Abstract
In response to ionizing radiation (IR)-induced DNA double-strand breaks (DSB), cells elicit an evolutionarily conserved checkpoint response that induces cell cycle arrest and either DNA repair or apoptosis, thereby maintaining genomic stability. DNA-dependent protein kinase (DNA-PK) is a central enzyme involved in DSB repair for mammalian cells that comprises a DNA-PK catalytic subunit and the Ku protein, which act as regulatory elements. DNA-PK also functions as a signaling molecule to selectively regulate p53-dependent apoptosis in response to IR. Herein, we demonstrate that the orphan nuclear receptor TR3 suppresses DSB repair by blocking Ku80 DNA-end binding activity and promoting DNA-PK-induced p53 activity in hepatoma cells. We find that TR3 interacts with Ku80 and inhibits its binding to DNA ends, which then suppresses DSB repair. Furthermore, TR3 is a phosphorylation substrate for DNA-PK and interacts with DNA-PK catalytic subunit in a Ku80-independent manner. Phosphorylated TR3, in turn, enhances DNA-PK-induced phosphorylation and p53 transcription activity, thereby enhancing IR-induced apoptosis in hepatoma cells. Together, our findings reveal novel functions for TR3, not only in DSB repair regulation but also in IR-induced hepatoma cell apoptosis, and they suggest that TR3 is a potential target for cancer radiotherapy.
The DNA double-strand breaks (DSB) are generally regarded as the most lethal form of DNA damage and are commonly caused by exogenous agents, such as ionizing radiation (IR) and mutagenic chemicals (1). IR-induced DSB have three possible outcomes: 1) the DSB are rapidly repaired, leading to cell survival; 2) the cell is unable to repair DSB and undergoes apoptosis; or 3) the cell survives with unrepaired or partially repaired DSB, leading to genetic instability, which, in extreme cases, produces cancerous cells (2, 3). DSB repair inhibition in cancer cells will thus enhance their radiosensitivity and facilitate successful radiotherapy.
In mammalian cells, DSB are principally repaired by the nonhomologous DNA end-joining (NHEJ) pathway. The DNA-dependent protein kinase (DNA-PK) is the primary component in the NHEJ pathway (1). DNA-PK belongs to a family of phosphatidylinositol 3-kinase-like kinases and is a serine/threonine kinase. Biochemical studies have revealed that DNA-PK is a multiprotein complex consisting of an approximately 465-kDa catalytic subunit, termed DNA-PKcs, and a DNA-binding Ku protein component that is composed of Ku70 and Ku80 (4).
The Ku proteins initially recognize and bind to the broken ends of double-strand DNA, and the DNA-PKcs is then recruited to the DNA via Ku end binding (1). An in vivo study using a laser system to introduce DSB showed that DNA-PKcs accumulation at DSB sites is Ku80 dependent, whereas Ku80 accumulation is DNA-PKcs independent (5, 6). Although Ku DNA-end binding activity is crucial for DNA-PKcs recruitment to DSB ends and their subsequent repair, evidence suggests that DNA-PKcs activity is somewhat stimulated by DNA in the absence of Ku (7). Evidence further indicates that DNA-PKcs is active independent of DNA, suggesting distinct regulatory mechanisms for DNA-PK activity.
Many DNA-PK substrates have been identified, such as p53. In response to IR-induced DNA damage, DNA-PKcs activates p53 via phosphorylation, which regulates distinct downstream pathways that control cell cycle progression and apoptosis (8). p53 phosphorylation is Ku independent, and even in the absence of DNA, DNA-PK can phosphorylate p53 at Ser15 in vitro, although the presence of DNA significantly enhances phosphorylation levels of p53 (9).
Nuclear orphan receptor TR3 (also known as Nur77) belongs to the steroid/thyroid/retinoid receptor superfamily. TR3 not only functions as both a positive and negative transcription factor to regulate its target gene expression (10) but also regulates protein function via protein-protein interactions (11). TR3 activity is controlled, in part, via phosphorylation. c-Jun N-terminal kinase phosphorylation induces TR3 ubiquitination and degradation, resulting in loss of its mitogenic activity in HEK293T cells (12). Akt can also phosphorylate cytoplasmic TR3, thereby blocking its mitochondrial targeting by disrupting its interaction with Bcl-2 and suppressing apoptosis (13).
Previously, we found in intact hepatoma cells that TR3 down-regulates p53 transcription activity by blocking its acetylation, which decreases murine double minute 2 (MDM2) expression; however, TR3 can bind to p53 and block MDM2-mediated ubiquitination and degradation, thereby enhancing p53 stability (11). Although we have also observed that TR3-enhanced p53-dependent apoptosis upon UV radiation is associated with the regulation of p53 transactivation, the mechanism by which TR3 regulates p53-dependent cell signaling pathways during apoptosis induction is still unclear. Herein, we first found that TR3 physically interacts with Ku80 to suppress its DNA-end binding activity, thereby retarding DSB repair in hepatoma cells. Although the suppression of Ku80 DNA-end binding activity by TR3 directly inhibits DNA-PK binding to DNA, DNA-PK can still phosphorylate TR3. This phosphorylation, in turn, induces TR3 enhancement of IR-induced p53 transcription activity via DNA-PK. The function of TR3 in two different pathways further clarifies the mechanism of IR-induced apoptosis in hepatoma cells. Together, our findings demonstrate a unique function for TR3 in regulating the radiosensitivity of hepatoma cells via Ku80 and DNA-PK. These data not only suggest that TR3 may be a suitable target for radiotherapy but also enhance our understanding of the role of TR3 in the DNA-PK-p53 regulatory network and DSB repair.
Results
TR3 interacts with Ku80 to repress its DNA-end binding
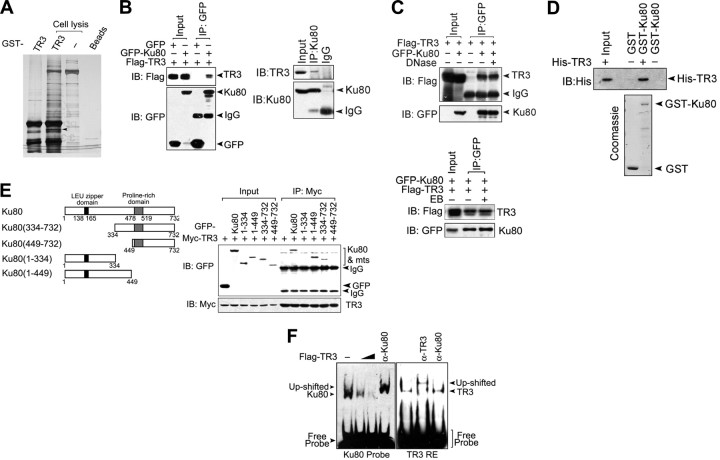
To identify novel TR3-binding proteins, the cell lysate from HepG2 cells was incubated with bacterially expressed glutathione S-transferase (GST)-TR3. Endogenous proteins interacting with TR3 were then pulled down using glutathione-agarose beads and visualized via silver staining on an SDS-PAGE gel. Several bands that bound specifically to GST-TR3 but not to GST were observed (Fig. 1A). One of these species, which migrated as an approximately 80-kDa band, was excised and sequenced using mass spectrometry. The result indicated that this band contained Ku80, which is a subunit of the Ku protein. To confirm this result, Flag-TR3 and green fluorescent protein (GFP)-Ku80 were transiently transfected into 293T cells, and coimmunoprecipitation (co-IP) assays were performed. Flag-TR3 bound to GFP-Ku80 but not GFP (Fig. 1B, left). We further tested this interaction at endogenous levels. As expected, TR3 was detected in the Ku80 immunoprecipitate but not in the IgG immunoprecipitate (Fig. 1C, right). Therefore, Ku80 is a novel TR3-binding protein.
Fig. 1.
TR3 physically interacts with Ku80 to repress its DNA binding. A, Identification of Ku80 as a TR3-associated protein. GST-TR3 was incubated with HepG2 cell lysate, and TR3 binding proteins were shown using silver staining. The specified band (indicated by arrow) was excised and analyzed via mass spectrometric sequencing. B, Co-IP experiments showing the interaction between TR3 and Ku80. Flag-TR3 and GFP-Ku80 were cotransfected into 293T cells, and the lysate was immunoprecipitated using an anti-GFP antibody. The immunoprecipitate was then examined via Western blot using an anti-Flag antibody. The input included 10% of the cell lysate used. GFP was used a control (left). HepG2 cell lysate was incubated with Ku80 antibody to immunoprecipitate endogenous Ku80 protein. For Western blotting of the immunoprecipitate, a TR3 antibody was used. IgG was used as a control (right). C, Effects of DNase and EB on TR3-Ku80 interactions. GFP-Ku80 and Flag-TR3 were transfected into 293T cells. Twenty-four hours after transfection, the cell extracts were incubated with either DNase (600 U/ml) or EB (50 μg/ml) and then immunoprecipitated with an anti-GFP antibody. Precipitated proteins were analyzed via Western blot using anti-Flag or anti-GFP antibodies. D, GST pull-down assay for examining the Ku80-TR3 interaction in vitro. GST-Ku80 was incubated with His-TR3 protein and then either stained with Coomassie brilliant blue or analyzed via Western blotting with an anti-His antibody to show either Ku or TR3 protein, respectively. E, Determination of the Ku80 region critical for TR3 binding. Schematic diagrams depict different Ku80 deletion constructs (left). Myc-TR3 and either GFP-Ku80 or its truncated mutants, as indicated, were cotransfected into 293T cells. The cell lysate was immunoprecipitated using Myc antibodies. The immunoprecipitate was then analyzed via Western blotting using anti-GFP antibodies (right). F, Effects of TR3 on Ku80 DNA binding. Different quantities of Flag-TR3 were transfected into 293T cells. Twenty-four hours after transfection, nuclear extracts were prepared and analyzed via EMSA using a biotin-labeled double-stranded oligonucleotide as a Ku80 probe and a biotin-labeled TR3 response element as a TR3 probe (negative control). The up-shifted band after incubation with the Ku80 or TR3 antibody confirmed specific binding of Ku or TR3 to DNA, respectively. IP, Immunoprecipitated; IB, immunoblotting; mts, mutants.
