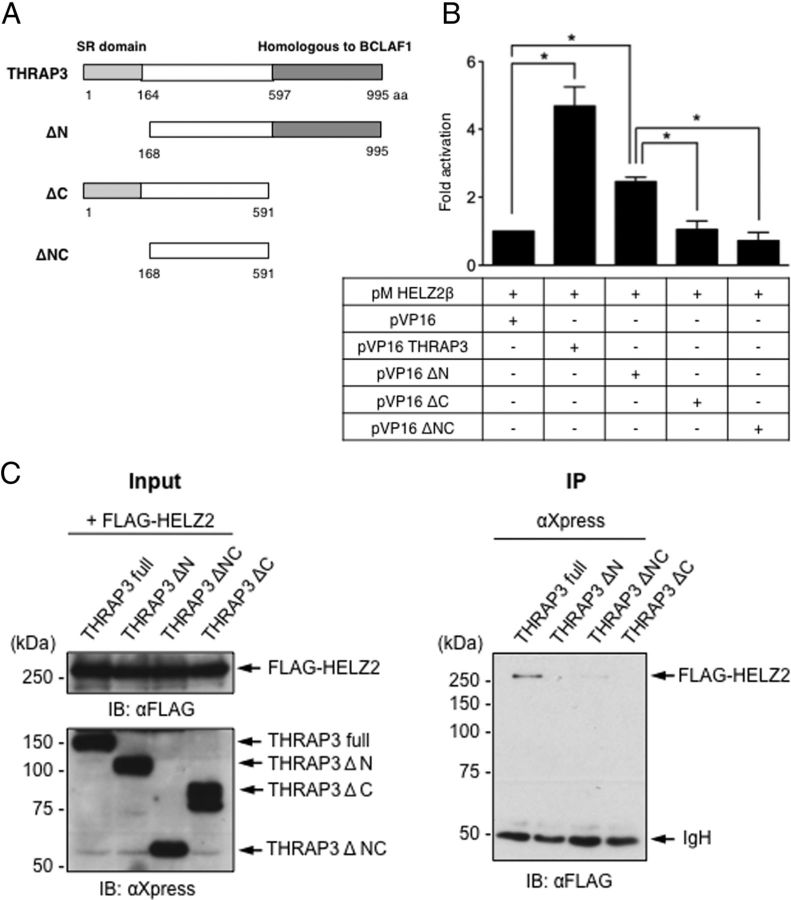
Human THRAP3 protein consists of 995 amino acids and possesses an S/R-rich domain (SR domain) at its N terminus and also carries a region homologous to BCLAF1 at its C terminus (28) (Figure 2A). To next determine the region necessary for binding to HELZ2β in THRAP3, the full-length and truncated forms of THRAP3 were expressed as VP16-fusion proteins, and their interaction with GAL4DBD-fused HELZ2β (pMHELZ2β) were examined using a mammalian two-hybrid assay in HeLa cells. As shown in Figure 2B, the activity of 3×UAS-TKLuc was significantly activated by cotransfection of pMHELZ2β with pVP16THRAP3. Significant, but lesser, activation was observed by cotransfection of pMHELZ2β with pVP16ΔN that lacks the SR domain. In contrast, cotransfection of pMHELZ2β with pVP16ΔC that lacks the region homologous to BCLAF1 or pVP16ΔNC that lacks both SR domain and BCLAF1 homologous region did not activate the reporter gene. To further confirm the THRAP3 domain necessary for interaction with HELZ2β, vector expressing HisMax-tagged ΔN, ΔC, or ΔNC THRAP3 was cotransfected with the FLAG-tagged HELZ2β expression vector, and IP was carried out using anti-FLAG Ab followed by immunoblotting with anti-Xpress Ab that recognizes HisMax-tag. Expression levels of FLAG-tagged HELZ2β and truncated THRAP3 proteins were verified by immunoblotting using anti-FLAG Ab and anti-Xpress Ab, respectively (left panel in Figure 2C). As shown in the right panel of Figure 2C, FLAG-tagged HELZ2β protein associated only with the full-length THRAP3, but not with N- or C-terminal truncated THRAP3 protein. These findings collectively indicated that HELZ2β requires both the N-terminal SR domain and the C-terminal BCLAF1 homologous region to effectively interact with THRAP3.
Figure 2.
A, Schematic representation of VP16 fusion constructs carrying the full-length and truncated versions of THRAP3. SR domain, and the region homologous to BCLAF1 in THRAP3 are indicated. ΔN and ΔC lack aa 1–167 and aa 592–995, respectively. ΔNC lacks both aa 1–167 and aa 592–995. B, HELZ2β interacts mainly with the C-terminal BCLAF1 homologous region in THRAP3 in mammalian two-hybrid (MTH) assay. MTH assay was performed using pMHELZ2β, and pVP16 fused full-length or truncated THRAP3 vector in HeLa cells. Data are expressed as fold activation of the luciferase activity in the presence of pMHELZ2β and empty VP16 vector, and represent mean ± SEM from 3 independent experiments. Asterisks denote significant difference between indicated groups (P < .05). C, HELZ2β requires both the SR domain and the region homologous to BCLAF1 to associate with THRAP3 in IP assays. FLAG-HELZ2β and HisMax-tagged full-length or truncated THRAP3 expression vectors were transfected to HeLa cells. WCL was prepared and subjected to IP with Xpress AB (αXpress) followed by IB using FLAG AK (αFLAG) (right panel). IB using αFLAG and αXpress of 10% of input WCL was shown in the left panel.
