Abstract
For decades, natural and synthetic glucocorticoids (GC) have been among the most commonly prescribed classes of immunomodulatory drugs. Their unsurpassed immunosuppressive and antiinflammatory activity along with cost-effectiveness makes these compounds a treatment of choice for the majority of autoimmune and inflammatory diseases, despite serious side effects that frequently accompany GC therapy. The activated GC receptor (GR) that conveys the signaling information of these steroid ligands to the transcriptional machinery engages a number of pathways to ultimately suppress autoimmune responses. Of those, GR-mediated apoptosis of numerous cell types of hematopoietic origin and suppression of proinflammatory cytokine gene expression have been described as the primary mechanisms responsible for the antiinflammatory actions of GC. However, along with the ever-increasing appreciation of the complex functions of the immune system in health and disease, we are beginning to recognize new facets of GR actions in immune cells. Here, we give a brief overview of the extensive literature on the antiinflammatory activities of GC and discuss in greater detail the unexpected pathways, factors, and mechanisms that have recently begun to emerge as novel targets for GC-mediated immunosuppression.
In 1948, a patient at St. Mary's Hospital in Duluth, MN, received the first injection of synthetic cortisol to treat rheumatoid arthritis (RA). Two years later, Edward Kendall, Tadeus Reichstein, and Philip Hench received the Nobel Prize in Physiology and Medicine for their roles in isolating, synthesizing, and delivering cortisol (1, 2); more generally, for discovering the antiinflammatory properties of glucocorticoids (GC). Since then, GC have been used to treat a great variety of inflammatory disorders, and their therapeutic uses are ever widening. In 2008, over 44 million prescriptions for oral, topical, or inhaled GC were written in the United States alone, and GC are a standard in any situation where immunosuppression is desired: after transplant surgery, during severe allergic reactions or autoimmune flare-ups, and as a supplement to certain chemotherapies (3). Much like the diseases for which they are administered, the mechanisms of action of these steroid molecules are extremely diverse. In fact, we now know that GC are immunomodulatory rather than indiscriminately immunosuppressive and that their molecular functions are far more complex than previously recognized. In this minireview, we discuss published examples of GC effects on cytokine-driven autoimmunity and highlight the emerging concepts of their therapeutic mechanisms in various disease states.
GC Receptor (GR) Signaling
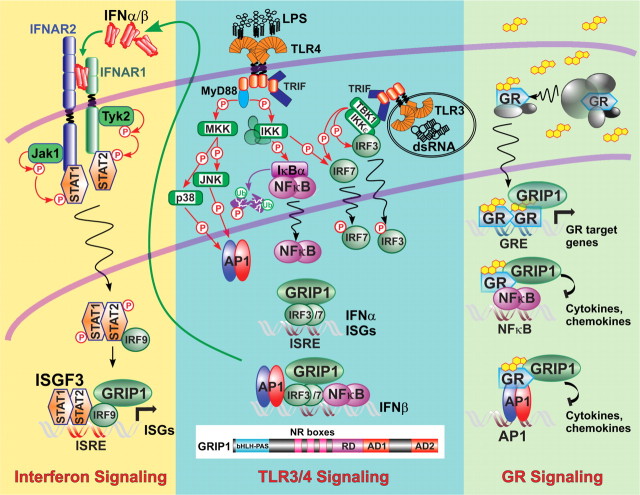
GC are a class of cholesterol-derived steroid molecules that elicit an array of responses in virtually every tissue; indeed, GC are important for metabolism, circadian rhythm, reproduction, and immunity. An early indication of the effect of GC on the immune system was based on the observation that acute or chronic stress induced thymic atrophy, which was later shown to result from cortisol-induced T-cell apoptosis (4). GC signal through the GR, a steroid receptor within a larger nuclear receptor (NR) family of ligand-dependent transcription factors. In the absence of GC hormone, GR is a transcriptionally inactive cytoplasmic protein residing in an “aporeceptor” complex that includes heat shock protiens 70 and 90, immunophilins, and p23 (5). Upon GR association with hormonal ligand, this complex partially dissociates, enabling GR to translocate into the nucleus, where it binds genomic GC-response elements (GRE) and regulates transcription of associated genes (Fig. 1, right panel).