Because DNA is required for Ku80 to interact with many binding partners, such as DNA-PKcs (14), we investigated whether the interaction between TR3 and Ku80 is DNA dependent. Either deoxyribonuclease (DNase), which is used to digest cellular DNA, or ethidium bromide (EB), which disrupts protein-DNA interactions by intercalating into the DNA double helix (15), was incubated with the cell lysate. The co-IP assays showed that TR3 still bound Ku80 in the presence of either DNase or EB (Fig. 1C), indicating that the TR3-Ku80 interaction is neither mediated by nor requires a DNA cofactor. Furthermore, bacterially expressed His-TR3 bound GST-Ku80 but not GST alone, as tested via GST pull-down assay (Fig. 1D). Thus, TR3 directly interacts with Ku80.
We next determined which domain of Ku80 binds to TR3. Different Ku80 truncation mutants were constructed (Fig. 1E, left). TR3 efficiently pulled down full-length Ku80, Ku80 (334–732), and Ku80 (1–449), but neither Ku80 (449–732) nor Ku80 (1–334) (Fig. 1E, right), indicating that the central region in Ku80 (amino acids 334–449) is required for TR3 binding. Interestingly, this region is also responsible for the Ku80 DNA-end binding (16). We therefore suspected that, upon binding to the central region of Ku80, TR3 impairs its DNA-end binding. After incubation with nuclear proteins extracted from HepG2 cells, a double-stranded DNA probe formed a strong complex with Ku80 as shown via EMSA supershift experiments using anti-Ku80 antibodies. However, the Ku80-DNA complex was clearly disrupted by transfection with TR3 (Fig. 1F, left). As a negative control, anti-Ku80 antibodies did not shift the TR3 DNA-binding band (with the TR3 response element used as a probe) (Fig. 1F, right). These results suggest that TR3 inhibits Ku80 DNA-end binding activity upon interaction.
TR3 interacts with DNA-PKcs independent of Ku80
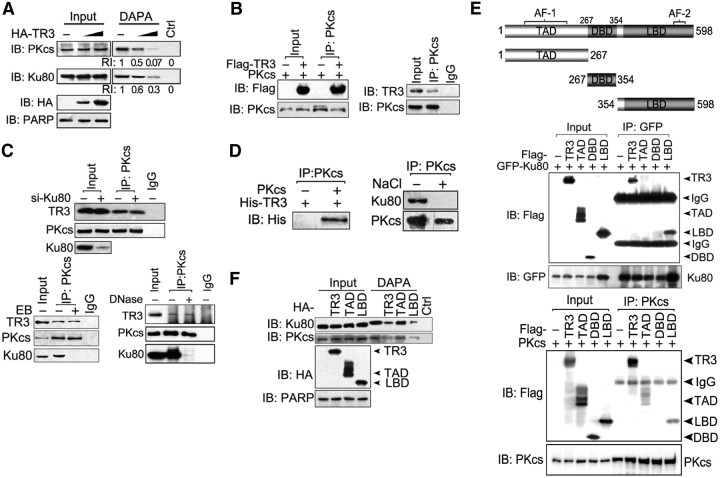
In the SDS-PAGE gel shown in Fig. 1A, we did not find a specific band that may represent the DNA-PKcs protein, probably because DNA-PKcs is too large (∼460 kDa) to be detected when 8% gel is used. However, based on the fact that the major function of Ku is the recruitment of DNA-PKcs to broken DNA ends to release the catalytic potential of DNA-PK (3), we suspected that TR3 inhibition in Ku80 DNA-end binding may also affect DNA-PKcs binding at these ends. To investigate this characteristic, DNA affinity precipitation assay (DAPA) assays were performed using an end-labeled 18-bp DNA fragment as a probe (7). The probe efficiently pulled down endogenous Ku80 and DNA-PKcs in HepG2 cells (Fig. 2A, first lane), suggesting that both Ku80 and DNA-PKcs form a complex with the DNA fragment. However, the formation of these Ku-DNA and DNA-PKcs-DNA complexes was obviously impaired by TR3 transfection (Fig. 2A), demonstrating that TR3 may inhibit the DNA-PKcs-DNA complex by repression of Ku80 DNA-end targeting. We also evaluated whether TR3 interacts with DNA-PKcs. When cotransfected with DNA-PKcs, TR3 was clearly detected in the DNA-PKcs immunoprecipitate (Fig. 2B, left), and this interaction under physiological conditions was also detected (Fig. 2B, right). Thus, DNA-PKcs is also a TR3-interacting protein.
Fig. 2.
Interaction of TR3 with DNA-PK, independent of Ku80. A, TR3 inhibits the DNA-end binding activity of both Ku80 and DNA-PKcs. HepG2 cells were transfected with HA-TR3. Nuclear extracts were incubated either with 18-bp biotinylated double-stranded oligonucleotides for Ku80 and DNA-PKcs or streptavidin-agarose beads without a probe as a control and then subjected to DNA-affinity precipitation assays. Specifically bound proteins were analyzed via Western blotting using antibodies specific for DNA-PKcs and Ku80; PARP expression levels were assessed as a protein loading control. RI, Relative intensity of TR3 expression. B, TR3 interacts with DNA-PKcs exogenously and endogenously. Left panel, DNA-PKcs and Flag-TR3 were cotransfected into 293T cells, and the cell lysate was immunoprecipitated with an anti-DNA-PKcs antibody. The immunoprecipitate was then examined via Western blotting using an anti-Flag antibody. Right panel, HepG2 cell lysate was immunoprecipitated with an anti-DNA-PKcs antibody. A TR3 antibody was used to examine the endogenous interaction between TR3 and DNA-PKcs via Western blotting. C, The interaction between TR3 and DNA-PKcs did not correlate with the presence of Ku80. Ku80-targeting siRNA was used to repress endogenous Ku protein in HepG2 cells, and the cell lysate was immunoprecipitated using anti-DNA-PKcs antibodies. For Western blotting of the immunoprecipitate, an anti-TR3 antibody was used (top). Extracts from HepG2 cells were incubated with either DNase (600 U/ml) or EB (50 μg/ml), as indicated, and then immunoprecipitated with an anti-DNA-PKcs antibody. Precipitated proteins were analyzed via Western blotting using anti-TR3 or anti-Ku80 antibodies (bottom). D, TR3 interacts directly with DNA-PKcs in vitro. DNA-PKcs protein was immunoprecipitated from commercially obtained DNA-PK (containing DNA-PKcs and Ku) using an anti-DNA-PKcs antibody and then incubated with NaCl (0.5 m) to separate DNA-PK and Ku80. The immunoprecipitated DNA-PKcs was then analyzed via Western blotting using anti-DNA-PKcs and anti-Ku80 antibodies (right). The immunoprecipitated DNA-PKcs was incubated with His-TR3 and then analyzed via Western blotting using an anti-His antibody to show the in vitro interaction between TR3 and DNA-PKcs (left). E, Determination of the TR3 region critical for Ku80 and DNA-PKcs binding. The schematic diagrams depict different TR3 deletion constructs (top). GFP-Ku80 or DNA-PKcs and Flag-TR3 or its truncated mutants, as indicated, were cotransfected into 293T cells. The cell lysate was immunoprecipitated with either an anti-GFP antibody (middle) or an anti-DNA-PKcs antibody (bottom). The immunoprecipitate was then analyzed by Western blotting using anti-Flag antibodies. F, Role of TR3 truncated mutants in the DNA-end binding activities of Ku80 and DNA-PKcs. HepG2 cells were transfected with HA-TR3 and its truncation mutants. DAPA were performed as described in Fig. 2A.
