Abstract
Background:
Isolation-reared mice show social encounter-induced hyperactivity with activation of prefrontal serotonergic and dopaminergic systems, but it is not known whether this stress response is observed in other pathological conditions. Here we examined whether the social encounter stimulation induces abnormal behavior during withdrawal in chronic methamphetamine-treated mice.
Methods:
To induce methamphetamine-induced behavioral sensitization, male mice were injected with methamphetamine (1 mg/kg) once daily for 7 days.
Results:
The encounter with an intruder elicited hyperactivity 24 h after the last injection of methamphetamine in methamphetamine-sensitized mice. This response was observed even as long as 2 weeks after withdrawal of methamphetamine. The encounter increased c-Fos expression in the prefrontal cortex, dorsal raphe nucleus and ventral tegmental area in methamphetamine-sensitized mice, while it did not in control mice. Furthermore, the encounter increased extracellular serotonin (5-HT) and dopamine, but not noradrenaline, levels in the prefrontal cortex in methamphetamine-sensitized mice. Local injection of 5,7-dihydroxytryptamine and 6-hydroxydopamine into the prefrontal cortex attenuated encounter-induced hyperactivity in methamphetamine-sensitized mice and it markedly decreased prefrontal 5-HT and dopamine levels, respectively. Pharmacological analysis showed that the encounter-induced hyperactivity is mediated by dopamine D1 receptors and 5-HT2A receptors and attenuated by anxiolytics and antidepressants such as diazepam, osemozotan and selective 5-HT reuptake inhibitors. The effect of paroxetine was blocked by the 5-HT3 receptor antagonist azasetron.
Conclusions:
The present study shows that psychological stress elicits hyperactivity with activation of prefrontal 5-HT and dopamine systems in methamphetamine-dependent mice and suggests that the abnormal behavior is associated with anxiety and depression.
Keywords: encounter, hyperactivity, methamphetamine, serotonin, dopamine
Significance Statement
Methamphetamine (METH) is a psychostimulant, and its prolonged use results in dependence or psychosis similar to paranoid schizophrenia. METH withdrawal causes depression and stress-induced anxiety, but the neurochemical mechanism is not fully understood. To study the roles of psychological stress in the expression of abnormal behaviors, we designed an encounter with an intruder to occur through a mesh partition, which avoids physical stress. The present study demonstrates that social encounter stimulation elicits hyperactivity during withdrawal in METH-sensitized mice. Furthermore, we found that the hyperactivity is accompanied by an activation of prefrontal serotonergic and dopaminergic systems and is attenuated by anxiolytic and antidepressant drugs. Our findings imply that encounter-induced behavioral alteration is useful as a novel approach to investigate pharmacotherapy for the symptoms of METH withdrawal.
Introduction
Aggressive behavior and impaired social interactions are observed in rodent models of psychiatric disorders such as schizophrenia (Sams-Dodd, 1999; Jones et al., 2011; Ago et al., 2014). The response to an encounter with an intruder, a psychological stress, is an example of these abnormal behaviors, and it often involves aggressive contact (i.e., biting attacks, wrestling, and lateral threats) between 2 rodents. To study the involvement of psychological stress in the expression of abnormal behaviors, we designed an encounter stimulation to occur through a mesh partition, which avoids physical stress. We found that the psychological stress elicits a restless and hyperexcitable state (hyperactivity) in isolation-reared mice, a model of psychiatric disorders, and it specifically activates prefrontal dopaminergic and serotonergic systems (Ago et al., 2013; Araki et al., 2014). Furthermore, we showed that the hyperactivity is attenuated by the anxiolytic diazepam and several antidepressants (Ago et al., 2013; Hasebe et al., 2015). The pharmacological profile of encounter-induced hyperactivity in isolation-reared mice suggests that the hyperactivity reflects an anxiety- and depression-like state. It is not known whether the encounter-induced abnormal behavior is observed in other rodent models of psychiatric disorders.
Methamphetamine (METH) is a psychostimulant, and its prolonged use results in dependence or psychosis similar to paranoid schizophrenia (Pierce and Kalivas, 1997). In rodents, chronic METH administration causes behavioral sensitization, a long-lasting augmented locomotor response (Robinson and Becker, 1986; Kalivas and Stewart, 1991; Ago et al., 2008). This behavioral sensitization is used as an animal model of METH dependence and psychosis, and the mesocorticolimbic dopamine and serotonin (5-HT) neurons play key roles as the neural substrates (Vanderschuren and Kalivas, 2000; Nestler, 2001; Ago et al., 2008). METH withdrawal causes sedation, depression, and stress-induced anxiety (London et al., 2004; Mancino et al., 2011; Shen et al., 2013; Hellem, 2016), and central dopamine and 5-HT systems as well as γ-aminobutyric acid (GABA) and glutamate systems play an important role in anxiety disorders (Kent et al., 2002; Nikolaus et al., 2010) and psychostimulant withdrawal and relapse (Ago et al., 2008; Collo et al., 2014; Neisewander et al., 2014; Filip et al., 2015; Li et al., 2015). These observations suggest that METH-dependent rodents should show abnormal behavior in response to encounter stimulation, a psychological stress. In contrast, Janetsian et al. (2015) reported that deficits in social interaction were not observed during withdrawal in chronic METH-treated mice. There is little information on the effect of psychological stress on behavior in psychostimulant-dependent rodents. The present study demonstrates that psychological stress elicits hyperactivity during withdrawal in METH-sensitized mice. Furthermore, we found that the prefrontal serotonergic and dopaminergic systems play a key role in the encounter-induced hyperactivity in METH-sensitized mice.
Materials and Methods
Animals and Drugs
All animal studies were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Osaka University. All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996). Every effort was made to minimize animal suffering and to reduce the number of animals used. Seven-week-old male ddY mice were obtained from SHIMIZU Laboratory Supplies Co., Ltd. (Kyoto, Japan) and housed in cages (28 cm × 17 cm × 12 cm) in groups of 5 or 6 animals under controlled environmental conditions (22 ± 1°C; 50 ± 10% relative humidity; 12-hour-ligh/-dark cycle, lights on at 8:00 am; food and water ad libitum) for at least 1 week before use in the experiments. The following drugs were used: methamphetamine hydrochloride (METH; Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan), MDL100907, desipramine hydrochloride, diazepam, escitalopram oxalate, fluvoxamine maleate, paroxetine maleate, ritanserin, 5,7-dihydroxytryptamine (5,7-DHT) creatinine sulfate, 6-hydroxydopamine hydrobromide (6-OHDA) (Sigma, St. Louis, MO), GR125487 sulfamate, SB399885 hydrochloride, SB269970 hydrochloride, RS102221 hydrochloride hydrate, SCH39166 hydrobromide, raclopride, SR57227 hydrochloride (Tocris Bioscience, Bristol, UK), azasetron hydrochloride, osemozotan, WAY100635 (Mitsubishi Tanabe Pharma Corp., Yokohama, Japan).
METH, azasetron, desipramine, escitalopram, fluvoxamine, GR125487, paroxetine, SB399885, and WAY100635 were dissolved in saline (0.9% wt/v NaCl solution). Diazepam, osemozotan, and ritanserin were suspended in 0.5% wt/v carboxymethylcellulose. MDL100907, raclopride, and SCH39166 were dissolved in saline containing <0.01% v/v dimethyl sulfoxide. RS102221 was first dissolved in sterile distilled water containing 0.1 M HCl, and the pH was adjusted to 7.4 using 0.1 M NaOH. 5,7-DHT and 6-OHDA were dissolved in Ringer’s solution (147.2 mM NaCl, 4.0 mM KCl, and 2.2 mM CaCl2 [pH 6.0]; Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) containing 0.1% w/v ascorbic acid. All drugs except 5,7-DHT and 6-OHDA were administered i.p. at a volume of 10 mL/kg body weight. In this study, a single dose of most of the drugs was used. Thus, the dosage and timing of drugs used here were determined referring to our and others’ previous studies where the drugs affected behaviors and/or neurotransmitter release (Moser et al., 1996; Ago et al., 2006a, 2006b, 2007, 2011b, 2013, 2015; Collins et al., 2010; Jones et al., 2010; Hiramatsu et al., 2013; Bétry et al., 2015; Hasebe et al., 2015). To induce METH-induced behavioral sensitization, mice were injected with METH (1 mg/kg) once daily for 7 days, as previously described (Ago et al., 2006b, 2007, 2012). Mice withdrawn for 7 days after chronic METH or saline treatment were used for the experiments unless otherwise stated.