Fig. 1.
A shared coregulator GRIP1 in the GR and type I IFN signaling networks. The three panels depict a GR signaling pathway (right) and the TLR3/4-induced type I IFN production (center) and signaling (left) cascades. The GRIP1 domain structure is diagrammed at the bottom center. Detailed description in the text. MKK, MAPK kinase; IKK, IkB kinase; JNK, Jun kinase; Tyk2, tyrosine kinase-2; MyD88, myeloid differentiation primary response gene 88; TRIF, TIR-domain-containing adapter-inducing IFN-β.
The GRE have been classified into three broad groups (6). “Simple” palindromic GRE are composed of two specific inverted hexamers (AGAACA) separated by a 3-bp linker to which GR binds, usually as a homodimer, and acts as the sole DNA-bound regulator. “Composite” GRE sequences provide a binding surface for GR along with another regulator and may not resemble conventional binding sites for either partner. “Tethering” GRE are binding elements for other transcription factors, e.g. activating protein (AP)1 or nuclear factor (NF)-κB, to which GR is recruited through protein-protein interactions. Although GR can either activate or repress transcription from GRE of all three types, GR binding to a palindromic GRE usually leads to transcriptional activation, whereas GR recruitment to tethering sites typically effects repression. Of note, repression of AP1 and NF-κB activities via GR tethering (also known as “transrepression”) is viewed as a critical component of the inhibitory effects of GC on inflammatory and immune responses.
Similar to other mammalian transcription factors, including NR, GR relies on cofactors (coactivators and corepressors) to transduce hormonal signal to basal transcriptional machinery and/or chromatin. These coregulators, which are critical for transcriptional control in eukaryotes, encompass a variety of proteins, whose molecular actions range from stabilizing DNA-bound regulator complexes and recruiting components of basal machinery to nucleosome remodeling and altering DNA topology. To date, nearly 250 coregulators have been described for NR alone (7). One extensively studied family of coregulators, the p160 proteins (steroid receptor coactivator-1/nuclear receptor coactivator (NCoA)1, TIF2/glucocorticoid receptor-interacting protein (GRIP)1/NCoA2, and NCoA3, were initially isolated in yeast two-hybrid screens with agonist-activated ligand-binding domains of several NR and shown to facilitate transcription by recruiting histone-modifying enzymes, including cAMP response element-binding protein-binding protein (CBP)/p300 acetyl transferases and coactivator-associated arginine methyl transferase-1 (reviewed in Refs. 8, 9). p160 proteins were later shown to function as coactivators for multiple transcription factors in addition to NR. Interestingly, different transcription factors preferentially interact with different p160, and these preferences are cell and target gene specific (reviewed in Ref. 9). Furthermore, although all three p160 family members can function as coactivators, TIF2/GRIP1 also serves as a GR agonist-dependent corepressor at tethering GRE (Fig. 1, right panel) (10, 11).
Multiple Pathways to Autoimmune Activation
GC have been regarded as potent wide-spectrum immunosuppressants for decades. However, the immune system is an intricate network of regulatory pathways and, as such, can be influenced by GC at multiple levels, sometimes with conflicting outputs. Interestingly, GC can modulate both arms of the immune system: innate, which functions as a first line of defense against invading pathogens; and adaptive, which is instructed by immune events that have already transpired and which responds by releasing high-affinity antibodies directed against foreign material. For example, by triggering apoptosis in both “innate” dendritic cells (DC) and “adaptive” T lymphocytes, GR simultaneously affects the activation and effector functions of immune cells through manipulation of their transcriptional pathways.
Innate immune cells, including granulocytes, macrophages (MΦ), and DC, recognize and are activated by molecules that are either nonhost-derived or associated with host cell injury or death. Upon activation, innate immune cells produce a battery of toxic chemicals, such as reactive oxygen and nitrogen species and complement proteins, which act as direct weapons against exogenous threats; chemokines, which attract leukocytes from the bloodstream to infiltrate infected tissues; and cytokines, which can activate or curb innate and/or adaptive responses. In addition, MΦ and DC act as antigen-presenting cells (APC), engulfing and degrading pathogens and then “presenting” molecular fragments (antigens) derived from the degradation process to T cells.