We further determined a role for Ku80 in the TR3-DNA-PKcs interaction. In si-Ku80-transfected HepG2 cells, endogenous TR3 was present and consistently immunoprecipitated with the anti-DNA-PKcs antibody, regardless of the small interfering RNA (siRNA)-reduced Ku80 levels (Fig. 2C, top). Moreover, when the cell lysate was preincubated with DNase or EB, the endogenous association between TR3 and DNA-PKcs was still evident, whereas the Ku80-DNA-PKcs complex was disrupted (Fig. 2C, bottom). Hence, TR3 binds DNA-PKcs independent of Ku80. To further test whether the TR3-DNA-PKcs interaction is direct, DNA-PKcs was immunoprecipitated by its antibody from commercially obtained DNA-PK (containing DNA-PKcs and Ku) and incubated with NaCl (0.5M) to separate DNA-PKcs and Ku (Fig. 2D, right). Purified DNA-PKcs was then incubated with bacterially expressed His-TR3, and they interacted (Fig. 2D, left). Thus, the interaction between TR3 and DNA-PKcs is direct.
We mapped the TR3 region that interacts with Ku80 and DNA-PKcs. Different truncated TR3 mutants were constructed, including transcription activation domain (TAD), DNA-binding domain (DBD), and ligand-binding domain (LBD) (Fig. 2E, top). Ku80 interacted with LBD but not with DBD or TAD (Fig. 2E, middle), whereas DNA-PKcs bound to both TAD and LBD (Fig. 2E, bottom). We further used these TR3-truncated mutants in the DAPA to verify their effects on Ku80 and DNA-PKcs DNA-end binding. Using this assay, we found that full-length TR3 and its LBD inhibited both Ku80 and DNA-PKcs DNA-end binding, whereas TAD, which interacts with DNA-PKcs but not with Ku80, failed to do so (Fig. 2F). Together, these results verify that the Ku80-DNA-end complex must be disrupted before TR3 can repress DNA-PKcs binding to DNA.
TR3 is a novel phosphorylation substrate for DNA-PKcs
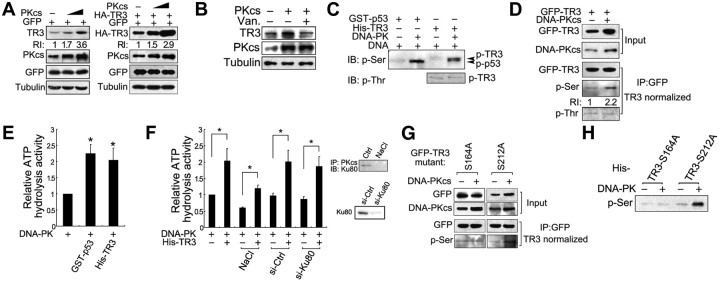
Interestingly, we unexpectedly found that DNA-PKcs positively regulates TR3 protein levels. Transfection of DNA-PKcs dramatically increased the protein levels for both endogenous (Fig. 3A, left) and exogenous (Fig. 3A, right) TR3 in HepG2 and 293T cells. This effect was correlated with DNA-PKcs kinase activity, because pretreatment with vanillin (Van), a specific DNA-PK inhibitor (17), abolished up-regulation of endogenous TR3 expression by DNA-PKcs (Fig. 3B). Given that TR3 is a hyperphosphorylated protein (18) and that DNA-PKcs is a serine/threonine kinase, we suspected that TR3 may be a DNA-PK substrate. We therefore assayed the phosphorylation of TR3 by DNA-PKcs using specific anti-phospho-Thr or -Ser antibodies in an in vitro phosphorylation assay. DNA-PKcs phosphorylated TR3 as effectively as p53, which is another well-characterized DNA-PK substrate, and this TR3 phosphorylation was clearly recognized by anti-phospho-Ser but not by an anti-phospho-Thr antibody (Fig. 3C). Similar results were also observed for transfected 293T cells, in which introduction of DNA-PKcs increased the GFP-TR3 protein levels (Fig. 3D, Input). After normalization for levels of TR3 protein, DNA-PK-induced phosphorylation of TR3 at Ser but not Thr could be clearly detected (Fig. 3D, IP). These in vitro and in vivo data suggest that DNA-PK primarily phosphorylates serine on TR3. Moreover, the results from a Kinase-Glo Luminescent Kinase assay, which is typically used to indicate substrate phosphorylation level by measuring the ATP hydrolysis activity (19), confirmed that TR3 is a DNA-PK substrate. In the presence of GST-p53, higher ATP hydrolysis activity was detected as expected (Fig. 3E), which indicates that DNA-PK phosphorylated p53 effectively (20). Similarly, this activity was greatly increased upon incubation with His-TR3 (Fig. 3E). Interestingly, this DNA-PKcs function was shown to be independent of Ku80, because knockdown of this protein by siRNA did not influence DNA-PKcs-induced TR3 phosphorylation (Fig. 3F). In addition, although NaCl disrupts the Ku80/DNA-PKcs complex (21) (Fig. 3F, inset), DNA-PKcs maintained its ability to phosphorylate TR3 in this buffer (Fig. 3F). Thus, DNA-PKcs directly phosphorylates TR3 in a Ku80-independent manner.
Fig. 3.
DNA-PK phosphorylates TR3 protein. A, Transfection of DNA-PKcs induces TR3 expression in a concentration-dependent manner. DNA-PKcs was transfected into HepG2 cells alone (left) or with HA-TR3 into 293T cells (right). After transfection, the cell lysate was analyzed via Western blotting using an anti-TR3 or anti-HA antibody to evaluate endogenous and exogenous TR3 expression levels. GFP was cotransfected to monitor transfection efficiency. RI, Relative intensity of TR3 expression. B, DNA-PKcs-induced TR3 expression is suppressed by its inhibitor vanillin (Van.). HepG2 cells were transfected with DNA-PKcs and then treated with vanillin (200 μmol/liter) for 12 h. The cell lysate was then analyzed via Western blotting using an anti-TR3 antibody. C, The in vitro assay to detect DNA-PK phosphorylation of TR3. Bacterially expressed His-TR3 was incubated with whole-enzyme DNA-PK, and the in vitro phosphorylation assays were performed as described in Materials and Methods. GST-p53 was used as a positive control. D, DNA-PKcs phosphorylates TR3 at a Ser, but not a Thr, residue. GFP-TR3 with or without DNA-PKcs was transfected into 293T cells. The cell lysate was immunoprecipitated using an anti-GFP antibody. After normalization for the loading of the immunoprecipitated TR3, TR3 phosphorylation was analyzed by Western blotting using specific anti-phospho-Ser and anti-phospho-Thr antibodies to detect TR3 phosphorylation levels. RI, Relative intensity of TR3 expression. E, Evaluation of the phosphorylation activity of DNA-PK toward TR3. The phosphorylation activity of DNA-PK was assessed by determining ATP hydrolysis activity. DNA-PK proteins were immunoprecipitated from the HepG2 cell lysate using the anti-DNA-PK antibody and then incubated with His-TR3. The ATP hydrolysis activity was then determined using the Kinase-Glo Luminescent Kinase assay as described in Materials and Methods. GST-p53 was used as a control. The ATP hydrolysis activity of DNA-PK alone was given a value of 1, and the experimental values were normalized against this value. The bars represent the means ± sd values from three independent experiments. *, P < 0.05. F, The kinase activity of DNA-PK for TR3 does not correlate with the presence of Ku80. HepG2 cells were pretransfected with si-Ku80 to repress the expression of endogenous Ku80. Scrambled siRNA was used as a control (si-Ctrl). The kinase activity of DNA-PK was determined as described above. The DNA-PK immunoprecipitate was preincubated with NaCl (0.5 m) to disrupt its association with Ku80. The right inset panels indicate the disruption of the endogenous Ku80-DNA-PKcs interaction by NaCl (0.5 m) (top) and the repression of endogenous Ku80 expression via si-Ku80 (bottom). The bars represent the means ± sd from three independent experiments. The ATP hydrolysis activity of DNA-PK alone was given a value of 1, and the experimental values were normalized against this value. *, P < 0.05. G, DNA-PKcs phosphorylates TR3 at S164 but not S212. The TR3 point mutants GFP-S164A and GFP-S212A were separately cotransfected with DNA-PKcs into 293T cells. The cell lysate was immunoprecipitated using anti-GFP antibodies, and the level of immunoprecipitated GFP-TR3 was normalized. The same quantity of immunoprecipitate was then analyzed via Western blotting using a specific anti-phospho-Ser antibody. H, The in vitro assay to detect the DNA-PK phosphorylation of S212A but not S164A in TR3. Bacterially expressed His-S164A and S212A were incubated with whole-enzyme DNA-PK, and the in vitro phosphorylation assays were performed as described above.
Based on the above data indicating that DNA-PKcs primarily phosphorylates Ser rather than Thr on TR3, we next precisely mapped the DNA-PKcs phosphorylation sites. Because DNA-PK displays a preference for the SQ phosphorylation motif (3), we searched for this motif in TR3 and found two potential motifs at Ser164-Gln and Ser212-Gln. We therefore constructed corresponding point mutants, i.e. S164A and S212A, and cotransfected each construct with DNA-PKcs into 293T cells. After normalization of the level of immunoprecipitated TR3, phosphorylation of S212A but not of S164A was clearly detected in the presence of DNA-PKcs (Fig. 3G, IP). Similarly, the in vitro phosphorylation assay also showed phosphorylation of S164A, but not S212A, by DNA-PK (Fig. 3H). Hence, Ser164 is the critical site for DNA-PK-induced phosphorylation of TR3. Importantly, DNA-PKcs failed to induce expression of S164A, whereas it still increased the protein levels of S212A (Fig. 3G, Input), which suggests a correlation between expression and phosphorylation of TR3 induced by DNA-PKcs.
