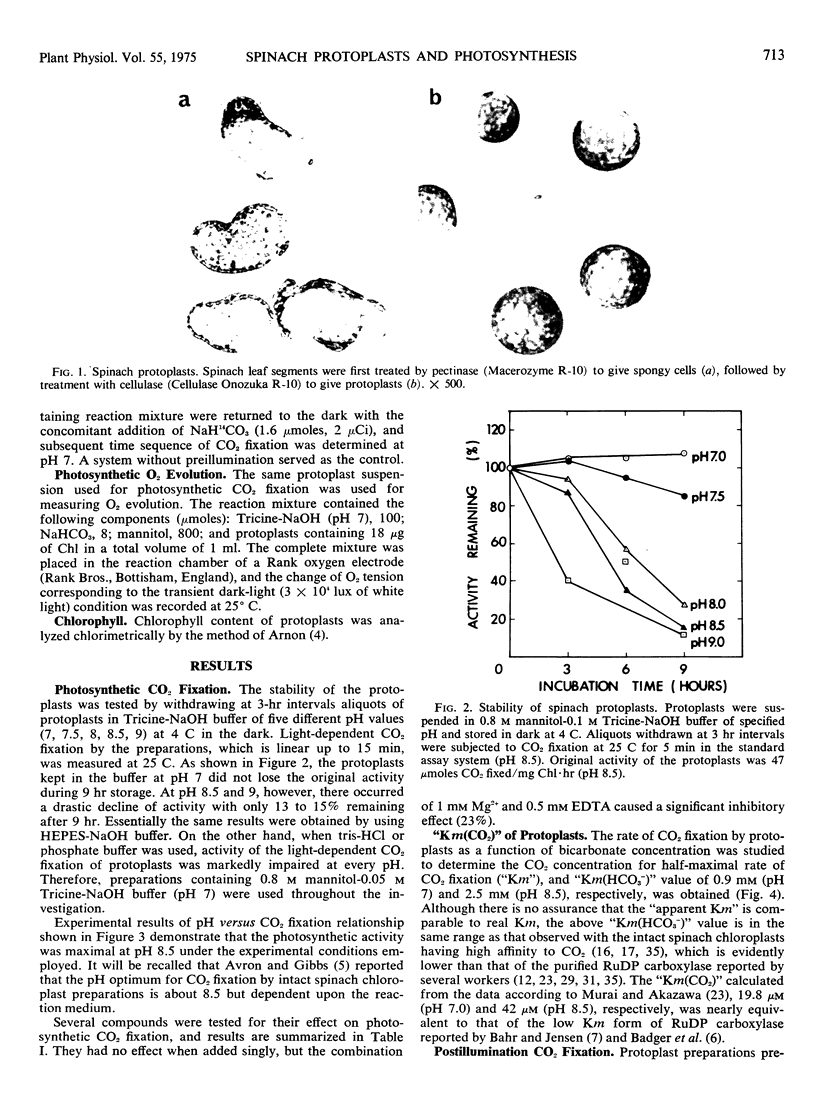
Abstract
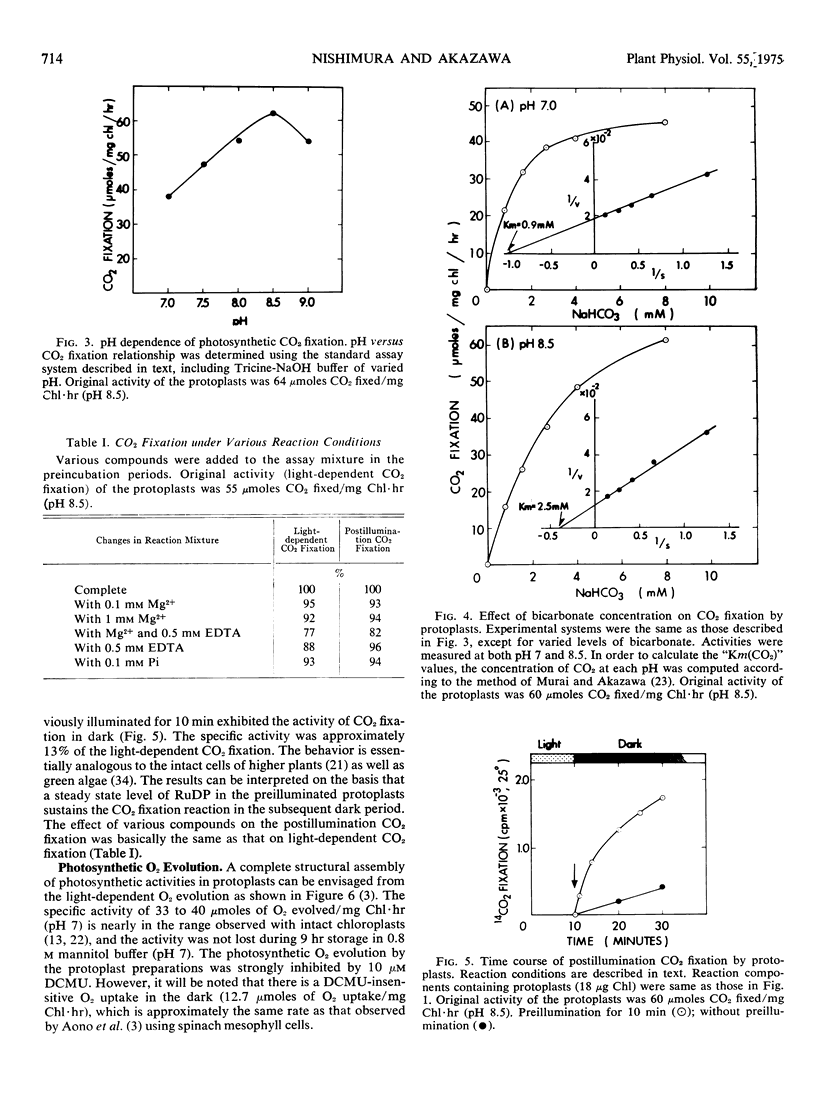
Photosynthetic activities of protoplasts isolated from spinach leaf (Spinacia oleracea L.) were investigated. The protoplasts were stable up to 9 hr, without loss of the original activity of CO2 fixation (33-75 μmoles CO2/mg Chl·hr) and light-dependent O2 evolution (33-40 μmoles O2/mg Chl·hr), when stored in 0.8 m mannitol-0.05 m N-tris (hydroxymethyl)-methylglycine-NaOH buffer, pH 7, at 4 C in dark. The optimum pH of 8.5 for CO2 fixation reaction carried out in the present experimental condition employed is about the same as that reported for intact spinach chloroplasts. The CO2 concentration for half-maximal rate of CO2 fixation by protoplasts. “Km (CO2),” were determined to be 19.8 μm (pH 7) and 42 μm (pH 8.5) and are similar to those observed for intact spinach chloroplasts. Protoplasts showed postillumination CO2 fixation. Over-all results indicate that spinach protoplasts are as active as the intact plant leaf tissues in their photosynthetic activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Takebe I. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus ribonucleic acid. Virology. 1969 Nov;39(3):439–448. doi: 10.1016/0042-6822(69)90092-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Chen T. M., Campbell W. H., Dittrich P., Black C. C. Distribution of carboxylation and decarboxylation enzymes in isolated mesophyll cells and bundle sheath strands of C 4 plants. Biochem Biophys Res Commun. 1973 Mar 17;51(2):461–467. doi: 10.1016/0006-291x(73)91279-5. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Roy H., Jagendorf A. T. Immunological identification of nascent subunits of wheat ribulose diphosphate carboxylase on ribosomes of both chloroplast and cytoplasmic origin. Arch Biochem Biophys. 1973 Nov;159(1):324–335. doi: 10.1016/0003-9861(73)90458-x. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber L. J., Latzko E., Gibbs M. Photosynthetic path of carbon dioxide in spinach and corn leaves. J Biol Chem. 1974 Jun 10;249(11):3436–3441. [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T., Akazawa T. Homotropic effect of CO 2 in ribulose-1,5-diphosphate carboxylase reaction. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2121–2126. doi: 10.1016/0006-291x(72)90768-1. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Akazawa T. Studies on spinach leaf ribulosebisphosphate carboxylase. Carboxylase and oxygenase reaction examined by immunochemical methods. Biochemistry. 1974 May 21;13(11):2277–2281. doi: 10.1021/bi00708a006. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I. Fluorescent antibody staining of tobacco mosaic virus antigen in tobacco mesophyll protoplasts. Virology. 1969 Jul;38(3):497–499. doi: 10.1016/0042-6822(69)90167-6. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I., Honda Y., Matsui C. Ultrastructure of infection of tobacco mesophyll protoplasts by tobacco mosaic virus. Virology. 1972 Jul;49(1):188–194. doi: 10.1016/s0042-6822(72)80020-5. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakayama N., Akazawa T. Structure and function of chloroplast proteins. V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch Biochem Biophys. 1968 Sep 10;126(3):737–745. doi: 10.1016/0003-9861(68)90465-7. [DOI] [PubMed] [Google Scholar]
- Takebe I., Otsuki Y. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus. Proc Natl Acad Sci U S A. 1969 Nov;64(3):843–848. doi: 10.1073/pnas.64.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togasaki R. K., Gibbs M. Enhanced Dark CO(2) Fixation by Preilluminated Chlorella pyrenoidosa and Anacystis nidulans. Plant Physiol. 1967 Jul;42(7):991–996. doi: 10.1104/pp.42.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]