Social Encounter Stimulation and Behavioral Analysis
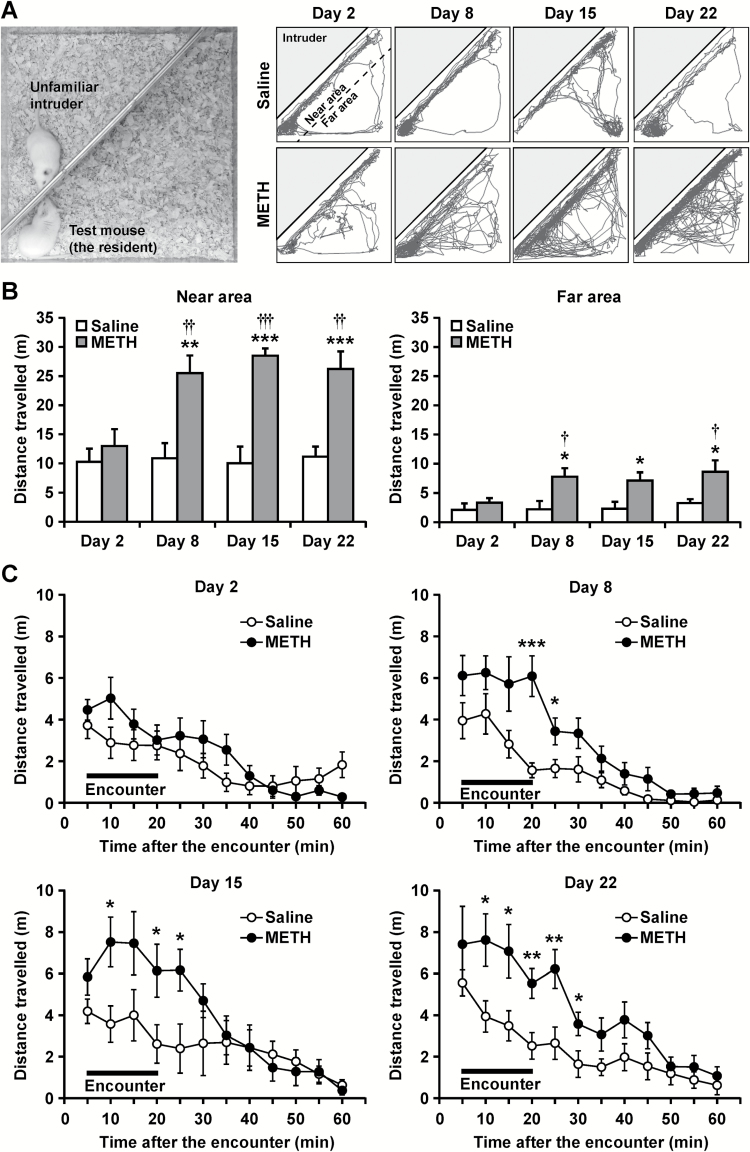
The social encounter stimulations and behavioral analyses were performed as previously described (Ago et al., 2013; Araki et al., 2014; Hasebe et al., 2015). A METH- or saline-pretreated male mouse was placed in the large compartment of a novel clear Plexiglas cuboid cage (30 × 30 × 35 cm), which was divided into 2 compartments by a mesh partition (Figure 1A). This allowed the animal to see, hear, and smell, but not physically contact, the neighbor. After a 3-hour habituation period, a METH- or saline-pretreated male mouse (the resident) was injected with the drug or vehicle. An unfamiliar age-matched male ddY mouse was introduced into the unoccupied smaller compartment as an intruder 30 minutes after the drug administration. The resident and intruder mice were allowed to interact through the partition for 20 minutes before the intruder mouse was removed. The behavior of the resident mouse during the 20-minute encounter was videotaped, and its locomotor path and distance travelled in the large compartment were automatically analyzed offline using the ANY-maze video tracking software (Stoelting Company, Wood Dale, IL). The locomotor activity of the resident mouse in areas near and far from the partition was analyzed (Figure 1B), and the distance travelled near the partition was used as an index of the encounter response.
Figure 1.
Effects of social encounter stimulation on the behavior of mice. (A) Experimental apparatus and representative locomotor paths of resident methamphetamine (METH)- or saline-treated mice during the 20-minute encounters. At 24 hours after a single administration of METH (1 mg/kg) or saline, each mouse was subjected to the encounter with the intruder (on day 2). Then, mice were administered METH (1 mg/kg/d) or saline once daily until day 7. At 24 hours and 8 and 15 days after the last METH injection, each mouse was subjected to the encounter with the intruder (on days 8, 15, and 22, respectively). (B) Total distance travelled during the 20-minute encounters in areas either near or far from the partition was analyzed. Results are expressed as the mean ± SEM of 12 mice/group. *P < .05, **P < .01, ***P < .001, compared with the saline-treated mice on each day. †P < .05, ††P < .01, †††P < .001, compared with the METH-treated mice on day 2. (C) Time-course of the distance travelled in the area near the partition during and after the 20-minute encounters was shown. Results are expressed as the mean ± SEM of 12 mice/group. *P < .05, **P < .01, ***P < .001, compared with the saline-treated mice on each time point.
In Vivo Microdialysis
Microdialysis experiments were performed as previously described (Ago et al., 2011b; Hiramatsu et al., 2013; Hara et al., 2016). Briefly, each mouse was anesthetized with a mixture of medetomidine (0.3 mg/kg, i.p.), midazolam (4 mg/kg, i.p.), and butorphanol (5 mg/kg, i.p.) and stereotaxically implanted unilaterally and counterbalanced left or right with a guide-cannula for a dialysis probe (Eicom Corp., Kyoto, Japan) in the prefrontal cortex (A +1.9 mm, L +/−0.5 mm, V −0.8 mm, from bregma and skull; Franklin and Paxinos, 1997). The cannula was cemented in place with dental acrylic, and the animal was kept warm and allowed to recover from anesthesia. Postoperative analgesia was performed with a single injection of buprenorphine (0.1 mg/kg, i.p.). Two days after surgery, the probe was perfused with Ringer’s solution at a constant flow rate of 1 µL/min. A stabilization period of 3 hours, which was identical to the 3-hour habituation period in the clear Plexiglas cage described above, was established before the onset of the experiment. Microdialysis samples (20 µL) were collected every 20 minutes and injected immediately onto a high-performance liquid chromatography column for simultaneous assaying of 5-HT, dopamine, and noradrenaline (Ago et al., 2011b; Hiramatsu et al., 2013; Hara et al., 2016).
5,7-DHT and 6-OHDA Treatment
Intracerebral drug administration was performed as previously described (Ago et al., 2011a). Twenty-four hours after repeated METH administration, mice were pretreated with desipramine (15 mg/kg, i.p.) 30 minutes prior to surgery to prevent the destruction of noradrenergic neurons by 5,7-DHT. For 6-OHDA infusion, mice were pretreated with desipramine (35 mg/kg, i.p.) and escitalopram (5 mg/kg, i.p.) 30 minutes prior to surgery to prevent the destruction of noradrenergic and serotonergic neurons (Bergamini et al., 2016). Mice were subsequently anesthetized with a mixture of medetomidine, midazolam, and butorphanol. Either 5,7-DHT or 6-OHDA was delivered bilaterally into the prefrontal cortex (stereotaxic coordinates: A +1.9 mm, L ±0.5 mm, V –2.5 mm, from bregma and skull; Franklin and Paxinos, 1997) using a 28-gauge stainless-steel injector connected to a microsyringe pump. A volume of 1 μL of 5,7-DHT (6 μg/μL) or 6-OHDA (1.5 μg/μL) was infused over a period of 10 minutes on each side. After infusion, the cannula was left in place for a further 5 minutes to allow diffusion of the neurotoxin and to avoid backflow of the solution up the injection path. Sham-operated mice underwent the same surgical procedure and received an equal volume of vehicle solution. Mice received an injection of buprenorphine (0.1 mg/kg, i.p.) and gentamicin (10 mg/kg, i.p.) and recovered within a few hours after the surgery. The mice were used for experiments 1 week after the surgery. Tissue levels of 5-HT, dopamine, and noradrenaline in the prefrontal cortex and striatum were determined as described previously (Kawasaki et al., 2006; Horiguchi et al., 2013; Ota et al., 2015).