IR stimulates phosphorylation of TR3 through DNA-PK
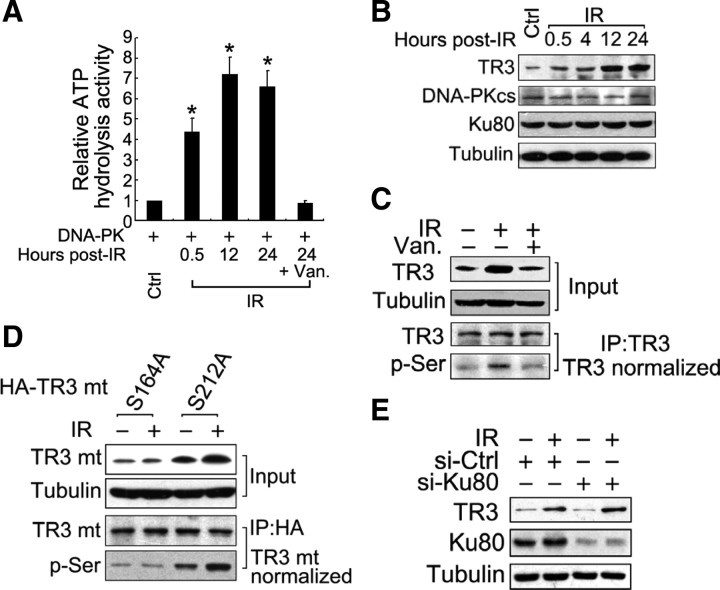
Because IR can activate DNA-PK kinase activity, we investigated whether TR3 is phosphorylated in response to IR. Consistent with a previous report (22), IR strongly induced DNA-PK activity (Fig. 4A). Under these conditions, the TR3 expression levels, but not DNA-PKcs and Ku80 levels, were remarkably increased, reaching a maximum level 12 h after IR exposure (Fig. 4B). Furthermore, up-regulation of TR3 protein accompanied IR-induced Ser phosphorylation of TR3 (Fig. 4C). However, the IR effects on TR3 phosphorylation and expression were almost abolished in the presence of vanillin, suggesting that IR likely stimulates TR3 phosphorylation via DNA-PK.
Fig. 4.
IR induces TR3 phosphorylation via DNA-PK. A, IR induces DNA-PK to phosphorylate p53. HepG2 cells were exposed to IR (3 Gy) and then harvested at the indicated time points. Kinase-Glo Luminescent Kinase assays were performed to assess the ATP hydrolysis activity of DNA-PK as described above. GST-p53 was used as a substrate. The bars represent the means ± sd from three independent experiments. *, P < 0.05. B, IR induces TR3 protein expression in a time-dependent manner. HepG2 cells were exposed to IR (3 Gy) and then harvested at the indicated time points. Western blotting was performed to determine the expression of endogenous TR3, DNA-PKcs, and Ku80. C, IR induces TR3 phosphorylation via DNA-PK in HepG2 cells. Cells were pretreated either with or without vanillin (Van.) (200 μmol/liter) and then exposed to IR (3 Gy). Twenty-four hours later, TR3 was immunoprecipitated from the cell lysate using an anti-TR3 antibody. After normalization of the quantity of TR3 in the immunoprecipitate, TR3 phosphorylation was analyzed using an anti-phospho-Ser antibody. D, Effects of IR on the phosphorylation and expression of the TR3 point mutants. HA-S164A and HA-S212A were separately transfected into HepG2 cells, and the transfected cells were subsequently exposed to IR (3 Gy). After 24 h, the cell lysate was prepared for Western blotting using an anti-HA antibody to demonstrate their expression levels and an anti-phospho-Ser antibody to determine their phosphorylation levels. E, IR-induced TR3 expression did not correlate with the presence of Ku80. HepG2 cells were transfected with si-Ku80 to inhibit endogenous Ku80 protein, and the scrambled siRNA was used as a control (si-Ctrl). The transfected cells were exposed to IR (3 Gy). After 24 h, Western blotting was performed. mt, Mutant.
We next determined whether IR-induced up-regulation of TR3 depends on DNA-PK-mediated phosphorylation. Upon transfection of the TR3 point mutants S164A and S212A into cells, IR increased the expression and phosphorylation only for S212A (Fig. 4D). However, when the Ku80 protein was knocked down, IR retained the ability to induce TR3 expression (Fig. 4E). We therefore conclude that IR-induced TR3 up-regulation depends on DNA-PKcs-induced phosphorylation but is independent of Ku80.
TR3 enhances DNA-PK-induced p53 phosphorylation and transcription activity
Because p53 is a known target for DNA-PK (9), we next explored the role of TR3 in the DNA-PK-activated p53 signaling pathway. Consistent with our and other reports, DNA-PKcs alone can activate p53 transcription activity (9), and this is suppressed by introduction of TR3 alone (11). However, we unexpectedly found that TR3 enhances, rather than represses, p53 transcription activity when coexpressed with DNA-PKcs (Fig. 5A). Moreover, this effect was related to DNA-PKcs kinase activity, because vanillin treatment entirely abolished the TR3-enhanced p53 transcription activity, even in the presence of DNA-PKcs. These results therefore suggest that TR3-enhanced p53 transcription activity is dependent on DNA-PKcs activity.
Fig. 5.
TR3 enhances DNA-PK-activated p53 phosphorylation and transactivation. A, TR3 enhances DNA-PK-induced p53 transcription activity. DNA-PKcs, p53-luciferase reporter, and β-gal gene expression vectors with different concentrations of HA-TR3 were cotransfected into 293T cells. The cells were treated either with or without vanillin (Van.) (200 μmol/liter) as indicated. A p53-luciferase reporter was used to monitor the transcription activity of p53. The p53 reporter gene activity was determined and normalized in relation to the β-gal activity. The bars represent the mean ± sd values from three independent experiments. *, P < 0.05. B, TR3 enhances DNA-PK-induced p53 transcription activity under different conditions. DNA-PKcs, p53-luciferase reporter, and β-gal gene expression vectors with HA-TR3 or its point mutants, as indicated, were cotransfected into HepG2 cells. The cells were then exposed to IR (3 Gy; lower panel) or treated with or without vanillin (200 μmol/liter) as indicated. p53 reporter gene activity was determined and normalized as described above. *, P < 0.05. C, TR3 enhances DNA-PKcs-induced p53 phosphorylation in vivo. HA-TR3 either with or without DNA-PKcs was transfected into 293T cells as indicated. The cell lysate was then analyzed via Western blot using an anti-phospho-Ser15–p53 antibody. D, TR3 enhances DNA-PKcs-induced p53 phosphorylation in vitro. GST-p53 and increasing quantities of His-TR3 were incubated with whole-enzyme DNA-PK in either the presence or absence of DNA. An in vitro phosphorylation assay was then performed as described in Materials and Methods. The phospho-Ser15–p53 antibody was used to detect p53 phosphorylation. E, Effects of TR3 point mutations on DNA-PK-induced p53 phosphorylation. S164A and S212A mutants either with or without DNA-PKcs were separately transfected into 293T cells as indicated. The cell lysate was then analyzed via Western blotting using a phospho-Ser15–p53 antibody. F, Effects of TR3 and its point mutations on DNA-PK-induced p53 phosphorylation in vitro. GST-p53 with either His-TR3 or its point mutants was incubated with whole-enzyme DNA-PK. An in vitro phosphorylation assay was then performed using a phospho-Ser15–p53 antibody. G, TR3 enhances IR-induced p53 phosphorylation. HepG2 cells were transfected either with or without Flag-TR3, exposed to IR (3 Gy) and then harvested at the various time points indicated. The cell lysate was analyzed via Western blotting with an anti-phospho-Ser15–p53 antibody. H, TR3 enhances the DNA-PKcs-p53 interaction after IR exposure. HepG2 cells were transfected either with or without Myc-TR3, exposed to IR (3 Gy), and then harvested after 24 h culture. The cell lysate was immunoprecipitated with an anti-DNA-PKcs antibody, followed by Western blotting using an anti-p53 antibody. I, IR enhances the TR3-DNA-PKcs interaction in HepG2 cells. Cells were exposed to IR (3 Gy) and harvested after 24 h culture. The cell lysate was immunoprecipitated with an anti-DNA-PKcs antibody. The immunoprecipitate was then analyzed via Western blotting using an anti-TR3 antibody. Luc, Luciferase.
We further examined whether the TR3 functional switch from p53 inhibition to p53 activation involves its phosphorylation by DNA-PKcs. TR3/S212A but not TR3/S164A retained the capacity to enhance p53 transcription activity in cells cotransfected with DNA-PKcs (Fig. 5B, top) concurrently with DNA-PKcs phosphorylation of TR3/S212A but not TR3/S164A. A similar result was also obtained upon IR exposure (Fig. 5B, bottom). Therefore, DNA-PK phosphorylation of TR3 is a prerequisite for its activation of p53 transcription activity.