THRAP3 directly binds the helicase motifs in HELZ2β
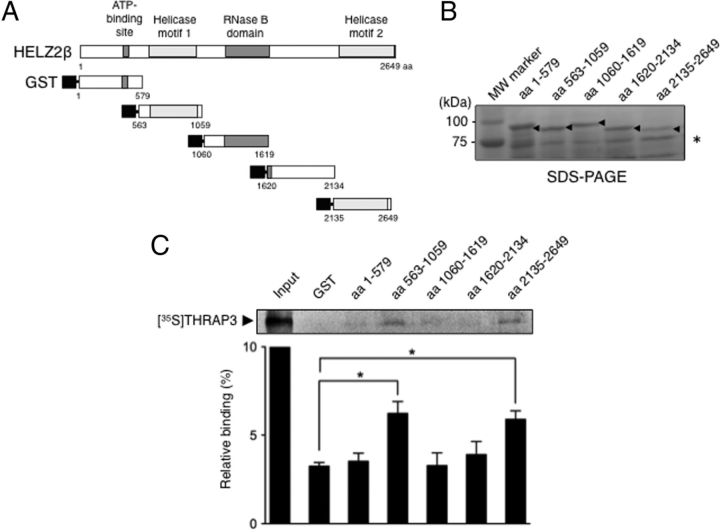
To next evaluate the region necessary for direct binding to THRAP3 in HELZ2β, partial polypeptides of HELZ2 were serially synthesized as GST-fusion proteins (Figure 3A), and GST pull-down assays were carried out using in vitro translated 35S-labeled full-length THRAP3 protein. Proper synthesis of GST-fused proteins was verified by SDS-PAGE analysis (Figure 3B). After extensive washing, the partial polypeptides corresponding to aa 563-1059 (helicase motif 1) and aa 2135–2649 (helicase motif 2), but not other 4 polypeptides, significantly retained with 35S-labeled THRAP3 (Figure 3C). These data indicated that THRAP3 directly binds two helicase motifs in HELZ2β.
Figure 3.
A, Schematic representation of GST-fused HELZ2β polypeptides. The positions of the ATP binding site, two helicase motifs, and RNase B domain in HELZ2β are indicated. Five serial HELZ2β polypeptides (aa 1–579, aa 563–1059, aa 1060–1619, aa 1620–2134, and aa 2135–2649) were bacterially synthesized, and proper synthesis of GST-fusion proteins was verified by SDS-PAGE. Arrowheads indicate individual GST-fused protein, and an asterisk represents nonspecific protein. B, THRAP3 binds helicase motifs in HELZ2β in GST pull-down assays. 35S-labeled THRAP3 protein was synthesized and incubated with equivalent amounts of GST-fused HELZ2β polypeptides. After extensive washing, bound proteins were resolved in SDS-PAGE. “Input” represent 10% of input 35S-labeled THRAP3 protein, and a representative autoradiography is shown above. Data represent mean ± SEM from 3 independent experiments and are expressed as percentage (%) of input THRAP3 protein. Asterisks indicate significant difference between indicated samples (P < .05).
THRAP3 and HELZ2β cooperatively enhance PPARγ-mediated gene activation
We next examined whether cotransfection of THRAP3 affects the PPARγ-mediated gene transcription that was augmented by HELZ2β using a transient transfection assay in HeLa cells (Supplemental Figure 1A). The activity of DR1-TKLuc was stimulated by cotransfected PPARγ in the presence of troglitazone. In agreement with our previous observation (20), the activation by liganded PPARγ was significantly augmented by cotransfection of HELZ2β. Although cotransfection of THRAP3 did not enhance the PPARγ-mediated gene activation in the absence of transfected HELZ2β, cotransfection of THRAP3 with HELZ2β further enhanced the promoter activity. In the absence of cotransfected PPARγ, cotransfection of HELZ2β and THRAP3 did not stimulate the reporter gene activity, indicating that PPARγ is required for the cooperative enhancement of the promoter activity by HELZ2 and THRAP3. As expected from the findings in mammalian two-hybrid assays (Figure 2B), THRAP3 lacking the C-terminal BCLAF1 homologous domain (THRAP3ΔC) did not reveal cooperative enhancement of the reporter activity with HELZ2β (Supplemental Figure 1B).