c-Fos Immunohistochemistry
Brain expression of the neuronal activity marker c-Fos was determined 2 hours after the 20-minute encounter with an intruder. Control mice were placed into a chamber with no neighbor. c-Fos immunostaining was performed as described previously (Ago et al., 2013, 2015). Each mouse was deeply anesthetized with isoflurane and perfused transcardially with saline, followed by a solution of 4% paraformaldehyde in phosphate-buffered saline (PBS). Serial 20-µm-thick coronal sections were cut using a cryostat microtome at −20°C. Free-floating sections were preincubated for 30 minutes in 0.3% hydrogen peroxide in PBS and then blocked in 1.5% goat serum in PBS for 20 minutes at room temperature. Thereafter, the sections were incubated with anti-c-Fos rabbit polyclonal primary antibodies (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at room temperature. Subsequently, the sections were washed in PBS and incubated with a secondary antibody solution containing biotinylated anti-rabbit IgG (1:500 dilution; Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature. The sections were then incubated with avidin-biotin-horseradish peroxidase complex (Vectastain ABC kit; Vector Laboratories) for 30 minutes at room temperature. Brown cytosolic products were obtained by reacting with 3,3ʹ-diaminobenzidine (Sigma, St. Louis, MO). Four independent sections per animal containing the prefrontal cortex, striatum, nucleus accumbens, dorsal raphe nucleus, ventral tegmental area, locus coeruleus, paraventricular hypothalamic nucleus, or central or basolateral nucleus of the amygdala were selected. c-Fos-positive cells were counted manually by experienced observers blinded to treatment and encounter conditions under bright-field illumination on an Axio Imager.M2 microscope (Carl Zeiss, Jena, Germany). The number of c-Fos-positive cells in each section was determined in a 500-μm×500-µm area in the left and right hemispheres and averaged using the ImageJ 1.41 software package (NIH, Bethesda, MD). The average of this average across 4 sections was then calculated for each subject.
Statistical Analysis
All results are presented as the mean ± SEM. For the microdialysis experiment, data were calculated as percentage change from baseline dialysate concentrations, with 100% defined as the average of three measurements before the encounter. Data for the time-course of the encounter responses (Figure 1) and microdialysis (Figure 2) were analyzed using 2-way ANOVA with treatment as the intersubject factor and repeated measures with time as the intrasubject factor, followed by the Bonferroni/Dunn posthoc test. For the other experiments, data were analyzed using 2-way ANOVA with the treatment and/or encounter as the independent variables, followed by the Bonferroni/Dunn posthoc test. Statistical analyses were performed using the software package Statview 5.0J for Apple Macintosh (SAS Institute Inc., Cary, NC). Values of P < .05 were considered statistically significant.
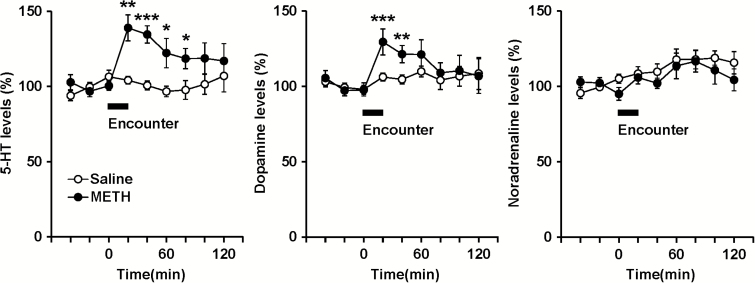
Figure 2.
Effects of social encounter stimulation on extracellular monoamine levels of mice. Mice were administered methamphetamine (METH; 1 mg/kg) or saline repeatedly for 7 days. At 7 days after the withdrawal period, each mouse was subjected to the 20-minute encounter with the intruder, as indicated by the horizontal bar. Results are expressed as the mean ± SEM of 8 mice/group. *P < .05, **P < .01, ***P < .001, compared with saline-pretreated mice at each time point.
Results
Effects of Social Encounter Stimulation on the Behavior of METH-Sensitized Mice
Figure 1 shows the effects of social encounter stimulation on the behavior of chronic METH-treated (METH-sensitized) and saline-treated (control) mice. Spontaneous locomotor activity in the cage decreased gradually during the 3-hour habituation period, and there was no difference in activity between chronic METH- and saline-treated mice during this time (data not shown). Figure 1A shows the representative locomotor paths of resident METH- and saline-treated mice during the 20-minute encounters. Both groups of mice moved around in their area, especially near the partition, and interacted with the intruder over the partition, suggesting behavioral reactivity to the intruder. At 24 hours after a single administration of METH (1 mg/kg) or saline (on day 2), locomotor activities of METH-treated mice in near or far area did not differ from those of saline-treated mice (Figure 1B). At 24 hours after the repeated administration of METH (1 mg/kg/d, once daily for 7 days) (on day 8), locomotor activities of METH-sensitized mice in the area near the partition were significantly greater than those of saline-treated control mice, suggesting hyperactivity in response to the intruder. This encounter-induced hyperactivity in the near area was also observed after a 7-day or 14-day withdrawal period (on days 15 and 22, respectively). Repeated measures 2-way ANOVA revealed significant main effects for METH treatment (F1,22 = 39.0, P < .0001) and time (F3,66 = 4.4, P < .01), and there was a significant interaction between treatment and time (F3,66 = 4.1, P < .01). The temporal pattern of locomotor activity of METH-sensitized mice in the area far from the partition was similar to that in the near area, but repeated-measures 2-way ANOVA indicated no significant interaction between treatment and time (F3,66 = 1.2, P > .05). Because of this small difference between areas, we used the distance travelled near the partition as an index of the encounter-induced behavioral response for the subsequent analyses. Figure 1C shows the time-course of the locomotor response in the area near the partition during and after the 20-minute encounters. Enhanced locomotor activity in METH-sensitized mice was still observed for several minutes following the cessation of the encounter on day 8 (interaction: F11,242 = 2.7, P < .01), 15 (interaction: F11,242 = 3.3, P < .001), and 22 (treatment: F1,22 = 14.8, P < .001).
Effects of Social Encounter Stimulation on Extracellular Monoamine Levels in the Prefrontal Cortex and Regional Neuronal Activity in the Brain
Figure 2 shows the effects of social encounter stimulation on extracellular levels of monoamines in the prefrontal cortex of mice. Baseline levels of extracellular 5-HT, dopamine and noradrenaline (not corrected for in vitro probe recovery) in the prefrontal cortex did not differ significantly between chronic METH- and saline-treated mice (analyzed using Student’s t test). The 5-HT levels (mean ± SEM) were 1.14 ± 0.22 (METH, n = 8) and 1.01 ± 0.17 (saline, n = 8) pg/20 μL. The dopamine levels were 0.68 ± 0.10 (METH) and 0.51 ± 0.09 (saline) pg/20 μL. The noradrenaline levels were 0.80 ± 0.24 (METH) and 0.78 ± 0.05 (saline) pg/20 μL. Encounter stimulation caused significant increases in extracellular 5-HT and dopamine levels, but not noradrenaline levels, in the prefrontal cortex of chronic METH-treated mice. In contrast, the stimulation did not affect extracellular monoamine levels in the prefrontal cortex of saline-treated mice (interaction between time and treatment: F8,112 = 3.0, P < .01 for 5-HT; F8,112 = 2.2, P < .05 for dopamine; F8,112 = 0.7, P > .05 for noradrenaline).