DNA-PKcs phosphorylates p53 at Ser15 and thus activates p53 transcription activity (23). If TR3 is involved in regulating p53 transcription activity, it is reasonable to speculate that TR3 may influence DNA-PKcs-induced phosphorylation of p53. As expected, transfection of DNA-PKcs induced the phosphorylation of endogenous p53 at Ser15, and this phosphorylation event was further enhanced by the transfection of TR3 (Fig. 5C). This finding was further verified using an in vitro phosphorylation assay. DNA-PK phosphorylated p53 at Ser15, which was further enhanced by TR3 in a concentration-dependent manner (Fig. 5D). Notably, TR3 also exerted such effects in the absence of DNA, suggesting that TR3 enhances a direct and DNA-independent regulation of DNA-PK-induced p53 phosphorylation. Importantly, the TR3 effect on p53 phosphorylation relies on DNA-PKcs-induced TR3 phosphorylation. Both in vivo (Fig. 5E) and in vitro (Fig. 5F) phosphorylation assays further showed that TR3/S164A, which cannot be phosphorylated by DNA-PKcs, lost the ability to regulate p53 phosphorylation. In contrast, TR3/S212A could still enhance DNA-PKcs-induced p53 phosphorylation. Accordingly, it is likely that TR3 and DNA-PKcs form a feedback loop to coregulate p53 phosphorylation and the transcription activity of p53.
TR3 effects on p53 phosphorylation were further tested using HepG2 cells exposed to IR. IR induced Ser15 phosphorylation without affecting p53 protein levels, and this IR-induced p53 phosphorylation was remarkably enhanced when TR3 was overexpressed (Fig. 5G). Additionally, we found that the endogenous interaction between DNA-PKcs and p53 was enhanced by TR3 upon IR exposure (Fig. 5H), which suggests that TR3 may reinforce DNA-PK-induced p53 phosphorylation and activation by promoting DNA-PK-p53 physical interaction. Furthermore, an IR-promoted endogenous interaction between TR3 and DNA-PKcs was observed (Fig. 5I). Taken together, our results clearly show that a feedback regulatory loop exists between DNA-PKcs and TR3. In this mechanism, DNA-PKcs phosphorylates TR3 and switches the role of TR3 from an inhibitor to an activator of p53 transcription activity. Phosphorylated TR3 further reinforces the DNA-PKcs-induced phosphorylation and activation of p53 through protein-protein interactions.
TR3-induced hepatoma cell apoptosis correlates with its phosphorylation after IR
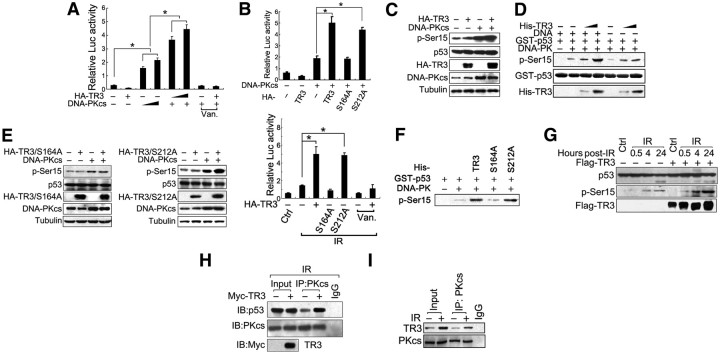
We evaluated the biological consequences of TR3 phosphorylation by DNA-PK in response to IR. DNA-PK can activate p53 transcription activity and then initiate cancer cell apoptosis (24, 25). Because TR3 coordinates DNA-PK to elevate p53 phosphorylation and activation, it was important to assess the TR3 function during IR-induced cell apoptosis. We detected the apoptotic rate via flow cytometry in various hepatoma HepG2 cell types, including wild-type, TR3-overexpressing (TR3), and TR3-knockdown (TR3 KD) HepG2 cells. Overexpression of TR3 enhanced IR-induced apoptosis. However, when endogenous TR3 was knocked down, IR exposure only marginally induced apoptosis (Fig. 6A), demonstrating that TR3 positively regulates the apoptotic induction of hepatoma HepG2 cells in response to IR.
Fig. 6.
TR3 promotes IR-induced cell apoptosis. A and B, Effects of TR3 on IR-induced apoptosis. HepG2 cells, either stably overexpressing TR3 and si-TR3 (A) or transfected with various TR3 point mutants (B), were exposed to IR (4 Gy) and then harvested 24 h later. Cells were stained with propidium iodide and analyzed via flow cytometry. The numbers indicate the apoptotic rate. C, TR3–p53 cross talk in IR-induced apoptosis. p53-null H1299 cells were transfected with TR3 or p53 as indicated. After 24 h transfection, cells were exposed to IR (4 Gy) and then harvested 24 h later. Cells were stained with propidium iodide and analyzed via flow cytometry. The numbers indicate the apoptotic rate.
We next examined whether this positive regulatory effect of TR3 on IR-induced apoptosis correlates with its phosphorylation. Transient transfection of TR3, but not TR3/S164A, significantly enhanced IR-induced apoptosis in HepG2 cells. However, the TR3/S212A mutant that can be phosphorylated by DNA-PKcs also enhanced IR-induced apoptosis (Fig. 6B). These data clearly indicate that phosphorylation by DNA-PKcs is required for TR3 to enhance IR-induced apoptosis.
Furthermore, we investigated whether the function of TR3 in IR-induced cell apoptosis is dependent on p53. p53-null H1299 cells were transfected with TR3 and then exposed to IR. IR exposure failed to induce apoptosis in H1299 cells, and transfection of TR3 did not enhance IR-induced apoptosis; however, when p53 was reintroduced into these cells, IR-induced cell apoptosis was rescued and enhanced by TR3 transfection (Fig. 6C). These results thus confirm the TR3-p53 cross talk in the regulation of IR-induced cell apoptosis.
TR3 represses the DSB repair pathway mediated by Ku80
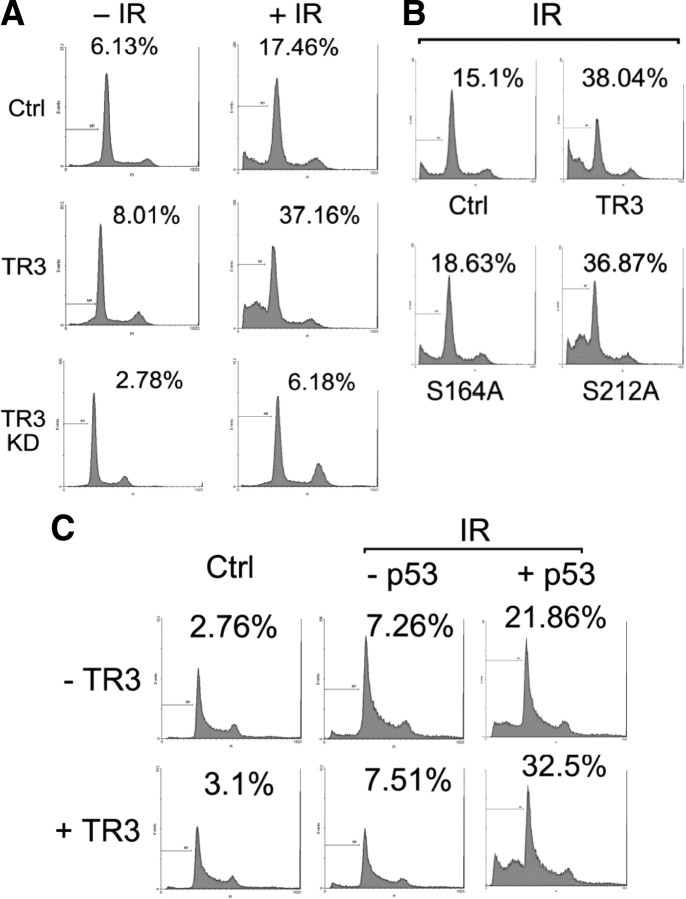
Because Ku has been characterized for its key role in DSB repair (26), we evaluated a possible role for TR3 in this process. DSB were induced using IR exposure, and the foci from phosphorylated histone H2AX (γ-H2AX), which is a sensitive marker for DSB, were assayed. Interestingly, γ-H2AX foci were potently induced in HepG2 cells 30 min after IR exposure. Twenty-four hours later, the number of γ-H2AX foci per cell was significantly reduced (Fig. 7, A, left, and B), indicating partial DSB repair. However, when TR3 was coexpressed in these cells, although the formation of γ-H2AX foci was not obviously influenced 30 min after IR exposure as compared with the control, DSB repair was clearly retarded 24 h after IR exposure, i.e. the number of γ-H2AX foci in TR3 transfected cells was similar at both the 30-min and 24-h time points (Fig. 7, A, right, and B). This finding suggests that TR3 repressed DSB repair. Similar results were also obtained by Western blot analysis, in which TR3 maintained the γ-H2AX levels even 24 h after IR exposure (Fig. 7C, left), and a significant difference was thus found between the curves obtained with transfected and nontransfected TR3 (Fig. 7C, right). Importantly, the inhibitory effect of TR3 on DSB repair depended on Ku80. When endogenous Ku80 protein was knocked down, the induction of γ-H2AX by IR was prolonged as compared with controls (Fig. 7D, si-Ctrl vs. si-Ku80). Under these conditions, overexpression of TR3 could longer maintain the γ-H2AX levels, and the curves obtained for the TR3-transfected and nontransfected cells nearly overlapped (Fig. 7D, si-Ku80 vs. si-Ku80+TR3). In addition, under IR exposure, the inhibitory effect of TR3 on the Ku80-DNA and DNA-PKcs-DNA association is clearly evident (Fig. 7E). Taken together, these data consistently demonstrate that TR3 represses Ku80-dependent repair of DSB under IR exposure.