To next examine whether knockdown of endogenous THRAP3 affects enhancement of PPARγ-mediated gene transcription by HELZ2β, siRNA-targeting THRAP3 (siTHRAP3) was introduced 24 hours before transfection of the reporter and expression vectors for PPARγ and HELZ2β. Lipofection of siTHRAP3 reduced levels of endogenous THRAP3 protein by approximately 30% after 24 hours, and the suppressive effect lasted for at least 4 days (Supplemental Figure 1C and data not shown). Knockdown of THRAP3 significantly attenuated the enhancement of PPARγ-mediated gene transcription by HELZ2β (Supplemental Figure 1D). These findings suggest that endogenous THRAP3 is required for enhancement of PPARγ-mediated gene transcription by HELZ2β.
Knockdown of Thrap3 attenuates terminal differentiation of 3T3-L1 cells
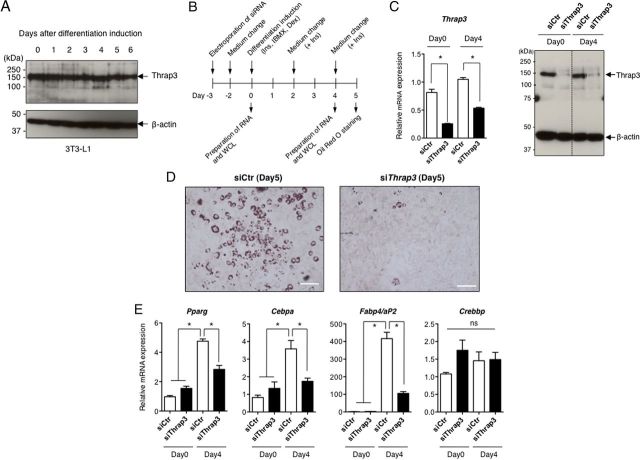
Because PPARγ is established to function as a master regulator of adipocyte differentiation (14–16), we next questioned whether siRNA-mediated knockdown of Thrap3 affects differentiation of 3T3-L1 preadipocytes. In Western blot analyses, Thrap3 protein was expressed at a constant level throughout the differentiation of 3T3-L1 adipocytes (Figure 4A) similarly with Helz2 as we previously described (20). Electroporation of siThrap3, but not siControl (siCtr), into 3T3-L1 cells 3 days before hormonal induction apparently reduced levels of Thrap3 mRNA and protein on day 0, and the effect lasted at least until day 4 (Figure 4, B and C). As depicted in Figure 4D, accumulation of lipid droplets stained with Oil Red O was apparently reduced in siThrap3-introduced 3T3-L1 cells on day 5. The expression of Pparg as well as Cebpa and Fabp4/aP2, two well-known PPARγ target genes (29, 30), significantly increased 4 days after differentiation induction in siCtr-introduced 3T3-L1 cells, but the expression of these 3 genes was significantly attenuated in siThrap3-introduced cells. In contrast, the expression of Crebbp gene, which has been reported to be unchanged during differentiation of 3T3-L1 cells (20, 31), was not affected by introduction of siThrap3 (Figure 4E).
Figure 4.
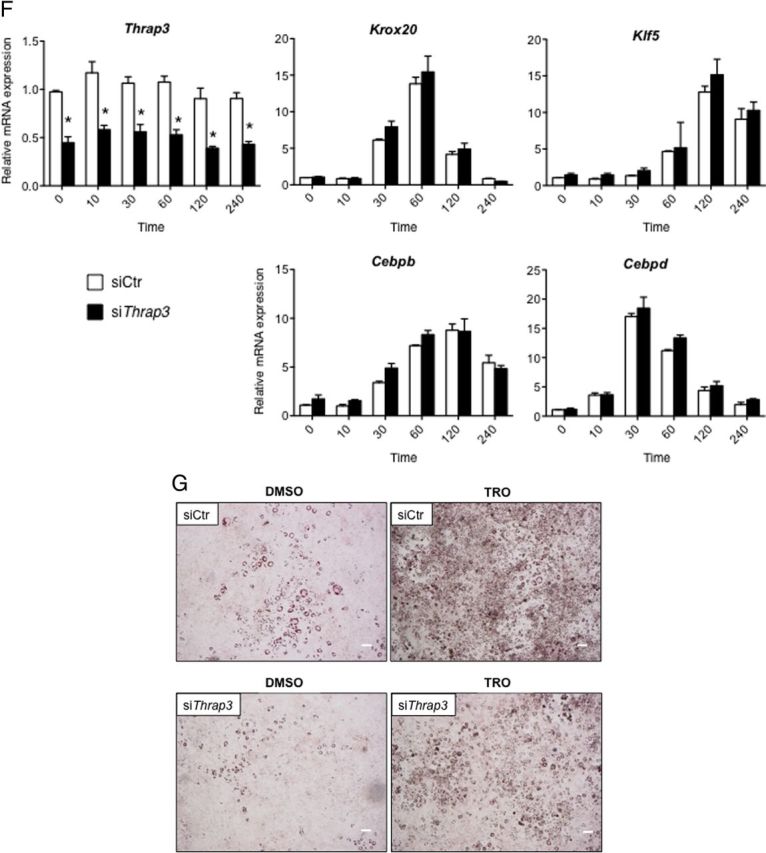
A, Levels of Thrap3 protein during 3T3–L1 cell differentiation[b]. WCL were prepared before and after induction of differentiation of 3T3–L1 cells. IB was performed using anti-THRAP3 Ab and anti-β-actin Ab. B, The experimental design to evaluate effects of knockdown of Thrap3 in 3T3–L1 cell differentiation. After electroporation of siRNA, differentiation of 3T3–L1 cells was induced, and total RNA and WCL were prepared on the indicated days. C, Effect of siThrap3 on levels of Thrap3 mRNA and protein on day 0 and day 4. siCtr and siThrap3 were introduced into 3T3–L1 cells as indicated above. WCL was prepared on the day before differentiation induction (Day 0) and 4 days later (Day 4). IB of the same membrane was performed using anti-THRAP3 Ab and anti-β-actin Ab. D, Differentiation of 3T3–L1 cells is attenuated by knockdown of Thrap3. Five days after differentiation induction of 3T3–L1 cells