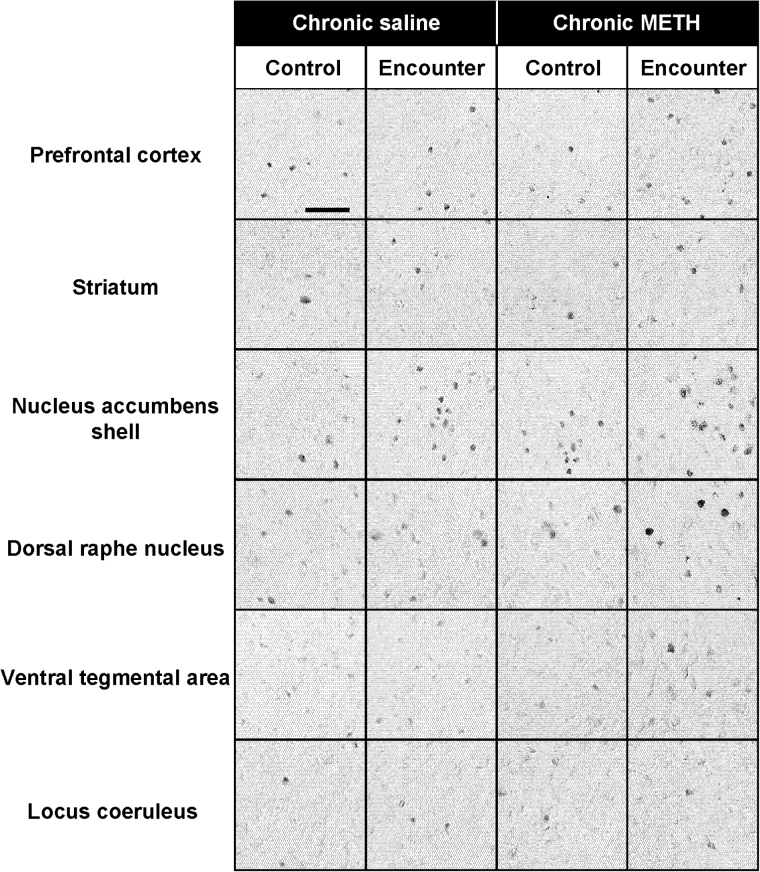
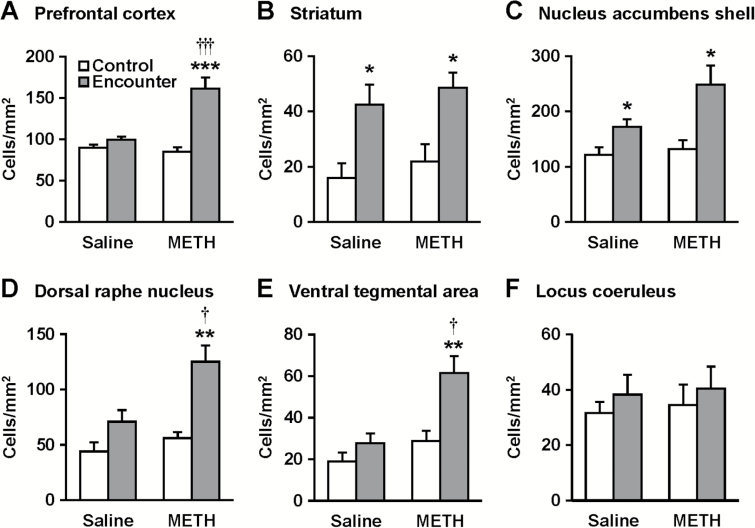
Expression of the neuronal activity marker c-Fos was determined 2 hours after the social encounters in the prefrontal cortex, striatum, nucleus accumbens, dorsal raphe nucleus, ventral tegmental area, and locus coeruleus of mice (Figures 3 and 4). Representative photomicrographs of c-Fos-positive cells are shown in Figure 3. Encounter stimulation caused a significant increase in the number of c-Fos-positive nuclei in the prefrontal cortex (main effect of the encounter: F1,36 = 30.4, P < .0001; interaction between the encounter and treatment: F1,36 = 18.1, P < .001), dorsal raphe nucleus (main effect: F1,36 = 21.7, P < .0001; interaction: F1,36 = 4.2, P < .05), and ventral tegmental area (main effect: F1,36 = 13.2, P < .001; interaction: F1,36 = 4.4, P < .05) of chronic METH-treated mice, but not saline-treated mice (Figure 4). Encounter stimulation increased c-Fos expression in the striatum (main effect of the encounter: F1,36 = 9.3, P < .01) and nucleus accumbens shell (main effect: F1,36 = 15.8, P < .001) of both chronic METH- and saline-treated mice, but there was no significant interaction between the encounter and METH treatment. Encounter stimulation did not affect c-Fos expression in the locus coeruleus (Figure 4F), nucleus accumbens core, paraventricular hypothalamic nucleus, or the central or basolateral nucleus of the amygdala (data not shown).
Figure 3.
Representative photomicrographs showing c-Fos-immunoreactive cells after encounter with an intruder in the brain of resident test mice. Mice that were not exposed to an intruder were used as controls (Control). Scale bar = 50 μm.
Figure 4.
Effects of social encounter stimulation on c-Fos expression in the prefrontal cortex (A), striatum (B), nucleus accumbens shell (C), dorsal raphe nucleus (D), ventral tegmental area (E), and locus coeruleus (F) of mice. Immunohistochemical localization of the neuronal activity marker c-Fos in the chronic methamphetamine (METH)- and saline-pretreated mice was determined 2 hours after the 20-minute encounter. Mice that were not exposed to an intruder were used as controls. Results are expressed as the mean ± SEM of 10 mice/group. *P < .05, **P < .01, ***P < .001, compared with the control in each group. †P < .05, †††P < .001, compared with saline-pretreated mice.
Effects of Prefrontal 5-HT and Dopamine Depletion on Encounter-Induced Hyperactivity in METH-Sensitized Mice
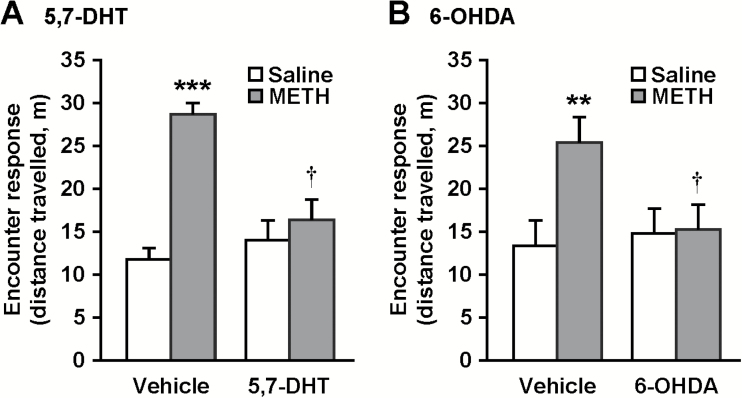
To clarify the roles of the prefrontal serotonergic and dopaminergic systems, we examined the effects of bilateral microinjection of either the serotonergic toxin 5,7-DHT, or dopaminergic toxin 6-OHDA into the prefrontal cortex on encounter-induced hyperactivity in METH-sensitized mice (Figure 5). Local injection of 5,7-DHT (6 μg/side) induced marked reductions in concentrations of 5-HT, but not dopamine or noradrenaline, in the prefrontal cortex of both chronic METH- and saline-treated mice (Table 1). This treatment did not affect the monoamine levels in the striatum (Table 1). Prefrontal 5-HT depletion by 5,7-DHT abrogated encounter-induced hyperactivity in chronic METH-treated mice (Figure 5A) (interaction between 5,7-DHT and METH treatments: F1,44 = 7.1, P < .05), while it did not affect the response to the encounter in saline-treated mice. Local injection of 6-OHDA (1.5 μg/side) induced marked reductions in concentrations of dopamine, but not 5-HT or noradrenaline, in the prefrontal cortex of both chronic METH- and saline-treated mice (Table 2). This treatment did not affect the monoamine levels in the striatum (Table 2). Prefrontal dopamine depletion by 6-OHDA abrogated encounter-induced hyperactivity in chronic METH-treated mice (Figure 5B) (interaction between 6-OHDA and METH treatments: F1,44 = 4.4, P < .05), while it did not affect the response to the encounter in saline-treated mice.
Figure 5.
Effects of local injection of either 5,7-DHT or 6-OHDA into the prefrontal cortex on encounter-induced hyperactivity in methamphetamine (METH)-sensitized mice. Twenty-four hours after repeated METH or saline administration, mice received bilateral microinjection of 5,7-DHT (6 μg/side) (A), 6-OHDA (1.5 μg/side) (B), or vehicle into the prefrontal cortex. At 7 days after surgery, each mouse was subjected to the encounter with an intruder. Total distance travelled in the area near the partition during the 20-minute encounters was analyzed as an index of the encounter response. Results are expressed as the mean ± SEM of 12 mice/group. **P < .01, ***P < .001, compared with vehicle-injected saline-pretreated mice. †P < .05, compared with vehicle-injected mice in each group.
Table 1.