Under normal circumstances, in addition to clearing pathogens, APC and other phagocytes eliminate necrotic, apoptotic, and senescent host cells. However, a sustained failure of APC to properly clear these cells and their components can trigger or contribute to autoimmunity (for review, see Ref. 12). The exact causes of autoimmunity are unknown, although certain genetic risk factors, gender, environmental factors, and infections appear to play some role. Autoimmune responses can be driven by a specific antigen: for example, myelin (a protein that covers and protects neuronal axons) is an antigenic target in multiple sclerosis (MS). Other autoimmune diseases manifest systemically: in the case of systemic lupus erythematosus (SLE), B cells produce antinuclear antibodies directed against numerous nuclear components, including double-stranded DNA, histones, and ribonuclear proteins. Despite the great diversity in pathogenesis, both innate and adaptive responses are affected in most autoimmune diseases.
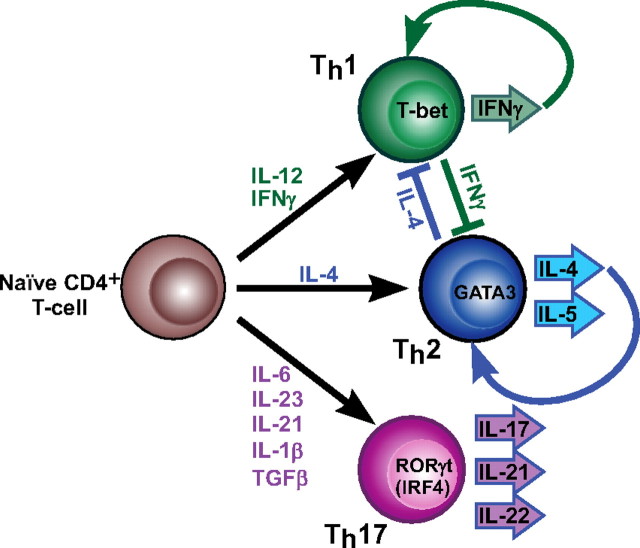
Under the guidance of innate immune cells, naïve T cells can differentiate into at least three T “helper” subsets: Th1, Th2, and Th17 (Fig. 2). The identity of each subtype is orchestrated by unique transcription factors [T-box expressed in T cells (T-bet), GATA3, and retinoic acid receptor-related orphan receptor (RORγt), respectively], which are instructed by the specific cytokine environment. Furthermore, each of the three Th subtypes produces a distinct milieu of cytokines that is inhibitory to the development of the other two. Although not fully understood (reviewed in Ref. 13), the cytokine combinations leading to Th cell specification are beginning to emerge. For example, Th1 cell lineage commitment is driven by IL-12 (14) and, in human cells, type II interferon (IFN) (IFNγ) (15); these cells also produce large quantities of IFNγ, which inhibits Th2 differentiation. Conversely, Th2 cells are specified in part by IL-4, which along with IL-5, is also secreted by these cells and is inhibitory to Th1 differentiation. Recently described Th17 cells (Fig. 2), which characteristically produce IL-17, IL-21, and IL-22, arise from naïve CD4+ T cells in response to a unique cytokine combination that has not been completely elucidated but includes IL-6, IL-23, IL-21, IL-1β, and TGFβ (16, 17).
Fig. 2.
Cytokine control of effector T-cell differentiation. The three effector T-cell subsets along with the cytokines driving their differentiation, key transcription factors identifying the lineage, and cytokines produced by each subset are shown.
The specific roles of Th cell subsets in autoimmunity and inflammation have been reviewed elsewhere and remain the focus of intense investigation (18). In brief, Th2 cells are associated with allergic reactions, such as asthma. Th1 cells secrete IFNγ, which is a potent activator of inflammatory MΦ and granulocytes; IFNγ also stimulates B cells to produce IgG2, an antibody subclass associated with pathogenic autoantibodies (19). Th1 cells were originally described as the predominant subtype dysregulated in autoimmunity and are still regarded as a driving factor in autoimmune pathogenesis. However, excessive Th17 cell numbers have suggested a role for these cells in inflammation and tissue injury in MS, inflammatory bowel disease, psoriasis, and RA (20–22). Indeed, peripheral blood and tissue levels of IL-17, prominently produced by Th17 cells, were found to be elevated in patients with these disorders (23–28). In addition, studies in murine models of MS (experimental autoimmune encephalomyelitis) and RA (collagen-induced arthritis) have implicated IL-17 in the pathogenesis of inflammation (29–32). Interestingly, T cells producing high levels of both IFNγ and IL-17 were found in the kidneys of lupus patients with nephritis (33), and plasma levels of both IL-12 and IL-17 correlated positively with disease severity in SLE (34). Thus, it appears that either Th1 or Th17 can drive autoimmunity and that disease severity can correlate with either or both (35).