in which siCtr or siThrap3 was introduced, accumulation of lipid droplets was microscopically analyzed by Oil Red O staining. Bars represent 100 μm. The experiment was repeated twice with similar results. E, Expression changes of Pparg, Cebpa, Crebbp, and Fabp4/aP2 genes in siCtr or siThrap3-introduced 3T3–L1 cells on days 0 and 4. Introduction of siRNA into 3T3–L1 cells was carried out at the same time as in Figure 4C. Total RNA was isolated on day 4 and subjected to qRT-PCR for Pparg, Cebpa, Crebbp, and Fabp4/aP2 genes. Actb gene was amplified in parallel and used as an internal control. Data represent the relative mRNA expression and mean ± SEM from triplicate samples. Experiments were repeated twice with similar results. Asterisks indicate significant difference between indicated groups (P < .05). ns, not statistically significant (P > .05). F, Expression changes of genes involved in early adipocyte differentiation in siCtr or siThrap3-introduced 3T3–L1 cells. siCtr and siThrap3 were introduced as described above, and total RNA was isolated at the indicated time course after differentiation induction. The expression of Thrap3, Cebpd, Cebpb, Kfl5, and Krox20 was quantitated using qRT-PCR in parallel. Data represent the mRNA expression relative to that of Actb and mean ± SEM from triplicate samples. The experiment was repeated twice with similar results. G, Effect of troglitazone on differentiation of siThrap3-introduced 3T3–L1 cells. siCtr and siThrap3 were introduced as described above, and 10 μM troglitazone (TRO) or dimethylsulfoxide (DMSO) was added on day 0, 2, and 4. Oil Red O staining of cells was carried out on day 5. Bars represent 300 μm. The experiment was repeated once with similar results. Ins, insulin; IBMX, isobutylmethylxanthine.
We next evaluated whether knockdown of Thrap3 affects the expression of several transcription factors essential in the early stage of adipogenic differentiation (32–34). The knockdown efficiency of Thrap3 was monitored to quantitate Thrap3 mRNA level in a parallel qRT-PCR (Figure 4F). In agreement with previous findings reported by others (32–34), the expression of Krox20, Klf5, Cebpb, and Cebpd immediately increased after hormonal induction in siCtr-treated 3T3-L1 cells, whereas no significant differences in the expression of these genes were observed in siThrap3-introduced cells in the indicated time periods (Figure 4F).
To next examine whether pharmacologic activation of Pparg could bypass the effect of Thrap3 knockdown on adipocyte differentiation, siThrap3-introduced 3T3-L1 cells were treated with troglitazone, and accumulation of fat droplets was monitored using Oil Red O staining. As depicted in Figure 4G, accumulation of lipid droplets could not be completely restored in siThrap3-introduced 3T3-L1 cells even in the presence of troglitazone when compared with siCtr-introduced cells. Taken together, these findings suggest that Thrap3 plays pivotal roles mainly in terminal differentiation of 3T3-L1 adipocytes, but not at early stage of differentiation.
