Abstract
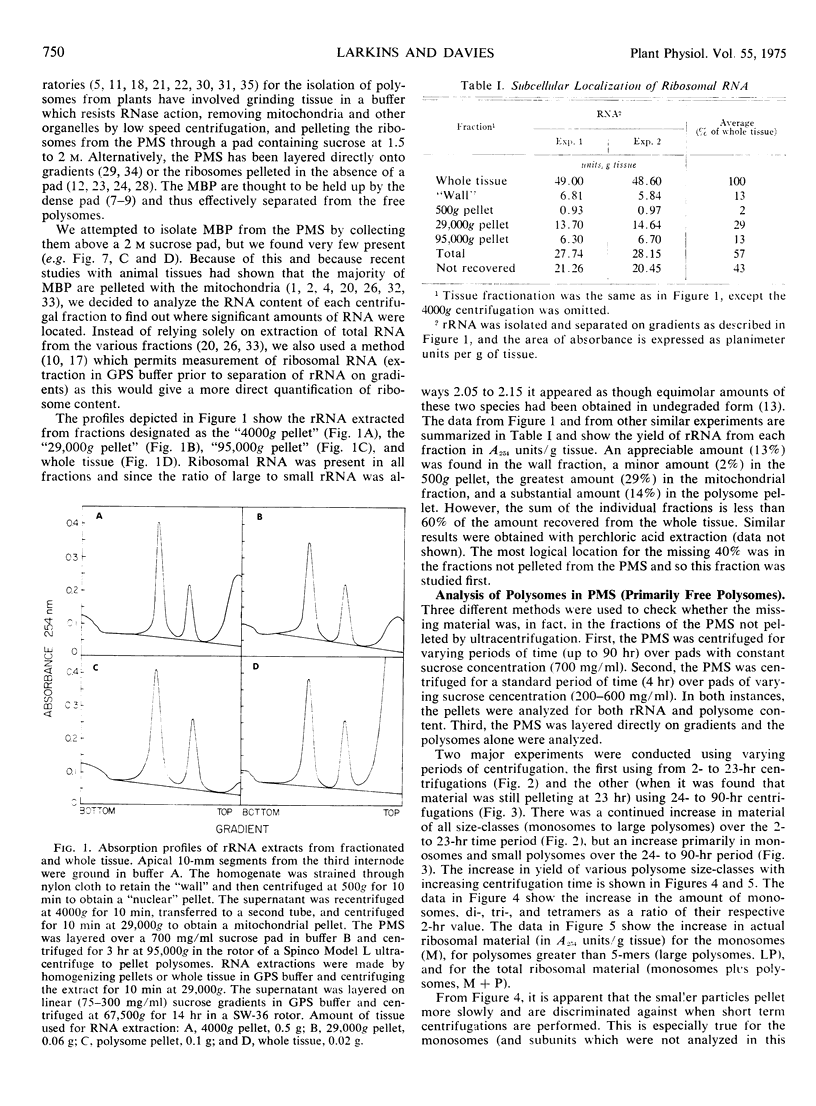
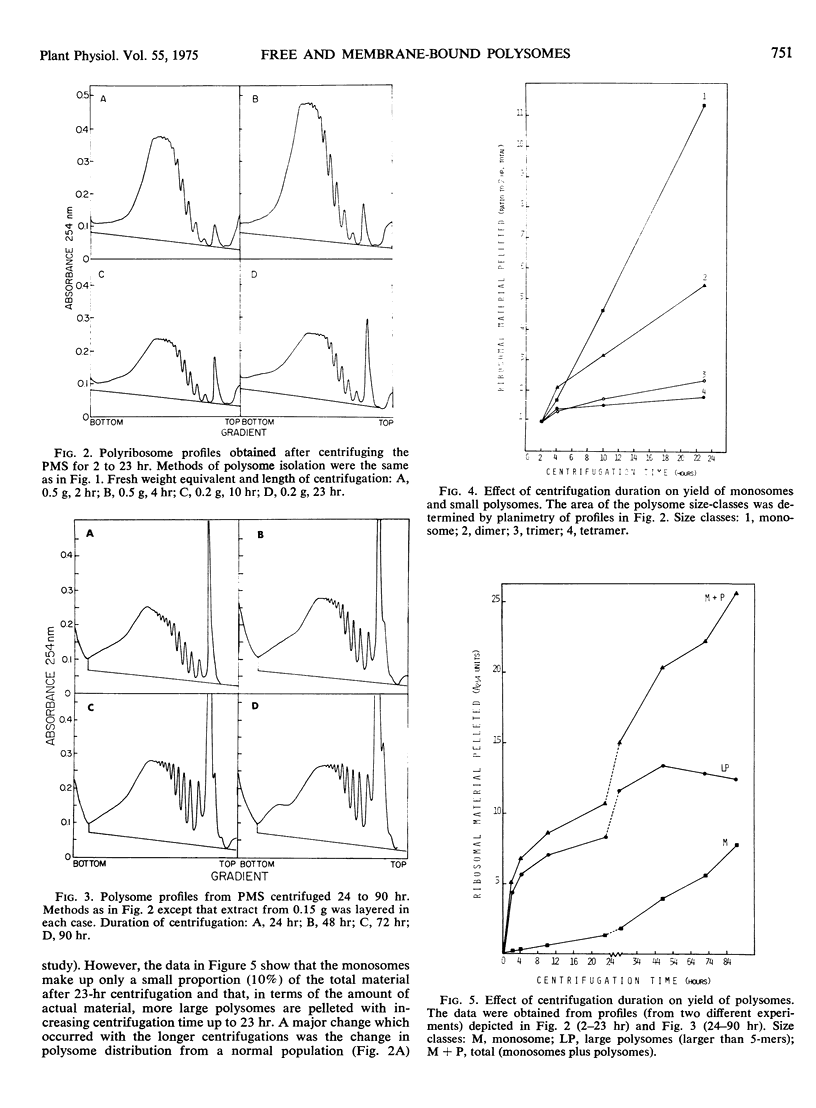
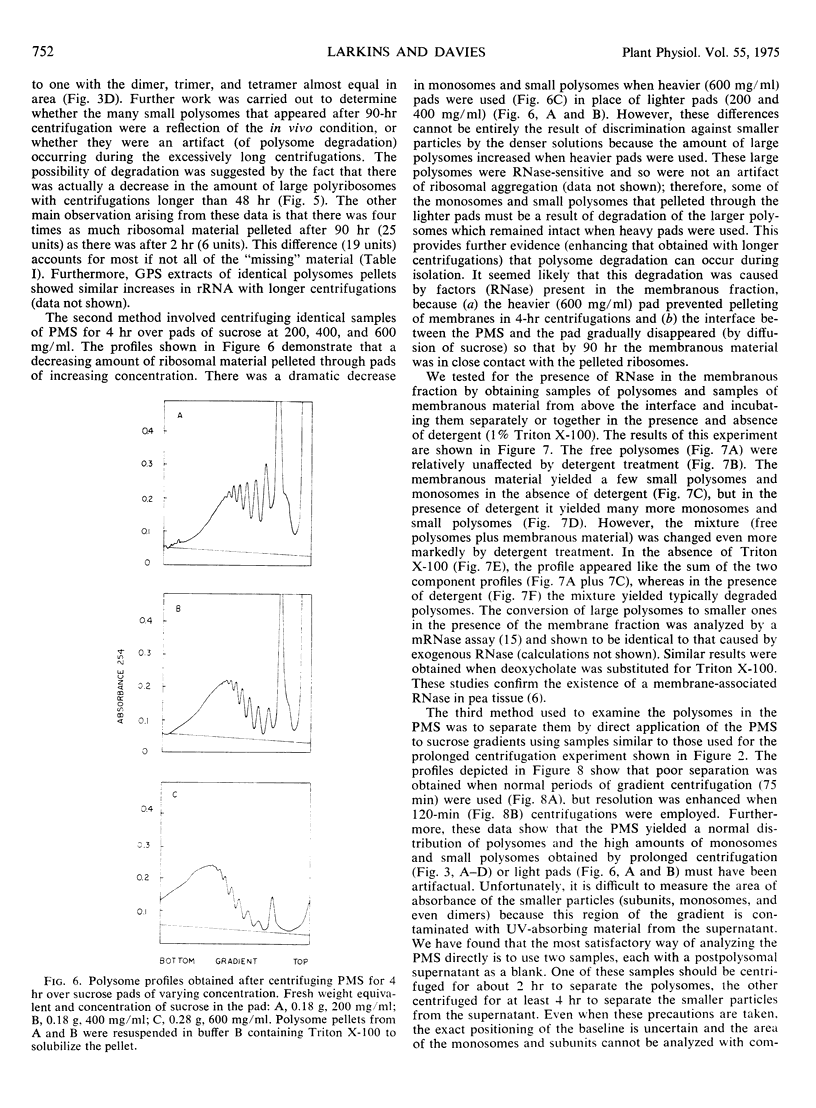
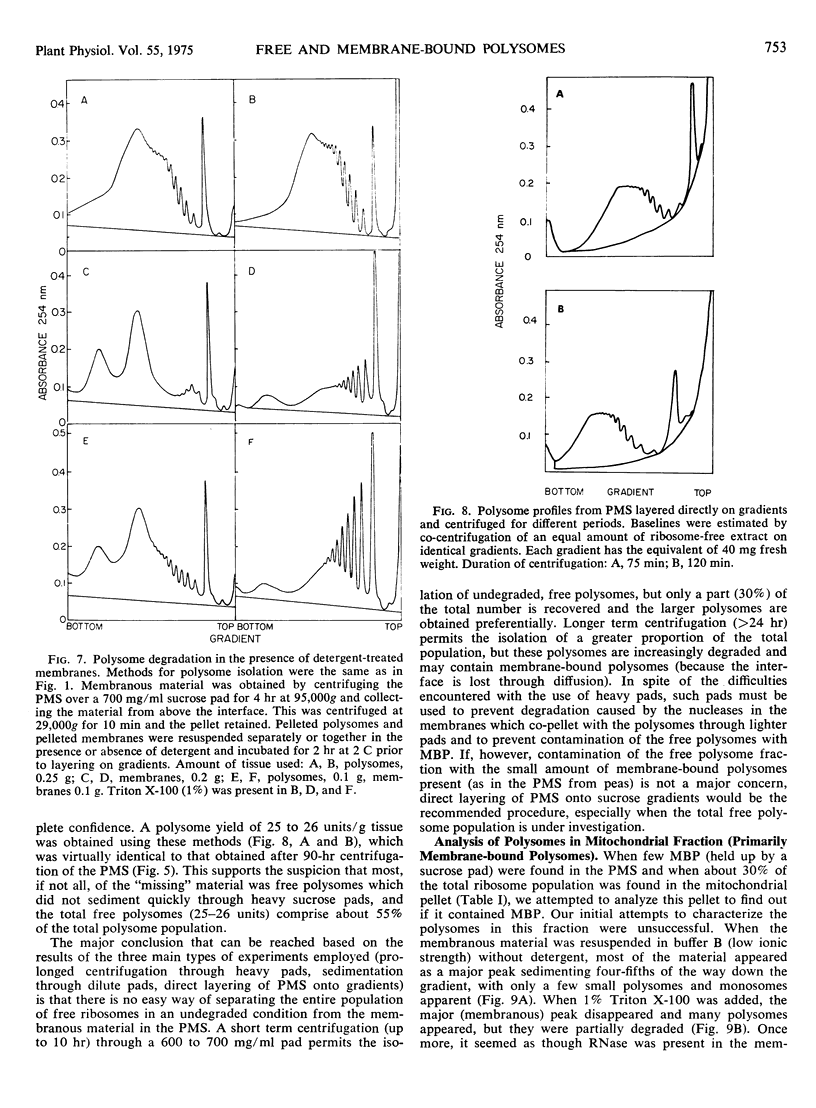
Attempts were made to isolate and characterize the total population of free and membrane-bound polysomes from the elongating region of dark-grown pea stems (Pisum sativum L.). Partial separation of free from membrane-bound polysomes was achieved by relatively low speed centrifugation of the homogenate. Complete separation was not achieved. Based on analysis of the rRNA content of various subcellular fractions, fractionated tissue yielded greater than 95% of the rRNA found in whole tissue. Approximately 45% of the ribosomal material was membrane-bound (released by detergent) and was found in the “wall” (13%), the “nuclear” pellet (2%), and the “mitochondrial” pellet (29%). The remaining 55%, consisting primarily of free polysomes, could be recovered free from membranous material by sedimentation through a dense (700 mg/ml) sucrose pad for 90 hours. The advantages and disadvantages of using sucrose pads for the separation of free and membrane-bound polysomes are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Blobel G., Sabatini D. D. An improved cell fractionation procedure for the preparation of rat liver membrane-bound ribosomes. J Cell Biol. 1973 Jan;56(1):191–205. doi: 10.1083/jcb.56.