Effects of 5,7-DHT on Contents of Monoamines in the Prefrontal Cortex and Striatum of Mice
| Chronic Saline | Chronic METH | |||
|---|---|---|---|---|
| Vehicle | 5,7-DHT | Vehicle | 5,7-DHT | |
| Prefrontal cortex | ||||
| 5-HT | 681 ± 62 | 75 ± 16*** | 759 ± 99 | 100 ± 39*** |
| Dopamine | 33 ± 7 | 29 ± 4 | 37 ± 2 | 28 ± 3 |
| Noradrenaline | 597 ± 38 | 490 ± 48 | 665 ± 49 | 515 ± 66 |
| Striatum | ||||
| 5-HT | 396 ± 46 | 508 ± 103 | 342 ± 56 | 415 ± 59 |
| Dopamine | 8782 ± 1412 | 10871 ± 1625 | 9927 ± 1753 | 10260 ± 1924 |
| Noradrenaline | 76 ± 7 | 95 ± 17 | 86 ± 7 | 88 ± 6 |
Results are expressed as the mean ± SEM of 11–12 mice/group. Values are expressed as ng/g tissue (wet weight). ***P < .001, compared with vehicle-treated mice.
Table 2.
Effects of 6-OHDA on Contents of Monoamines in the Prefrontal Cortex and Striatum of Mice
| Chronic Saline | Chronic METH | |||
|---|---|---|---|---|
| Vehicle | 6-OHDA | Vehicle | 6-OHDA | |
| Prefrontal Cortex | ||||
| 5-HT | 706 ± 69 | 691 ± 69 | 699 ± 41 | 681 ± 76 |
| Dopamine | 52 ± 7 | 16 ± 2*** | 46 ± 5 | 16 ± 2*** |
| Noradrenaline | 397 ± 39 | 347 ± 40 | 417 ± 38 | 367 ± 41 |
| Striatum | ||||
| 5-HT | 369 ± 31 | 375 ± 22 | 354 ± 27 | 335 ± 12 |
| Dopamine | 13445 ± 638 | 12401 ± 783 | 12892 ± 1049 | 12992 ± 902 |
| Noradrenaline | 80 ± 8 | 72 ± 7 | 79 ± 8 | 72 ± 8 |
Results are expressed as the mean ± SEM of 12 mice/group. Values are expressed as ng/g tissue (wet weight). ***P < .001, compared with vehicle-treated mice.
Involvement of 5-HT and Dopamine Receptors in Encounter-Induced Hyperactivity in METH-Sensitized Mice
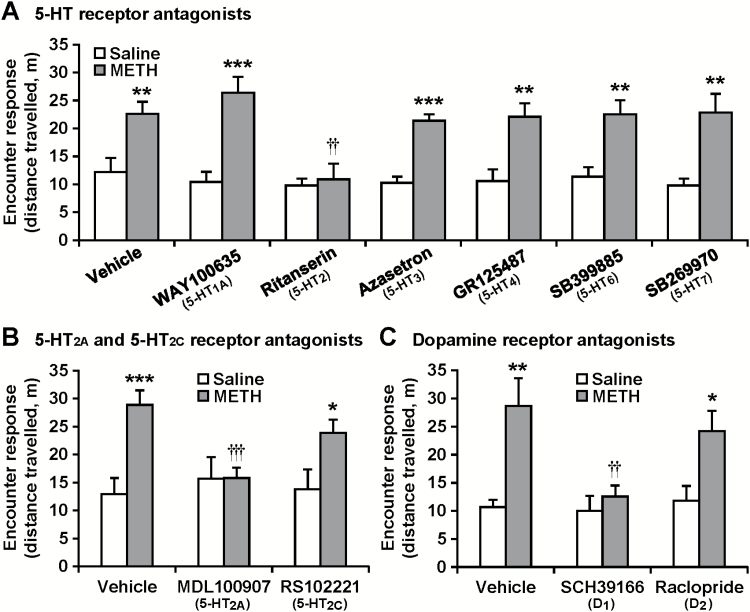
To investigate which subtypes of 5-HT and/or dopamine receptors are involved in the encounter-induced hyperactivity, we examined the effects of antagonists of various 5-HT or dopamine receptor subtypes on the encounter-induced hyperactivity in METH-sensitized mice (Figure 6). Ritanserin (3 mg/kg), a nonselective 5-HT2 receptor antagonist, blocked the encounter-induced hyperactivity in chronic METH-treated mice (interaction between the METH and antagonist treatments: F6,154 = 2.2, P < .05) (Figure 6A). None of the other 5-HT receptor antagonists, 5-HT1A receptor antagonist (WAY100635, 1 mg/kg), 5-HT3 receptor antagonist (azasetron, 3 mg/kg), 5-HT4 receptor antagonist (GR125487, 3 mg/kg), 5-HT6 receptor antagonist (SB399885, 3 mg/kg) or 5-HT7 receptor antagonist (SB269970, 1 mg/kg) affected the hyperactivity. The 5-HT2A receptor antagonist MDL100907 (1 mg/kg), but not the 5-HT2C receptor antagonist RS102221 (1 mg/kg), inhibited the encounter-induced hyperactivity in chronic METH-treated mice (F2,66 = 3.7, P < .05) (Figure 6B). Furthermore, the dopamine-D1/5 receptor antagonist SCH39166 (0.2 mg/kg), but not the dopamine-D2/3 receptor antagonist raclopride (0.1 mg/kg), inhibited the encounter-induced hyperactivity in chronic METH-treated mice (F2,66 = 3.2, P < .05) (Figure 6C).
Figure 6.
Effects of serotonin (5-HT) and dopamine receptor antagonists on encounter-induced hyperactivity in methamphetamine (METH)-sensitized mice. The resident chronic METH- or saline-pretreated mice were injected (i.p.) with the 5-HT receptor subtype antagonists WAY100635 (1 mg/kg), ritanserin (3 mg/kg), azasetron (3 mg/kg), GR125487 (3 mg/kg), SB399885 (3 mg/kg), or SB269970 (1 mg/kg) (A), MDL100907 (1 mg/kg) or RS102221 (1 mg/kg) (B), the dopamine receptor antagonists SCH39166 (0.2 mg/kg) or raclopride (0.1 mg/kg) (C), or vehicle 30 minutes before the encounter with the intruder. Total distance travelled in the area near the partition during the 20-minute encounters was analyzed as an index of the encounter response. Results are expressed as the mean ± SEM of 12 mice/group. *P < .05, **P < .01, ***P < .001, compared with saline-pretreated mice in each antagonist-treated group. ††P < .01, †††P < .001, compared with vehicle-injected METH-pretreated mice.
Effects of Anxiolytics and Antidepressants on Encounter-Induced Hyperactivity in METH-Sensitized Mice
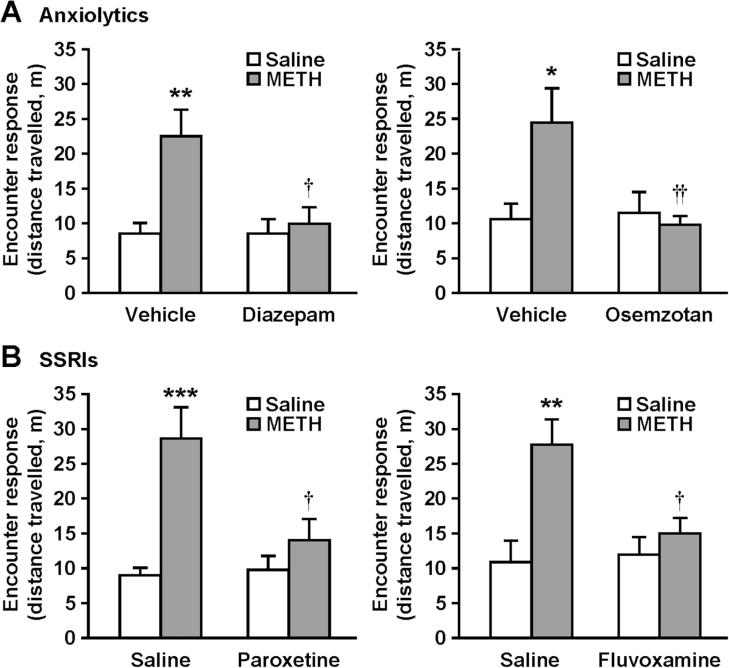
We previously found that encounter-induced hyperactivity in isolation-reared mice is attenuated by the anxiolytic diazepam and several antidepressants (Ago et al., 2013; Hasebe et al., 2015). In addition, the 5-HT1A receptor agonist osemozotan, which shows anxiolytic activities in rodent models (Sakaue et al., 2001, 2003; Matsuda, 2013; Ago et al., 2014), also inhibited encounter-induced hyperactivity in isolation-reared mice (Ago et al., 2013). To investigate the pharmacological profile of encounter-induced hyperactivity in METH-sensitized mice, we examined the effects of the GABAA receptor agonist diazepam (1 mg/kg), the 5-HT1A receptor agonist osemozotan (0.3 mg/kg), and the selective 5-HT reuptake inhibitors (SSRIs) paroxetine (10 mg/kg) and fluvoxamine (30 mg/kg) on encounter-induced hyperactivity (Figure 7). All drugs tested abrogated the encounter-induced hyperactivity in chronic METH-treated mice (interaction between the treatments: F1,44 = 5.8, P < .05 for diazepam; F1,44 = 6.2, P < .05 for osemozotan; F1,44 = 6.8, P < .05 for paroxetine; F1,44 = 5.7, P < .05 for fluvoxamine), while they did not affect the encounter response in saline-treated mice.