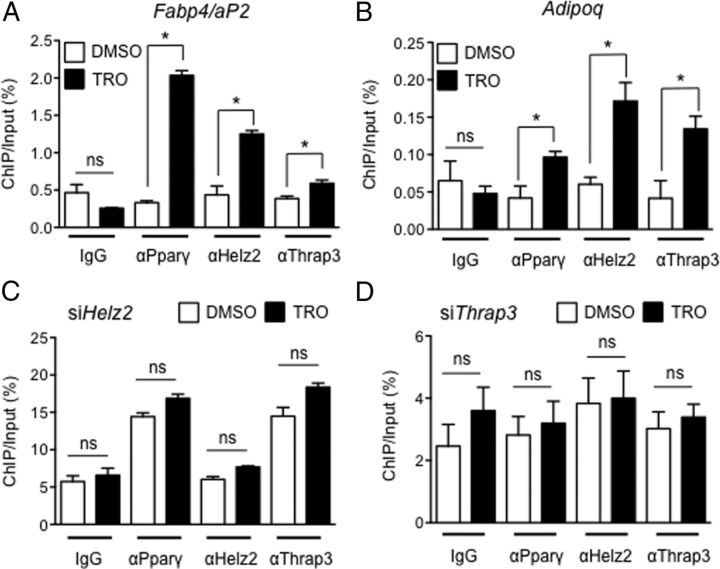
Recruitments of Pparγ, Helz2, and Thrap3 to the PPREs in the Fabp4/aP2 and Adipoq gene enhancers
To lastly examine whether endogenous Helz2 and Thrap3 could be recruited to the PPREs in Fabp4/aP2 and Adipoq gene enhancers (26, 27) in differentiated 3T3-L1 cells, troglitazone was added on day 4, and ChIP assays were performed 24 hours later. As shown in Figures 5, A and B, recruitments of Pparg, Helz2, and Thrap3 to the PPREs in two different enhancer regions were significantly increased in the presence of troglitazone. In contrast, when endogenous Helz2 or Thrap3 protein was knocked down by preintroduction of siHelz2 or siThrap3, respectively, ligand-dependent recruitments of Pparg, Helz2, or Thrap3 to the enhancer region in Fabp4/aP2 gene were diminished (Figure 5, C and D). These findings demonstrated that endogenous Helz2 and Thrap3 could be corecruited in a ligand-dependent manner with Pparg to the PPREs in Fabp4/aP2 and Adipoq gene enhancers in differentiated 3T3-L1 cells. In addition, Helz2 and Thrap3 appeared to cooperatively stabilize the binding of Pparg to DNA element in the presence of ligand.
Figure 5.
A and B, Recruitments of Pparg, Helz2, and Thrap3 to the PPREs in Fabp4/aP2 and Adipoq gene enhancers in differentiated 3T3–L1 cells. Troglitazone (TRO) (10 μM) or dimethylsulfoxide (DMSO) was added 3 days after differentiation induction of 3T3–L1 cells and ChIP assay was started 24 hours later. Data represent promoter occupancy of three proteins in the absence or presence of TRO expressed as a percentage of input DNA samples (mean ± SEM, n = 3). The ChIP assay was repeated once with similar results. C and D, Recruitments of Pparg, Helz2, and Thrap3 to the PPRE in Fabp4/aP2 gene enhancer in siHelz2 or siThrap3-introduced 3T3–L1 cells. siHelz2, or siThrap3 was introduced by electroporation on day 3, and TRO or DMSO was added on day 4. ChIP assay was started 24-hours later. Knockdown efficiency of siHelz2 evaluated by qRT-PCR was approximately 40% on day 0. Data represent promoter occupancy of 3 proteins in the absence or presence of TRO expressed as described above (mean ± SEM, n = 3). The ChIP assay was repeated once with similar results. ns, not statistically significant (P > .05).
Discussion
HELZ2, possessing characteristic structures including two ATP-binding motifs, an RNaseB domain, and dual DNA/RNA helicase motifs, belongs to the DNA2/NAM7 helicase family involved in gene transcription, RNA processing, and DNA repair (35, 36). Initially, PRIC285 and PDIP1 were independently isolated as a protein interacting with PPARα and PPARγ, respectively (20, 21). In cell culture system, HELZ2 has been shown to augment ligand-dependent gene activation mediated by PPARγ (20, 21), whereas siRNA-mediated knockdown of endogenous HELZ2 attenuated PPARγ-mediated gene activation in HeLa cells (20). Interestingly, HELZ2 has been suggested to be involved in the pathogenesis of type III familial partial lipodystrophy caused by heterozygous PPARG mutation in human (37). However, the detailed mechanism whereby HELZ2 enhances PPARγ-mediated gene transcription remains to be elucidated. As a step toward understanding the molecular function of HELZ2, we identified THRAP3 as a protein stably associating with HELZ2β in the present study. THRAP3 was originally identified as a component of the Mediator/TRAP complex that interacts with the liganded TRα (22). However, in recent studies, THRAP3 has been reported to play roles in precursor-mRNA alternative splicing as well as in DNA damage response apart from its transcription-regulatory function (38–40). These cumulative data suggest that THRAP3 is a multifunctional protein involved in gene transcription, RNA processing, and DNA repair similarly with HELZ2. Nonetheless, direct roles of THRAP3 in PPARγ-mediated transcriptional regulation or in the differentiation program of adipocytes have never been explored.