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. M., Tata J. R. Protein synthesis by membrane-bound and free ribosomes of secretory and non-secretory tissues. Biochem J. 1971 Feb;121(4):683–694. doi: 10.1042/bj1210683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnstein H. R., Cox R. A., Gould H., Potter H. A comparison of methods for the isolation and fractionation of reticulocyte ribosomes. Biochem J. 1965 Aug;96(2):500–506. doi: 10.1042/bj0960500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., BENEDETTI E. L. PREPARATION OF RAT-LIVER POLYSOMES WITHOUT THE UTILIZATION OF DETERGENT. Biochim Biophys Acta. 1964 May 18;87:177–180. doi: 10.1016/0926-6550(64)90064-7. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Bleiberg I., Zauderer M. Assembly of membrane-bound polyribosomes. Nat New Biol. 1971 Jul 7;232(27):8–12. doi: 10.1038/newbio232008a0. [DOI] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Maclachlan G. A. Generation and suppression of microsomal ribonuclease activity after treatments with auxin and cytokinin. Plant Physiol. 1972 Mar;49(3):371–375. doi: 10.1104/pp.49.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Bont W. S., Geels J., Huizinga A., Mekkelholt K., Emmelot P. The influence of the physical state of microsomal membranes from rat liver on translational processes of polyribosomes. Biochim Biophys Acta. 1972 Apr 12;262(4):514–524. doi: 10.1016/0005-2787(72)90495-9. [DOI] [PubMed] [Google Scholar]