Figure 7.
Effects of anxiolytic and antidepressant drugs on encounter-induced hyperactivity in methamphetamine (METH)-sensitized mice. The resident chronic METH- or saline-treated mice were i.p. injected with diazepam (1 mg/kg), osemozotan (0.3 mg/kg) (A), paroxetine (10 mg/kg), fluvoxamine (30 mg/kg) (B), or vehicle 30 minutes before the encounter with the intruder. Total distance travelled in the area near the partition during the 20-minute encounters was analyzed as an index of the encounter response. Results are expressed as the mean ± SEM of 12 mice/group. *P < .05, **P < .01, ***P < .001, compared with vehicle/saline-injected saline-pretreated mice. †P < .05, ††P < .01, compared with vehicle/saline-injected METH-pretreated mice.
Role of the 5-HT3 Receptor in the Inhibitory Effect of Paroxetine on Encounter-Induced Hyperactivity
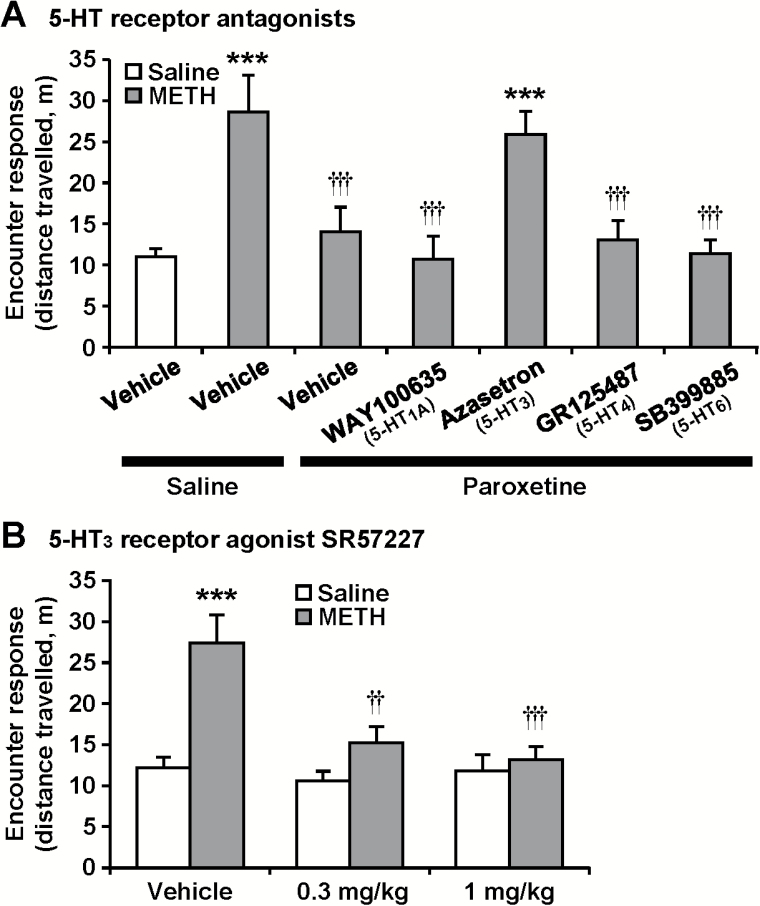
Finally, we examined which subtypes of 5-HT receptors are involved in the effect of paroxetine (Figure 8). The 5-HT3 receptor antagonist azasetron blocked the inhibitory effect of paroxetine on the encounter-induced hyperactivity in chronic METH-treated mice (F6,77 = 7.3, P < .0001), while the other 5-HT receptor antagonists used here did not impact the effect of paroxetine (Figure 8A). In support of the involvement of the 5-HT3 receptor, the 5-HT3 receptor agonist SR57227 (0.3 and 1 mg/kg) attenuated the encounter-induced hyperactivity in METH-sensitized mice (F2,66 = 6.3, P < .01) (Figure 8B).
Figure 8.
Involvement of 5-HT3 receptor activation in the effect of paroxetine on encounter-induced hyperactivity. The resident chronic methamphetamine (METH)- or saline-treated mice were i.p. injected with paroxetine (10 mg/kg) (A), SR57227 (0.3, 1 mg/kg) (B), or saline 30 minutes before the encounter with the intruder. WAY100635 (1 mg/kg), azasetron (3 mg/kg), GR125487 (3 mg/kg), SB399885 (3 mg/kg), or vehicle was i.p. injected 30 minutes before the paroxetine treatment (A). Total distance travelled in the area near the partition during the 20-minute encounters was analyzed as an index of the encounter response. Results are expressed as the mean ± SEM of 12 mice/group. ***P < .001, compared with vehicle-injected saline-pretreated mice. ††P < .01, †††P < .001, compared with vehicle/saline-injected METH-pretreated mice.
Discussion
Using a cage divided into 2 compartments by a mesh partition to prevent direct physical interactions, we have found that an encounter with an intruder elicits hyperactivity in isolation-reared mice (Ago et al., 2013). Although the exact profile of the encounter-induced response is not known, previous pharmacological studies suggested that the behavior is associated with anxiety and depression, because it is attenuated by diazepam and antidepressants (Ago et al., 2013; Hasebe et al., 2015). However, it is not known whether the encounter-induced hyperactivity is observed in other models of psychiatric disorders. As withdrawal from psychostimulant use is accompanied by symptoms of anxiety and depression in both humans and animal models (Barr and Markou, 2005; Kitanaka et al., 2008; Barr et al., 2010; Vuong et al., 2010), it is possible that mice chronically treated with psychostimulants show encounter-induced hyperactivity. Building on our previous studies on METH-sensitized mice (Ago et al., 2006b, 2007, 2012), the present study examined the effect of encounter stimulation on behavior in METH-sensitized mice. The present study demonstrated that a psychological stress elicits hyperactivity during withdrawal in chronic, but not acute, METH-treated mice. The hyperactivity was observed at both 24 hours and 2 weeks after the last injection of METH, and the time course of the hyperactivity was similar to expression of behavioral sensitization as reported previously (Ago et al., 2006b, 2007, 2012). Therefore, it appears that the encounter stimulation-induced hyperactivity observed during withdrawal in METH-sensitized mice is a phenotype leading to relapse.