In the present study, transfection of THRAP3 in the absence of HELZ2β did not enhance PPARγ-mediated gene activation, whereas its cotransfection with HELZ2β cooperatively augmented gene transcription activated by PPARγ. siRNA-mediated knockdown of endogenous THRAP3 abolished the enhancement of PPARγ-mediated gene activation by HELZ2β. A truncated form of THRAP3 lacking the BCLAF1 homologous region that was necessary for interaction with HELZ2β failed to enhance transcriptional activation mediated by PPARγ in the presence of cotransfected HELZ2β. These findings indicate that HELZ2β requires direct interaction with THRAP3 to augment PPARγ-mediated gene activation and that THRAP3 may function as an adaptor molecule to tether other TCRs to PPARγ-bound HELZ2β.
Accumulated evidence, established largely by using immortal cell lines such as 3T3-L1 cells, demonstrates that differentiation of adipocytes from fibroblast-like precursor cells induced by hormonal stimuli is orchestrated by coordinated and sequential expression of a variety of transcription factors at early and late stages of adipogenesis (16, 41, 42). However, knowledge about roles of TCRs required for gene-regulatory functions of these transcription factors remains to be limited in a complex process of adipocyte differentiation. Among these adipogenic transcription factors, genome-wide studies utilizing a ChIP-chip method recently uncovered that PPARγ and C/EBPα function to optimize adipocyte maturation by coordinately activating transcription of a number of common target genes in differentiated 3T3-L1 cells (43, 44). In the present study, we showed that accumulation of lipid-laden droplets and the expression of Pparg as well as Cebpa and Fabp4/aP2, well-characterized PPARγ target genes (29, 30), were significantly attenuated by knockdown of Thrap3 in 3T3-L1 cells. In contrast, the expression of transcription factors involved in the early adipogenesis including Krox20, Klf5, Cebpb, or Cebpd (31–33) was not significantly altered by introduction of siThrap3. The present ChIP assay revealed that Helz2 and Thrap3 could be corecruited, in a ligand-dependent manner, to Prarg on the DR1-elements in Fabp4/aP2 and Adipoq gene enhancers in differentiated 3T3-L1 cells. In addition, troglitazone treatment could not fully restore the differentiation of siThrap3-introduced 3T3-L1 cells. These findings collectively suggest that Thrap3 might be necessary for terminal differentiation of adipocytes by enhancing Pparg-mediated gene transcription, rather than early adipogenesis.
Surapureddi et al (21) previously reported that PRIC285 (Helz2α) was biochemically copurified with several components of the Mediator/TRAP complex such as TRAP230, Sur-2, and TRAP100 by using liganded PPARα as a bait, raising the possibility that HELZ2 may cooperatively stimulate PPARγ-mediated gene activation with the Mediator/TRAP complex. Several core subunits of the Mediator/Trap complex have been reported to play indispensable roles both at early and late stages of adipocyte differentiation (47–50). Med1/Trap220 and Med14/Trap170 are shown to be required for adipocyte differentiation in part by activating PPARγ-mediated gene activation through direct interaction with the C-terminal ligand-binding domain and the N-terminal A/B domain of PPARγ, respectively (47, 48). The expression of Cebpb at early stage of adipocyte differentiation was suppressed by knockdown of Med14/Trap170 (49), and knockdown of Med23/Trap150β reduced the expression of Krox20 in 3T3-L1 cells (50), thereby speculated to attenuate adipocyte differentiation. In the present study, we showed that the expression of Cebpb or Krox20 was not affected by knockdown of Thrap3. These findings taken together suggest that several core subunits of the Mediator/Trap complex play nonoverlapping and overlapping roles with Thrap3 in early and late stages of adipocyte differentiation, respectively.
PPARγ has been established to stimulate the expression of a downstream transcription factor, CCAAT enhancer binding protein (C/EBP)α, through direct transcriptional stimulation of Cebpa (32). The enhanced expression of C/EBPα by PPARγ reciprocally activates the expression of an upstream Pparg by directly binding to the CCAAT box present in the Pparg2 promoter, resulting in robust enhancement of terminal differentiation of adipocytes (32). Moreover, it has recently been reported by utilizing a ChIP-chip assay that PPARγ by itself up-regulates the expression of Pparg2 by binding to the DR-1 element located in the Pparg2 promoter in 3T3-L1 cells (45). Taken together, it was speculated that reduced transactivation function of PPARγ caused by Thrap3 knockdown in the present study could result, in part, in malfunction of these feed-forward loops, thereby suppressing the expression of Pparg, Cebpa, and Fabp4/aP2 and maturation of 3T3-L1 cells. Alternatively, because glucocorticoid receptor and TR could stimulate the expression of Pparg2 and Cebpα during early adipogenesis (43, 46, 51), it is possible that the reduced expression of Pparg, Cebpa, and Fabp4/aP2 might result from impaired transactivation function of glucocorticoid receptor and/or TR by knockdown of Thrap3. Because Helz2 and Thrap3 are readily expressed in 3T3-L1 preadipocytes before induction of differentiation, further study is necessary to investigate whether Helz2/Thrap3 complex can enhance other NR-mediated gene activation at early differentiation stages.