- Brakke M. K. Slowly sedimenting infectious entities of southern bean mosaic virus. Virology. 1972 Dec;50(3):669–680. doi: 10.1016/0042-6822(72)90421-7. [DOI] [PubMed] [Google Scholar]
- Breen M. D., Whitehead E. I., Kenefick D. G. Requirement for extraction of polyribosomes from barley tissue. Plant Physiol. 1972 May;49(5):733–739. doi: 10.1104/pp.49.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Hackett D. P. The isolation of ribonucleic acid from plant, bacterial or animal cells. Biochim Biophys Acta. 1966 Oct 24;129(1):74–84. doi: 10.1016/0005-2787(66)90010-4. [DOI] [PubMed] [Google Scholar]
- DIENER T. O. Isolation of an infectious, ribonuclease-sensitive fraction from tobacco leaves recently inoculated with tobacco mosaic virus. Virology. 1962 Feb;16:140–146. doi: 10.1016/0042-6822(62)90289-1. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosomes from Peas: II. Polyribosome Metabolism during Normal and Hormone-induced Growth. Plant Physiol. 1973 Oct;52(4):339–345. doi: 10.1104/pp.52.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: III. Stimulation of Polysome Degradation by Exogenous and Endogenous Calcium. Plant Physiol. 1973 Dec;52(6):655–659. doi: 10.1104/pp.52.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L., Bracker C. E. Association of D-RNA with Polyribosomes in the Soybean Root. Plant Physiol. 1966 Jun;41(6):976–982. doi: 10.1104/pp.41.6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Marei N., Romani R. Ribosomes from fig fruits: physical properties and the occurrence of transient dimers. Biochim Biophys Acta. 1971 Oct 14;247(2):280–290. doi: 10.1016/0005-2787(71)90676-9. [DOI] [PubMed] [Google Scholar]
- O'Toole K. Contamination of free-ribosome fractions by detached previously membrane-bound ribosomes. Biochem J. 1974 Feb;138(2):305–307. doi: 10.1042/bj1380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole K., Pollak J. K. Changes in free and membrane-bound ribosomes during the development of chick liver. A new cell-fractionation approach. Biochem J. 1974 Mar;138(3):359–371. doi: 10.1042/bj1380359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E. Identification of polysomes synthesizing thyroglobulin. Eur J Biochem. 1973 Jan 15;32(2):322–330. doi: 10.1111/j.1432-1033.1973.tb02613.x. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Marcus A. Polyribosome isolation in the presence of diethyl pyrocarbonate. Plant Physiol. 1969 Sep;44(9):1291–1294. doi: 10.1104/pp.44.9.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]