Concerning the mechanism for the encounter-induced hyperactivity in METH-sensitized mice, the present study examined the effect of the encounter stimulation on prefrontal monoamine levels and c-Fos expression. The encounter increased prefrontal 5-HT and dopamine, but not noradrenaline, release. Furthermore, it increased c-Fos expression in the prefrontal cortex, dorsal raphe, and ventral tegmental area. These results suggest that prefrontal serotonergic and dopaminergic systems play a role in encounter-induced hyperactivity, as reported in isolation-reared mice (Ago et al., 2013). We also found that microinjection of either 5,7-DHT or 6-OHDA into the prefrontal cortex blocked the encounter-induced hyperactivity. Treatment with 5,7-DHT and 6-OHDA selectively decreases the 5-HT and dopamine levels, respectively, in the prefrontal cortex. This result therefore supports an important role for the prefrontal serotonergic and dopaminergic systems in encounter-induced hyperactivity. Furthermore, the hyperactivity was attenuated by the 5-HT2 receptor antagonist ritanserin, the 5-HT2A receptor antagonist MDL100907, and the dopamine-D1 receptor antagonist SCH39166, but not by other 5-HT receptor subtype antagonists and the dopamine-D2 receptor antagonist raclopride. These results suggest that 5-HT2A and dopamine-D1 receptors are involved in the hyperactivity in METH-sensitized mice. Furthermore, the observation that MDL100907 and SCH39166 show a complete blockade of the encounter-induced hyperactivity suggests that simultaneous activation of 5-HT2A and dopamine-D1 receptors plays a key role in the expression of the abnormal behavior in METH-sensitized mice. It is not known how the activation of these receptors results in the expression of the abnormal behavior in METH-sensitized mice. With respect to the effects of ritanserin, MDL100907, and osemozotan, Martin-Ruiz et al. (2001) demonstrated that 5-HT1A and 5-HT2A receptors are colocalized in cortical pyramidal neurons and they have opposing regulatory effects on prefrontal serotonergic transmission. Therefore, the possibility remains that the effects of ritanserin, MDL100907, and osemozotan are owing to inhibition of prefrontal 5-HT release.
In this study, neurotoxic lesions and any 5-HT and dopamine receptor antagonists did not alter the locomotor response to the encounter in saline-pretreated mice. The effects of 6-OHDA and 5,7-DHT microinjection into the prefrontal cortex on locomotor activity were similar to the previous studies (Li et al., 2010; Sziray et al., 2010; Wanchoo et al., 2010). Furthermore, the receptor antagonists did not affect the baseline locomotor activity, although Collins et al. (2010) reported that the dopamine-D1 receptor antagonist SCH39166 and the dopamine-D2 receptor antagonist eticlopride decreased the locomotor activity in the open-field. The difference may be due to that in the experimental conditions: the previous study was performed under no habituation to the novel environment, while the locomotor response in this study was determined after a 3-hour habituation period. The lack of the effects of 5-HT1A, 5-HT2, and 5-HT3 receptor antagonists was similar to the previous study without habituation (Tanyeri et al., 2013).
This study further pharmacologically characterized the encounter-induced hyperactivity in METH-sensitized mice. We showed that encounter-induced hyperactivity in METH-sensitized mice was attenuated by drugs with anxiolytic or antidepressant-like activity, such as diazepam, SSRIs, and the 5-HT1A receptor agonist osemozotan. Osemozotan, like buspirone, a nonbenzodiazepine anxiolytic, has an anxiolytic activity in preclinical models (Sakaue et al., 2001, 2003; Matsuda, 2013; Ago et al., 2014). These results are in agreement with previous observations in isolation-reared mice (Ago et al., 2013; Hasebe et al., 2015). Furthermore, the encounter-induced changes in prefrontal 5-HT and dopamine release and c-Fos expression are similar between METH-sensitized and isolation-reared mice (Ago et al., 2013). However, the 5-HT receptor subtype involved in the effect of the SSRIs differed between METH-sensitized and isolation-reared mice. The effect of paroxetine was blocked by a 5-HT3 receptor antagonist in METH-sensitized mice, while the effect of fluvoxamine was blocked by a 5-HT4 receptor antagonist in isolation-reared mice (Hasebe et al., 2015). Therefore, it is likely that SSRIs attenuate the hyperactivity via activation of the 5-HT3 receptor in METH-sensitized mice, in contrast to previous finding in isolation-reared mice that showed that the effect of SSRIs is mediated by the 5-HT4 receptor. This observation may reflect a difference in the neurochemical basis between the 2 models. It is possible that isolation-reared mice have higher sensitivity of the 5-HT4 receptor, while METH-sensitized mice have higher sensitivity of the 5-HT3 receptor, but the exact mechanism is not known. The 5-HT4 receptor has received considerable interest as a target for the treatment of depression (Lucas et al., 2007; Bockaert et al., 2008; Mendez-David et al., 2014). Conversely, Kondo et al. (2015) reported that the 5-HT3 receptor plays a key role in exercise-induced antidepressant effects. The important role of the 5-HT3 receptor is also demonstrated in the antiepileptic effect of SSRIs (Payandemehr et al., 2012; Alhaj et al., 2015).
Withdrawal from psychostimulant use is accompanied by symptoms of anxiety and depression, and the increased anxiety states are associated with greater conditioned place preference for cocaine in rats (Pelloux et al., 2009), and negative affect during withdrawal is thought to be a critical factor leading to craving and relapse in humans (Koob et al., 2004). Here, we demonstrated that the psychological stress encounter elicits hyperactivity during withdrawal in METH-sensitized mice, and the hyperactivity is attenuated by anxiolytics and antidepressants. The pharmacological results suggest that the behavior is associated with a state of anxiety or depression. It is likely that the psychological stress-induced hyperactivity is a factor leading to craving and relapse. Taken together, the neurochemical studies on the hyperactivity may contribute to development of pharmacotherapy for withdrawal symptoms of METH dependence.
In conclusion, the present study demonstrates that an encounter with an intruder, a psychological stress, elicits hyperactivity during withdrawal in METH-sensitized mice. Furthermore, we found that the hyperactivity is accompanied by an activation of prefrontal serotonergic and dopaminergic systems and is attenuated by anxiolytic and antidepressant drugs. The results of the present study imply that encounter-induced behavioral alteration is useful as a novel approach to investigate pharmacotherapy for withdrawal syndromes in METH-sensitized mice.
Statement of Interest
None.
Acknowledgments
This study was supported in part by JSPS KAKENHI [JP14J06155 (to T.T.), JP25460099 (to Y.A.), JP16K08268 (to Y.A.), JP16K19183 (to S.H.), JP26293020 (to H.H.), JP26670122 (to H.H.), JP15H01288 (to H.H.), and JP16K15126 (to K.T.)], the Neuropsychiatry Drug Discovery Consortium established by Dainippon Sumitomo Pharma Co., Ltd. (Japan) with Osaka University (to T.M. and H.H.), Takeda Science Foundation (Japan) (to Y.A.), Research Foundation for Pharmaceutical Sciences (Japan) (to Y.A.), the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (S2603 to H.H.), and the SRPBS from AMED (to H.H.).