Acknowledgments
We thank Aya Osaki, Yuko Tagaya, and Atsuko Miura (Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine, Maebashi, Japan) and Katsuya Dezaki (Division of Integrative Physiology, Department of Physiology, Jichi Medical University School of Medicine, Shimotsuke City, Japan) for their helpful technical assistance.
This work was supported by a Grant-in-Aid from the Ministry of Science, Education, Sports and Culture of Japan (to T.S.) and the Global Center of Excellence Program of Japan (to M.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aa
- amino acid
- Ab
- antibody
- ChIP
- chromatin immunoprecipitation
- C/EBP
- CCAAT enhancer-binding protein
- DBD
- DNA-binding domain
- DR1-TKLuc
- firefly luciferase reporter vector driven by 3 copies of direct repeat with 1-bp spacer
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- HELZ2
- helicase with zinc finger 2
- IP
- immunoprecipitation
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- NE
- nuclear extract
- PDIP1
- PPARγ-DBD-interacting protein 1
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- PPARγ-response element
- qRT-PCR
- quantitative RT-PCR
- siCtr
- control siRNA
- siRNA
- short interfering RNA
- siTHRAP3
- short interfering THRAP3
- TCR
- transcriptional coregulator
- THRAP3
- TR-associated protein 3
- TR
- thyroid hormone receptor
- TRAP
- TR-associated protein
- WCL
- whole cycle lysate
- YTH
- yeast two-hybrid.
References
- 1. Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994;15:391–407. [DOI] [PubMed] [Google Scholar]
- 2. Ribeiro RC , Kushner PJ , Baxter JD. The nuclear hormone receptor gene superfamily. Annu Rev Med. 1995;46:443–453. [DOI] [PubMed] [Google Scholar]
- 3. Glass CK , Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 4. Lonard DM , Lanz RB , O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. [DOI] [PubMed] [Google Scholar]
- 5. Lonard DM , O'Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caelles C , González-Sancho JM , Muñoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desai-Yajnik V , Hadzic E , Modlinger P , Malhotra S , Gechlik G , Samuels HH. Interactions of thyroid hormone receptor with the human immunodeficiency virus type 1 (HIV-1) long terminal repeat and the HIV-1 Tat transactivator. J Virol. 1995;69:5103–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee Y , Nadal-Ginard B , Mahdavi V , Izumo S. Myocyte-specific enhancer factor 2 and thyroid hormone receptor associate and synergistically activate the alpha-cardiac myosin heavy-chain gene. Mol Cell Biol. 1997;17:2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfitzner E , Kirfel J , Becker P , Rolke A , Schüle R. Physical interaction between retinoic acid receptor and the oncoprotein myb inhibits retinoic acid-dependent transactivation. Proc Natl Acad Sci USA. 1998;95:5539–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi JS , Desai-Yajnik V , Yuan Y , Samuels HH. Constitutive activation of gene expression by thyroid hormone receptor results from reversal of p53-mediated repression. Mol Cell Biol. 1997;17:7195–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishizuka T , Satoh T , Monden T , et al. Human immunodeficiency virus type 1 Tat binding protein-1 is a transcriptional coactivator specific for TR. Mol Endocrinol. 2001;15:1329–1343. [DOI] [PubMed] [Google Scholar]
- 12. Satoh T , Ishizuka T , Tomaru T , et al. Tat-binding protein-1 (TBP-1), an ATPase of 19S regulatory particles of the 26S proteasome, enhances androgen receptor function in cooperation with TBP-1-interacting protein/Hop2. Endocrinology. 2009;150:3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satoh T , Ishizuka T , Yoshino S , et al. Roles of proteasomal 19S regulatory particles in promoter loading of thyroid hormone receptor. Biochem Biophys Res Commun. 2009;386:697–702. [DOI] [PubMed] [Google Scholar]
- 14. Lehrke M , Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. [DOI] [PubMed] [Google Scholar]
- 15. Michalik L , Auwerx J , Berger JP , et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. [DOI] [PubMed] [Google Scholar]
- 16. Siersbaek R , Nielsen R , Mandrup S. PPARgamma in adipocyte differentiation and metabolism–novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–3249. [DOI] [PubMed] [Google Scholar]
- 17. Puigserver P , Wu Z , Park CW , Graves R , Wright M , Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. [DOI] [PubMed] [Google Scholar]
- 18. Louet JF , O'Malley BW. Coregulators in adipogenesis: what could we learn from the SRC (p160) coactivator family? Cell Cycle. 2007;6:2448–2452. [DOI] [PubMed] [Google Scholar]
- 19. Koppen A , Kalkhoven E. Brown vs white adipocytes: the PPARγ coregulator story. FEBS Lett. 2010;584:3250–3259. [DOI] [PubMed] [Google Scholar]
- 20. Tomaru T , Satoh T , Yoshino S , et al. Isolation and characterization of a transcriptional cofactor and its novel isoform that bind the deoxyribonucleic acid-binding domain of peroxisome proliferator-activated receptor-γ. Endocrinology. 2006;147:377–388. [DOI] [PubMed] [Google Scholar]