Fig. 7.
TR3 represses DSB repair in response to IR. A, The overexpression of TR3 results in the suppression of DSB repair. HepG2 cells (left) and HA-TR3-overexpressing HepG2 cells (right) were exposed to IR (3 Gy). The cells were then harvested at various time points as indicated. DSB repair was determined by analyzing γ-H2AX via immunostaining with a specific primary γ-H2AX antibody followed by a fluorescein isothiocyanate-conjugated secondary antibody. HA-TR3 proteins were detected by incubation with an anti-HA antibody followed by a Texas-Red-conjugated secondary antibody. Stained cells were visualized using confocal microscopy. B, The number of γ-H2AX foci per cell was determined on a cell-to-cell basis by quantitative analysis of the above samples. C, Effects of TR3 on the IR-induced expression of γ-H2AX. HepG2 cells and Myc-TR3 expressing HepG2 cells were exposed to IR (3 Gy). After 24 h, the cell lysate was analyzed via Western blotting using anti-γ-H2AX antibodies. The relative expression levels for γ-H2AX were quantified using densitometry. The γ-H2AX levels at 1 h after IR exposure in each panel were normalized to 1. D, TR3-enhanced γ-H2AX expression depends on the presence of Ku80. HepG2 cells were transfected with si-Ku80 and TR3 as indicated, and the scrambled siRNA was used as a control (si-Ctrl). The methods of detection and quantification were the same as those described above. E, TR3 inhibits Ku80 and DNA-PK binding to DNA upon IR. HepG2 cells were transfected with HA-TR3. The nuclear extracts were incubated with either 18-bp biotinylated double-stranded oligonucleotides for Ku80 and DNA-PKcs or streptavidin-agarose beads, but no probe, as a control and then analyzed via DAPA. Specifically bound proteins were analyzed via Western blot analysis using antibodies specific for DNA-PKcs and Ku80, and the expression level of PARP was assessed as a loading control. DAPI, 4′,6-Diamidino-2-phenylindole.
Discussion
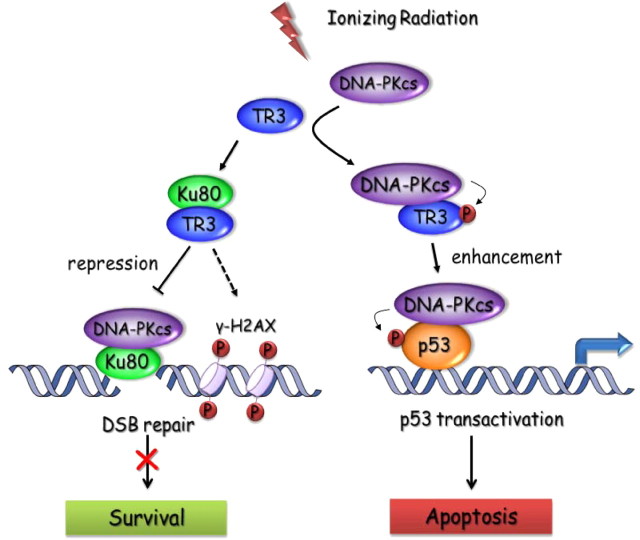
IR-induced DNA DSB may activate two mutually exclusive responses: DNA repair, which leads to cell survival; and apoptosis, which leads to cell death. DNA-PK, which is composed of Ku proteins and DNA-PKcs, is involved in both of these responses. DNA-PK not only regulates DSB-induced p53 transactivation and apoptosis (24, 25) but also directly mediates DSB repair and suppresses apoptosis (27). Thus, selective activation of the proapoptotic function and suppression of the prosurvival function of DNA-PK may be an effective approach to cancer therapy. In the study herein, TR3 is shown to be important in regulating these paradoxical functions of DNA-PK. On the one hand, TR3 impairs the DNA-end binding activity of Ku80 by blocking their physical interaction, and as a result, Ku80 loses its ability to recruit DNA-PKcs to DNA. Under this circumstance, TR3 repressed Ku80-mediated DSB repair in hepatoma cells in response to IR. On the other hand, although TR3 also inhibits the DNA binding of DNA-PK via its effect on Ku80, DNA-PK still phosphorylated TR3 and further increased its expression in a Ku80-independent manner. Consequently, phosphorylated TR3, in turn, enhanced DNA-PK-induced p53 phosphorylation and transcription activity, thereby increasing IR-induced and p53-dependent apoptosis in hepatoma cells (Fig. 8). Our data thus demonstrate important functions for TR3 in Ku80-mediated DSB repair and p53-dependent apoptosis.
Fig. 8.
Working model for repression of DSB repair and enhancement of DNA-PK-induced p53 activation by TR3 in response to IR.
The first step in DSB repair via NHEJ is mediated by Ku, which binds to the DNA ends and recruits DNA-PKcs and other NHEJ factors to complete the repair (1). Genetic ablation of Ku80 could diminish the capacity for DSB repair in cells (28). The DNA-end binding activity of Ku is essential in DSB repair. For example, Bcl-2 suppresses DSB repair by down-regulating the DNA-end binding activity of Ku (2). Similarly, we showed that TR3 directly binds the DNA-end binding region of Ku80 and decreases its DNA-end binding activity, thereby abolishing the association of DNA-PKcs with DNA ends, which suppresses DSB repair in hepatoma cells irradiated by IR. Accordingly, the TR3-Ku80 interaction may be an important event in DSB repair repression.
Nuclear hormone receptors, including the glucocorticoid and progesterone receptors, have been shown to be targets of DNA-PKcs (29, 30). Recently, estrogen receptor-α (ER-α) was also reported to be phosphorylated by DNA-PKcs, which protects ER-α from proteasomal degradation (31). In the experiments herein, we found that DNA-PKcs binds the N terminus of TR3 and phosphorylates Ser164 in this region. This phosphorylation clearly increased TR3 protein levels, suggesting a correlation between DNA-PK-mediated phosphorylation and TR3 protein stability. Interestingly, the DNA-PKcs phosphorylation of TR3 is likely Ku80 independent. Further supporting this conclusion, DNA-PKcs formed a complex with TR3 in the absence of Ku80. In fact, although Ku80 is known as the regulatory domain of DNA-PK, there is evidence that DNA-PKcs exhibits kinase activity without Ku80. For example, DNA-PKcs bound linear DNA fragments in the absence of Ku, which resulted in self-activation and thus the promotion of the phosphorylation of protein targets such as p53 (7).
Phosphorylated TR3 could, in turn, enhance the kinase effect of DNA-PKcs on p53. We further verified this observation using our in vitro phosphorylation assay, in which TR3 clearly enhanced p53 phosphorylation at Ser15 that was catalyzed by DNA-PK. Consistently, when the DNA-PKcs phosphorylation site at Ser164 of TR3 was mutated, TR3 lost its ability to enhance DNA-PK-induced p53 phosphorylation. Augmentation of DNA-PK-induced p53 phosphorylation by TR3 did not correlate with the presence of DNA ends. Instead, phosphorylated TR3 may facilitate the DNA-PKcs-p53 interaction. Supporting this conclusion, TR3 promoted the interaction of DNA-PKcs with p53 upon IR exposure. Other reports have also demonstrated that DNA-PK can be activated by nuclear nucleic acid-binding protein C1D in the absence of linear DNA and thereby mediates the p53 phosphorylation (32). Thus, DNA-PK and TR3 may form a feedback loop to regulate p53 phosphorylation, wherein DNA-PKcs phosphorylates TR3, and the phosphorylated TR3, in turn, enhances DNA-PK phosphorylation of p53 through protein-protein interactions.
In our previous study, we found that in intact hepatoma cells, TR3 negatively regulates the transcription activity of p53 by blocking its acetylation and protects p53 from degradation by MDM2 (11). However, DNA-PK-induced phosphorylation may promote a functional switch in TR3. Upon DNA damage, from IR exposure for example, DNA-PK is activated, TR3 is phosphorylated, and this phosphorylated TR3 significantly enhances p53 transcription activity. This phenomenon also occurs when DNA-PK is overexpressed, because DNA-PK has protein kinase activity and directly phosphorylates TR3. Importantly, TR3 S164A, which could not be phosphorylated by DNA-PK, lost its ability to enhance DNA-PK-induced p53 phosphorylation and transcription activity. Thus, we demonstrate a dual function for TR3 in regulating p53 activity under stressed and normal conditions. Under normal conditions, DNA-PK activity is maintained at a low level (22); therefore, hypophosphorylated TR3 may maintain p53 protein levels at a certain threshold by inhibiting p53-mediated MDM2 transcription and hence protecting p53 from MDM2 degradation. Under conditions of stress, such as IR exposure or DNA-PK introduction, however, TR3 is phosphorylated by DNA-PK. This phosphorylation switches TR3 from a p53 repressor to an activator by promoting DNA-PK-mediated phosphorylation at Ser15 on p53, ultimately inducing apoptosis in a p53-dependent manner.