References
- Ago Y, Nakamura S, Hayashi A, Itoh S, Baba A, Matsuda T. (2006. a) Effects of osemozotan, ritanserin and azasetron on cocaine-induced behavioral sensitization in mice. Pharmacol Biochem Behav 85:198–205. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, Matsuda T. (2006. b) Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology 51:914–922. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, Matsuda T. (2007) Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse 61:757–763. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Baba A, Matsuda T. (2008) Neuropsychotoxicity of abused drugs: effects of serotonin receptor ligands on methamphetamine- and cocaine-induced behavioral sensitization in mice. J Pharmacol Sci 106:15–21. [DOI] [PubMed] [Google Scholar]
- Ago Y, Araki R, Yano K, Hiramatsu N, Kawasaki T, Chaki S, Nakazato A, Onoe H, Hashimoto H, Baba A, Takuma K, Matsuda T. (2011. a) Activation of metabotropic glutamate 2/3 receptors attenuates methamphetamine-induced hyperlocomotion and increase in prefrontal serotonergic neurotransmission. Psychopharmacology (Berl) 217:443–452. [DOI] [PubMed] [Google Scholar]
- Ago Y, Yano K, Hiramatsu N, Takuma K, Matsuda T. (2011. b) Fluvoxamine enhances prefrontal dopaminergic neurotransmission in adrenalectomized/castrated mice via both 5-HT reuptake inhibition and σ1 receptor activation. Psychopharmacology (Berl) 217:377–386. [DOI] [PubMed] [Google Scholar]
- Ago Y, Tanaka T, Kita Y, Tokumoto H, Takuma K, Matsuda T. (2012) Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacology 62:1634–1639. [DOI] [PubMed] [Google Scholar]
- Ago Y, Araki R, Tanaka T, Sasaga A, Nishiyama S, Takuma K, Matsuda T. (2013) Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology 38:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Takuma K, Matsuda T. (2014) Potential role of serotonin1A receptors in post-weaning social isolation-induced abnormal behaviors in rodents. J Pharmacol Sci 125:237–241. [DOI] [PubMed] [Google Scholar]
- Ago Y, Hasebe S, Nishiyama S, Oka S, Onaka Y, Hashimoto H, Takuma K, Matsuda T. (2015) The female encounter test: a novel method for evaluating reward-seeking behavior or motivation in mice. Int J Neuropsychopharmacol 18:pyv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S, Takuma K, Matsuda T. (2014) Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychopharmacol 17:883–893. [DOI] [PubMed] [Google Scholar]
- Alhaj MW, Zaitone SA, Moustafa YM. (2015) Fluvoxamine alleviates seizure activity and downregulates hippocampal GAP-43 expression in pentylenetetrazole-kindled mice: role of 5-HT3 receptors. Behav Pharmacol 26:369–382. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A. (2005) Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev 29:675–706. [DOI] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. (2010) Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology 59:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G, Sigrist H, Ferger B, Singewald N, Seifritz E, Pryce CR. (2016) Depletion of nucleus accumbens dopamine leads to impaired reward and aversion processing in mice: relevance to motivation pathologies. Neuropharmacology 109:306–319. [DOI] [PubMed] [Google Scholar]
- Bétry C, Etiévant A, Pehrson A, Sánchez C, Haddjeri N. (2015) Effect of the multimodal acting antidepressant vortioxetine on rat hippocampal plasticity and recognition memory. Prog Neuropsychopharmacol Biol Psychiatry 58:38–46. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. (2008) 5-HT4 receptors: history, molecular pharmacology and brain functions. Neuropharmacology 55:922–931. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, Hockemeyer J, Müller CE, Salamone JD. (2010) Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: effects on locomotor activity. Behav Brain Res 211:148–155. [DOI] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Spano P. (2014) Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: critical role of BDNF and dopamine. Front Pharmacol 5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Sadakierska-Chudy A, Suder A, Szumiec L, Mierzejewski P, Bienkowski P, Przegaliński E, Cryan JF. (2015) GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators. Neuropharmacology 88:36–47. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (1997) The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc. [Google Scholar]
- Hara Y, Ago Y, Taruta A, Katashiba K, Hasebe S, Takano E, Onaka Y, Hashimoto H, Matsuda T, Takuma K. (2016) Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid-induced autism. Autism Res 9:926–939. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Ago Y, Nishiyama S, Oka S, Hashimoto H, Takuma K, Matsuda T. (2015) Pharmacological profile of encounter-induced hyperactivity in isolation-reared mice. Behav Pharmacol 26:681–690. [DOI] [PubMed] [Google Scholar]
- Hellem TL. (2016) A review of methamphetamine dependence and withdrawal treatment: a focus on anxiety outcomes. J Subst Abuse Treat 71:16–22. [DOI] [PubMed] [Google Scholar]
- Hiramatsu N, Ago Y, Hasebe S, Nishimura A, Mori K, Takuma K, Matsuda T. (2013) Synergistic effect of 5-HT1A and σ1 receptor activation on prefrontal dopaminergic transmission under circulating steroid deficiency. Neuropharmacology 75:53–61. [DOI] [PubMed] [Google Scholar]
- Horiguchi N, Ago Y, Hasebe S, Higashino K, Asada K, Kita Y, Takuma K, Matsuda T. (2013) Isolation rearing reduces mechanical allodynia in a mouse model of chronic inflammatory pain. Pharmacol Biochem Behav 113:46–52. [DOI] [PubMed] [Google Scholar]
- Janetsian SS, McCane AM, Linsenbardt DN, Lapish CC. (2015) Methamphetamine-induced deficits in social interaction are not observed following abstinence from single or repeated exposures. Behav Pharmacol 26:786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. (2011) Animal models of schizophrenia. Br J Pharmacol 164:1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Brennan KA, Colussi-Mas J, Schenk S. (2010) Tolerance to 3,4-methylenedioxymethamphetamine is associated with impaired serotonin release. Addict Biol 15:289–298. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ishihara K, Ago Y, Nakamura S, Itoh S, Baba A, Matsuda T. (2006) Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Eur J Pharmacol 542:92–99. [DOI] [PubMed] [Google Scholar]
- Kent JM, Mathew SJ, Gorman JM. (2002) Molecular targets in the treatment of anxiety. Biol Psychiatry 52:1008–1030. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. (2008) Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: a review. Neurochem Res 33:204–219. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nakamura Y, Ishida Y, Shimada S. (2015) The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry 20:1428–1437. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. (2004) Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27:739–749. [DOI] [PubMed] [Google Scholar]
- Li CR, Huang GB, Sui ZY, Han EH, Chung YC. (2010) Effects of 6-hydroxydopamine lesioning of the medial prefrontal cortex on social interactions in adolescent and adult rats. Brain Res 1346:183–189. [DOI] [PubMed] [Google Scholar]
- Li X, Caprioli D, Marchant NJ. (2015) Recent updates on incubation of drug craving: a mini-review. Addict Biol 20:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. (2004) Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61:73–84. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Piñeyro G, Sadikot AF, Debonnel G. (2007) Serotonin4 (5-HT4) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55:712–725. [DOI] [PubMed] [Google Scholar]
- Mancino MJ, Gentry BW, Feldman Z, Mendelson J, Oliveto A. (2011) Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: a pilot field study. Am J Drug Alcohol Abuse 37:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. (2001) Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 21:9856–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T. (2013) Neuropharmacologic studies on the brain serotonin1A receptor using the selective agonist osemozotan. Biol Pharm Bull 36:1871–1882. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Römer S, Gardier AM, Hen R. (2014) Rapid anxiolytic effects of a 5-HT4 receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology 39:1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser PC, Moran PM, Frank RA, Kehne JH. (1996) Reversal of amphetamine-induced behaviours by MDL 100,907, a selective 5-HT2A antagonist. Behav Brain Res 73:163–167. [DOI] [PubMed] [Google Scholar]
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press. [Google Scholar]
- Neisewander JL, Cheung TH, Pentkowski NS. (2014) Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: implications for medications development. Neuropharmacology 76:301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Müller HW. (2010) Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev Neurosci 21:119–139. [DOI] [PubMed] [Google Scholar]
- Ota Y, Ago Y, Tanaka T, Hasebe S, Toratani Y, Onaka Y, Hashimoto H, Takuma K, Matsuda T. (2015) Anxiolytic-like effects of restraint during the dark cycle in adolescent mice. Behav Brain Res 284:103–111. [DOI] [PubMed] [Google Scholar]
- Payandemehr B, Bahremand A, Rahimian R, Ziai P, Amouzegar A, Sharifzadeh M, Dehpour AR. (2012) 5-HT3 receptor mediates the dose-dependent effects of citalopram on pentylenetetrazole-induced clonic seizure in mice: involvement of nitric oxide. Epilepsy Res 101:217–227. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. (2009) Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res 197:311–316. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25:192–216. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11:157–198. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Murakami C, Sowa C, Sakamoto Y, Koyama Y, Baba A, Matsuda T. (2001) Involvement of benzodiazepine binding sites in an antiaggressive effect by 5-HT1A receptor activation in isolated mice. Eur J Pharmacol 432:163–166. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Sowa C, Koyama Y, Baba A, Matsuda T. (2003) The 5-HT1A receptor agonist MKC-242 increases the exploratory activity of mice in the elevated plus-maze. Eur J Pharmacol 458:141–144. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. (1999) Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev Neurosci 10:59–90. [DOI] [PubMed] [Google Scholar]
- Shen H, Mohammad A, Ramproop J, Smith S. (2013) A stress steroid triggers anxiety via increased expression of α4βδ GABAA receptors in methamphetamine dependence. Neuroscience 254:452–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziray N, Kuki Z, Nagy KM, Markó B, Kompagne H, Lévay G. (2010) Effects of single and simultaneous lesions of serotonergic and noradrenergic pathways on open-space and bright-space anxiety-like behavior in two animal models. Behav Brain Res 209:93–98. [DOI] [PubMed] [Google Scholar]
- Tanyeri P, Buyukokuroglu ME, Mutlu O, Ulak G, Yıldız Akar F, Komsuoglu Celikyurt I, Erden BF. (2013) Involvement of serotonin receptor subtypes in the antidepressant-like effect of beta receptor agonist Amibegron (SR 58611A): an experimental study. Pharmacol Biochem Behav 105:12–16. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. (2010) Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res 208:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchoo SJ, Lee MJ, Swann AC, Dafny N. (2010) Bilateral six-hydroxydopamine administration to PFC prevents the expression of behavioral sensitization to methylphenidate. Brain Res 1312:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]