- 21. Surapureddi S , Yu S , Bu H , et al. Identification of a transcriptionally active peroxisome proliferator-activated receptor α -interacting cofactor complex in rat liver and characterization of PRIC285 as a coactivator. Proc Natl Acad Sci USA. 2002;99:11836–11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fondell JD , Ge H , Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93:8329–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malik S , Roeder RG. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem Sci. 2000;25:277–283. [DOI] [PubMed] [Google Scholar]
- 24. Tagaya Y , Miura A , Okada S , Ohshima K , Mori M. Nucleobindin-2 is a positive modulator of EGF-dependent signals leading to enhancement of cell growth and suppression of adipocyte differentiation. Endocrinology. 2012;153:3308–3319. [DOI] [PubMed] [Google Scholar]
- 25. Oh-I S , Shimizu H , Satoh T , et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. [DOI] [PubMed] [Google Scholar]
- 26. Guan HP , Ishizuka T , Chui PC , Lehrke M , Lazar MA. Corepressors selectively control the transcriptional activity of PPARγ in adipocytes. Genes Dev. 2005;19:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lefebvre B , Benomar Y , Guédin A , et al. Proteasomal degradation of retinoid X receptor α reprograms transcriptional activity of PPARγ in obese mice and humans. J Clin Invest. 2010;120:1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarras H , Alizadeh Azami S , McPherson JP. In search of a function for BCLAF1. ScientificWorld J. 2010;10:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tontonoz P , Hu E , Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. 1995;5:571–576. [DOI] [PubMed] [Google Scholar]
- 30. Tontonoz P , Hu E , Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi N , Kawada T , Yamamoto T , et al. Overexpression and ribozyme-mediated targeting of transcriptional coactivators CREB-binding protein and p300 revealed their indispensable roles in adipocyte differentiation through the regulation of peroxisome proliferator-activated receptor γ. J Biol Chem. 2002;277:16906–16912. [DOI] [PubMed] [Google Scholar]
- 32. Mandrup S , Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–5370. [DOI] [PubMed] [Google Scholar]
- 33. Chen Z , Torrens JI , Anand A , Spiegelman BM , Friedman JM. Krox20 stimulates adipogenesis via C/EBPβ-dependent and -independent mechanisms. Cell Metab. 2005;1:93–106. [DOI] [PubMed] [Google Scholar]
- 34. Oishi Y , Manabe I , Tobe K , et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. [DOI] [PubMed] [Google Scholar]
- 35. Altamura N , Groudinsky O , Dujardin G , Slonimski PP. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J Mol Biol. 1992;224:575–587. [DOI] [PubMed] [Google Scholar]
- 36. Kang YH , Lee CH , Seo YS. Dna2 on the road to Okazaki fragment processing and genome stability in eukaryotes. Crit Rev Biochem Mol Biol. 2010;45:71–96. [DOI] [PubMed] [Google Scholar]
- 37. Agostini M , Schoenmakers E , Mitchell C , et al. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee KM , Hsu IaW , Tarn WY. TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation. Nucleic Acids Res. 2010;38:3340–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heyd F , Lynch KW. Phosphorylation-dependent regulation of PSF by GSK3 controls CD45 alternative splicing. Mol Cell. 2010;40:126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beli P , Lukashchuk N , Wagner SA , et al. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol Cell. 2012;46:212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siersbæk R , Nielsen R , Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab. 2012;23:56–64. [DOI] [PubMed] [Google Scholar]
- 43. Lefterova MI , Zhang Y , Steger DJ , et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen R , Pedersen TA , Hagenbeek D , et al. Genome-wide profiling of PPARγ:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wakabayashi K , Okamura M , Tsutsumi S , et al. The peroxisome proliferator-activated receptor γ/retinoid X receptor α heterodimer targets the histone modification enzyme PR-Set7/Setd8 gene and regulates adipogenesis through a positive feedback loop. Mol Cell Biol. 2009;29:3544–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steger DJ , Grant GR , Schupp M , et al. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010;24:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ge K , Guermah M , Yuan CX , et al. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. [DOI] [PubMed] [Google Scholar]
- 48. Ge K , Cho YW , Guo H , et al. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor γ-stimulated adipogenesis and target gene expression. Mol Cell Biol. 2008;28:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grøntved L , Madsen MS , Boergesen M , Roeder RG , Mandrup S. MED14 tethers mediator to the N-terminal domain of peroxisome proliferator-activated receptor gamma and is required for full transcriptional activity and adipogenesis. Mol Cell Biol. 2010;30:2155–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang W , Huang L , Huang Y , et al. Mediator MED23 links insulin signaling to the adipogenesis transcription cascade. Dev Cell. 2009;16:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mishra A , Zhu XG , Ge K , Cheng SY. Adipogenesis is differentially impaired by thyroid hormone receptor mutant isoforms. J Mol Endocrinol. 2010;44:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]