As a death factor, TR3 is induced by apoptotic stimuli and migrates to the mitochondria, where it binds Bcl2 and conformationally converts Bcl2 into its proapoptotic state to trigger apoptosis (33). TR3 also exerts its proapoptotic action, at least in part, via its nuclear function, i.e. the regulation of the transcription activity of its target genes. We recently reported that the octaketide cytosporone B analogs that bound TR3 not only stimulated its transactivation activity but also initiated mitochondrial apoptosis via repression of the transcription of its downstream target gene, brain and reproductive organ-expressed protein (BRE), which encodes an antiapoptotic protein (34). Herein, we provide another example where TR3 exerts its proapoptotic action by neither migrating to the mitochondria nor regulating its target genes; in this case, the proapoptotic effect occurred via cross talk with DNA-PK and p53. When TR3 is involved in p53-dependent apoptosis, it acts as a regulator rather than an effecter. Previous reports have shown a similarly indirect function for TR3 in apoptosis induction. In butyrate-treated colon cancer cells, TR3 translocates only into the cytoplasm, where TR3 displaces other proapoptotic cytosolic proteins, such as Bax, from their binding partners, thereby ensuring that these proteins target the mitochondria to induce apoptosis (35). Therefore, the paths by which TR3 can induce apoptosis are varied.
In summary, our findings suggest the following two potential roles for TR3 in the IR-induced DNA damage signaling pathway: 1) via negative regulation of DSB repair, TR3 may block the survival pathway and reduce the minimum radiation dose required to induce tumor cell apoptosis; and 2) via positive regulation of IR-induced p53 activation, TR3 may enhance apoptosis in tumor cells under lethal doses of irradiation. Overall, these TR3-mediated regulatory mechanisms may result in the enhanced radiosensitivity of tumor cells. Hence, TR3 may be a critical switch that determines whether cells undergo DSB repair or apoptosis in response to IR. In addition, high TR3 expression may provide a radiosensitive phenotype, and up-regulation of TR3 in a radioresistant organ, such as the liver (36), could be beneficial for the treatment of tumors using radiotherapy.
Materials and Methods
Cell culture and transfection
Human embryonic kidney HEK293T cells and human non-small-cell lung carcinoma H1299 (p53-null) cells were purchased from the American Type Culture Collection (Manassas, VA). Human hepatoma HepG2 cells were purchased from the Institute of Cell Biology, China. Cells were maintained in DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin. Transfection assays were performed using the calcium phosphate precipitation method for 293T cells and TurboFect Transfection Reagent (Fermentas, CA) for HepG2 and H1299 cells.
Identification of novel TR3 binding proteins
To identify novel TR3 binding proteins, GST-TR3 fusion proteins bound to beads were incubated with 500 μl whole-cell lysate from HepG2 cells for 6 h. The bound proteins were eluted by boiling for 10 min in loading buffer, resolved by SDS-PAGE, and visualized by silver staining. The specific band was excised and analyzed via mass spectrometry sequencing.
Luciferase reporter assay
Cells were transfected with a p53-luciferase reporter plasmid, β-galactosidase (β-gal), and additional expression vectors as required. After transfection, the cell lysate was prepared in luciferase cell lysis buffer and measured for luciferase and β-gal activity. Luciferase activity was normalized for transfection efficiency using the corresponding β-gal activity. The ratios of luciferase/β-gal activity were used as indicators of transcription activity at the p53 promoter. The bars represent the means ± sem from three independent experiments.
Co-IP assay
Cells were lysed in lysis buffer supplemented with protease inhibitors. Immunoprecipitation and Western blotting were performed as described previously (11). Briefly, the cell lysate was incubated with the appropriate antibody and protein A-agarose beads for 3 h. The immunoprecipitate was collected, washed three times with lysis buffer, and then analyzed using Western blotting and different antibodies as required after separation by SDS-PAGE. The immunoreactive products were visualized via enhanced chemiluminescence.
GST pull-down assay
The GST pull-down assay was performed as described previously (37). Approximately 1 μg GST or GST-Ku80 immobilized in 10 μl glutathione-Sepharose 4B was incubated with 1.0 μg His-TR3 in 500 μl lysis buffer for 3 h at 4 C. After extensive washing with lysis buffer, the bound proteins were fractionated by 10% SDS-PAGE and either stained with Coomassie brilliant blue or analyzed via Western blot.
For the in vitro TR3-DNA-PKcs binding assay, 100 U commercial DNA-PK proteins (Promega, Madison, WI) was incubated with 5 μl DNA-PKcs antibody (Thermo Scientific, Pittsburgh, PA) and protein A-agarose beads for 3 h at 4 C in 500 μl lysis buffer with 0.5 m NaCl. The immunoprecipitate was collected and washed three times with lysis buffer containing 0.5 m NaCl and then incubated with 1.0 μg His-TR3 in 500 μl lysis buffer for 3 h at 4 C. After extensive washing with lysis buffer, the bound proteins were fractionated by 10% SDS-PAGE and analyzed via Western blotting.
DNA affinity precipitation assay
DAPA were performed as described previously (38). Nuclear extracts (200 μg) were mixed with 2 μg specific, biotinylated double-stranded DNA probes (forward, 5′-AGGCTGTGTCCTCAGAGG-3′; reverse, 5′-CCTCTGAGGACACAGCCT-3′) and 10 μg poly-deoxyinosine-deoxycytosine in 500 μl DAPA buffer for 4 h at 4 C. Next, 20 μl streptavidin-agarose beads (Invitrogen, Carlsbad, CA) was added, and the samples were agitated for 2 h at 4 C. The agarose bead-protein complexes were collected via brief centrifugation and washed twice in DAPA buffer. The protein-DNA probe complexes recovered on beads were analyzed via Western blot.
Electrophoretic mobility shift assay
EMSA was performed as described previously (39) using biotin-labeled double-stranded oligonucleotides (5′-GGGCCAAGAATCTTAGCAGTTTCGGG-3′) for Ku80 binding. The biotin-labeled TR3 response element (5′-GATCCGTGACCTTTATTCTCAAAGGTCA-3′) was used as a negative control for TR3 binding. For the supershift experiment, 5 μg nuclear extract was incubated with 200 ng of either anti-Ku80 antibody (Thermo Scientific) or anti-TR3 antibody (Cell Signaling Technology, Danvers, MA) for 2 h before incubation with the probe.
Kinase-Glo Luminescent Kinase assay
The assay was performed as described previously (40). Briefly, DNA-PKcs was immunoprecipitated from cell extracts using an anti-DNA-PKcs antibody (Thermo Scientific). Kinase reactions were performed for 30 min at 37 C after adding 100 μl kinase buffer [20 mm Tris-HCl (pH 7.9), 50 mm KCl, 10 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 2 mm dithiothreitol, 0.02% Tween 20, and 10% glycerol] and 20 μm ATP to each sample. Purified protein (500 ng) was added as a substrate. The ATP hydrolysis activities, which indicate substrate phosphorylation levels, were measured by using the Kinase-Glo Luminescent Kinase Assay Platform kit (Promega) to quantify the amount of ATP remaining in solution after the kinase reaction. The bars represent the means ± sem from three independent experiments.
Apoptosis and cell cycle analysis
Cells were fixed with 70% ethanol and incubated with DNase-free ribonuclease A (50 μg/ml) for 30 min, and they were then stained with propidium iodide (50 μg/ml; Sigma Chemical Co., St. Louis, MO) for 30 min in the dark. The stained cells were analyzed using a FACSCater-Plus flow cytometer (Beckman Coulter, Fullerton, CA).
Immunofluorescent staining and microscopic observation
To stain endogenous γ-H2AX and transfected hemagglutinin (HA)-TR3 proteins, cells were incubated with either anti-γ-H2AX (Upstate Biotechnology, Lake Placid, NY) or anti-HA antibody (Sigma) followed by either a fluorescein isothiocyanate-conjugated or Texas Red-conjugated secondary antibody. Cells were then stained with 4′,6-diamidino-2-phenylindole (50 μg/ml) to indicate the nuclei and visualized using a confocal microscope.
The in vitro phosphorylation assay
The assay was performed according to the protocol for a DNA-dependent protein kinase (Promega). Purified protein (500 ng) was incubated with DNA-PK (20 U; Promega) for 30 min in a total volume of 50 μl with DNA-PK reaction buffer containing 10 mm ATP, 0.5 mg BSA, and 0.5 μg salmon sperm DNA. Phosphorylation levels were detected via Western blot with an antiphosphorylation antibody.
Acknowledgments
We sincerely thank Professor Robert J. G. Haché (Department of Medicine and Biochemistry, Microbiology, and Immunology, University of Ottawa, Ottawa, Canada) for providing the full-length DNA-PKcs and GFP-Ku80 expression vectors. We also thank Dr. Yih-Cherng Liou (Department of Biological Sciences, National University of Singapore) for the mass spectrometry analysis.
This work was supported by grants from the Department of Education for Fujian Province and the Department of Public Health for Fujian Province (WKJ2008-253), the National Natural Science Fund of China (30871281, 30971525, and 30630070), the “973” Project of the Ministry of Science and Technology in China (2007CB914402), a Science Planning program grant from Fujian Province (2009J1010), and the “111” Project of the Ministry of Education, China (B06016).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: Nur77.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource.
- Co-IP
- Coimmunoprecipitation
- DAPA
- DNA affinity precipitation assay
- DBD
- DNA-binding domain
- DNA-PK
- DNA-dependent protein kinase
- DNA-PKcs
- DNA-PK catalytic subunit
- DNase
- deoxyribonuclease
- DSB
- double-strand break
- EB
- ethidium bromide
- ER-α
- estrogen receptor-α
- β-gal
- β-galactosidase
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- HA
- hemagglutinin
- γ-H2AZ
- phosphorylated histone H2AX
- IR
- ionizing radiation
- LBD
- ligand-binding domain
- MDM2
- murine double minute 2
- NHEJ
- nonhomologous DNA end-joining
- siRNA
- small interfering RNA
- TAD
- transcription activation domain.
References
- 1. Mahaney BL , Meek K , Lees-Miller SP. 2009. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J 417:639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Q , Gao F , May WS , Zhang Y , Flagg T , Deng X. 2008. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell 29:488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith GC , Jackson SP. 1999. The DNA-dependent protein kinase. Genes Dev 13:916–934 [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb TM , Jackson SP. 1993. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell 72:131–142 [DOI] [PubMed] [Google Scholar]
- 5. Uematsu N , Weterings E , Yano K , Morotomi-Yano K , Jakob B , Taucher-Scholz G , Mari PO , van Gent DC , Chen BP , Chen DJ. 2007. Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks. J Cell Biol 177:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mari PO , Florea BI , Persengiev SP , Verkaik NS , Brüggenwirth HT , Modesti M , Giglia-Mari G , Bezstarosti K , Demmers JA , Luider TM , Houtsmuller AB , van Gent DC. 2006. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA 103:18597–18602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yaneva M , Kowalewski T , Lieber MR. 1997. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J 16:5098–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vousden KH. 2000. p53: death star. Cell 103:691–694 [DOI] [PubMed] [Google Scholar]
- 9. Shieh SY , Ikeda M , Taya Y , Prives C. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325–334 [DOI] [PubMed] [Google Scholar]
- 10. Mangelsdorf DJ , Evans RM. 1995. The RXR heterodimers and orphan receptors. Cell 83:841–850 [DOI] [PubMed] [Google Scholar]
- 11. Zhao BX , Chen HZ , Lei NZ , Li GD , Zhao WX , Zhan YY , Liu B , Lin SC , Wu Q. 2006. p53 mediates the negative regulation of MDM2 by orphan receptor TR3. Embo J 25:5703–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu B , Wu JF , Zhan YY , Chen HZ , Zhang XY , Wu Q. 2007. Regulation of the orphan receptor TR3 nuclear functions by c-Jun N terminal kinase phosphorylation. Endocrinology 148:34–44 [DOI] [PubMed] [Google Scholar]
- 13. Chen HZ , Zhao BX , Zhao WX , Li L , Zhang B , Wu Q. 2008. Akt phosphorylates the TR3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis 29:2078–2088 [DOI] [PubMed] [Google Scholar]
- 14. Dynan WS , Yoo S. 1998. Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res 26:1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai JS , Herr W. 1992. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci USA 89:6958–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu X , Lieber MR. 1996. Protein-protein and protein-DNA interaction regions within the DNA end-binding protein Ku70-Ku86. Mol Cell Biol 16:5186–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durant S , Karran P. 2003. Vanillins: a novel family of DNA-PK inhibitors. Nucleic Acids Res 31:5501–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winoto A , Littman DR. 2002. Nuclear hormone receptors in T lymphocytes. Cell 109(Suppl):S57–S66 [DOI] [PubMed] [Google Scholar]
- 19. Shakir SM , Bryant KM , Larabee JL , Hamm EE , Lovchik J , Lyons CR , Ballard JD. 2010. Regulatory interactions of a virulence-associated serine/threonine phosphatase-kinase pair in Bacillus anthracis. J Bacteriol 192:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lees-Miller SP , Chen YR , Anderson CW. 1990. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol 10:6472–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suwa A , Hirakata M , Takeda Y , Jesch SA , Mimori T , Hardin JA. 1994. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci USA 91:6904–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shangary S , Brown KD , Adamson AW , Edmonson S , Ng B , Pandita TK , Yalowich J , Taccioli GE , Baskaran R. 2000. Regulation of DNA-dependent protein kinase activity by ionizing radiation-activated abl kinase is an ATM-dependent process. J Biol Chem 275:30163–30168 [DOI] [PubMed] [Google Scholar]
- 23. Achanta G , Pelicano H , Feng L , Plunkett W , Huang P. 2001. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res 61:8723–8729 [PubMed] [Google Scholar]
- 24. Woo RA , McLure KG , Lees-Miller SP , Rancourt DE , Lee PW. 1998. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature 394:700–704 [DOI] [PubMed] [Google Scholar]
- 25. Wang S , Guo M , Ouyang H , Li X , Cordon-Cardo C , Kurimasa A , Chen DJ , Fuks Z , Ling CC , Li GC. 2000. The catalytic subunit of DNA-dependent protein kinase selectively regulates p53-dependent apoptosis but not cell-cycle arrest. Proc Natl Acad Sci USA 97:1584–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taccioli GE , Gottlieb TM , Blunt T , Priestley A , Demengeot J , Mizuta R , Lehmann AR , Alt FW , Jackson SP , Jeggo PA. 1994. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science 265:1442–1445 [DOI] [PubMed] [Google Scholar]
- 27. Burma S , Chen DJ. 2004. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) 3:909–918 [DOI] [PubMed] [Google Scholar]
- 28. Nussenzweig A , Sokol K , Burgman P , Li L , Li GC. 1997. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc Natl Acad Sci USA 94:13588–13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giffin W , Kwast-Welfeld J , Rodda DJ , Préfontaine GG , Traykova-Andonova M , Zhang Y , Weigel NL , Lefebvre YA , Haché RJ. 1997. Sequence-specific DNA binding and transcription factor phosphorylation by Ku autoantigen/DNA-dependent protein kinase. Phosphorylation of Ser-527 of the rat glucocorticoid receptor. J Biol Chem 272:5647–5658 [DOI] [PubMed] [Google Scholar]
- 30. Weigel NL , Carter TH , Schrader WT , O'Malley BW. 1992. Chicken progesterone receptor is phosphorylated by a DNA-dependent protein kinase during in vitro transcription assays. Mol Endocrinol 6:8–14 [DOI] [PubMed] [Google Scholar]
- 31. Medunjanin S , Weinert S , Schmeisser A , Mayer D , Braun-Dullaeus RC. 2010. Interaction of the double-strand break repair kinase DNA-PK and estrogen receptor-α. Mol Biol Cell 21:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yavuzer U , Smith GC , Bliss T , Werner D , Jackson SP. 1998. DNA end-independent activation of DNA-PK mediated via association with the DNA-binding protein C1D. Genes Dev 12:2188–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin B , Kolluri SK , Lin F , Liu W , Han YH , Cao X , Dawson MI , Reed JC , Zhang XK. 2004. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116:527–540 [DOI] [PubMed] [Google Scholar]
- 34. Liu JJ , Zeng HN , Zhang LR , Zhan YY , Chen Y , Wang Y , Wang J , Xiang SH , Liu WJ , Wang WJ , Chen HZ , Shen YM , Su WJ , Huang PQ , Zhang HK , Wu Q. 2010. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res 70:3628–3637 [DOI] [PubMed] [Google Scholar]
- 35. Wilson AJ , Arango D , Mariadason JM , Heerdt BG , Augenlicht LH. 2003. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res 63:5401–5407 [PubMed] [Google Scholar]
- 36. Erster S , Mihara M , Kim RH , Petrenko O , Moll UM. 2004. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol 24:6728–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lei NZ , Zhang XY , Chen HZ , Wang Y , Zhan YY , Zheng ZH , Shen YM , Wu Q. 2009. A feedback regulatory loop between methyltransferase PRMT1 and orphan receptor TR3. Nucleic Acids Res 37:832–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao S , Venkatasubbarao K , Li S , Freeman JW. 2003. Requirement of a specific Sp1 site for histone deacetylase-mediated repression of transforming growth factor β type II receptor expression in human pancreatic cancer cells. Cancer Res 63:2624–2630 [PubMed] [Google Scholar]
- 39. Zhao WX , Tian M , Zhao BX , Li GD , Liu B , Zhan YY , Chen HZ , Wu Q. 2007. Orphan receptor TR3 attenuates the p300-induced acetylation of retinoid X receptor-α. Mol Endocrinol 21:2877–2889 [DOI] [PubMed] [Google Scholar]
- 40. Bonnans C , Fukunaga K , Keledjian R , Petasis NA , Levy BD. 2006. Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury. J Exp Med 203:857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]