T Helper Cell Polarization Is Regulated by GC
The Th2-polarizing properties of GC have been extensively documented and are known to target T cells both indirectly, by affecting the immunomodulatory properties of APC (36–39), and by direct action on T cells themselves. Indeed, in naïve CD4+ T cells, treatment with the synthetic GC dexamethasone (Dex) induces IL-4 mRNA expression (37). Conversely, pretreatment with Dex for as little as 30 min renders naïve T cells incapable of responding to the Th1-polarizing cytokine IL-12, possibly by down-regulating the IL-12R β1- and β2-chain gene expression (40, 41). Studies from the Umetsu group in the late 1990s revealed that GC inhibit Th1 polarization indirectly in both mice and humans by specifying the cytokine repertoir produced by innate immune cells (38, 39). Human monocytes or murine splenic cells that were preexposed to GC and challenged with lipopolysaccharide (LPS) or heat-killed Listeria monocytogenes displayed a marked decrease in IL-12 production. Furthermore, naïve CD4+ T lymphocytes cocultured with the Dex-primed APC produced significantly more IL-4/IL-5 and less IFNγ relative to T cells cultured with unprimed APC, suggesting that the naïve T cells had been polarized by the APC to the Th2 phenotype.
The effects of GC on Th17 development are unknown, in part because the events leading to Th17 differentiation are not entirely understood. Overall, however, the indirect evidence available suggests that GC are likely to prohibit Th17 polarization: to wit, the expression of IL-23, which promotes the differentiation of Th17 cells, is inhibited by the GC prednisolone in DC (42, 43). Similarly, several groups, including ours, demonstrated that the induction of IL-6 by cytokines and pathogenes in several cell types was GC-sensitive (44–47). Interestingly, GC administration has been shown to reduce IL-6 and TGFβ expression in the joints of arthritic mice as well as IL-17 levels in their joints and lymph nodes (48), suggesting that both Th17 differentiation and function may be affected by GC. Furthermore, independent studies reported GC suppression of IL-17 production by purified T cells in vitro (49) and by peripheral blood mononuclear cells of patients suffering from Vogt-Koyanagi-Harada syndrome, an inflammatory autoimmune disorder (50). However, whether and to what extent Th17 differentiation and function are affected by GC, as well as the underlying molecular mechanisms and, ultimately, the possible impact on autoimmune disease, remain to be determined.
DC play a critical role in the activation of Th cells, and DC populations of distinct origins have been proposed to differentially regulate the Th subsets. For example, DC raised in an environment with IL-10, IL-6, and TNFα hinder Th1 cell activation (51). Interestingly, the authors later reported that exposure of DC to Dex induced the expression of the Toll-like receptor (TLR)2, a sensor of bacterial lipoprotein, on their surface and that a subsequent stimulation of these cells with a TLR2 ligand initiated the secretion of IL-10, IL-6, and TNFα (52). The provocative conclusion of this study is that GC may inhibit Th1 cell activation indirectly, through manipulation of DC subset identity. Mutual signaling between the TLR2 and GR pathways was further supported by the unexpected observation that TLR2 knockout mice display deficiencies in adrenal architecture and corticosteroid production (53).
GR Interferes with Inflammatory Cytokine Production
In addition to affecting differentiation of specific T-cell subsets, GC are widely known for their ability to suppress, directly or indirectly, the activation of proinflammatory cytokine genes. GC-mediated suppression of TNFα and IL-1β production has long been considered the basis for their efficacy in relieving symptoms of RA, inflammatory bowel disease, and psoriasis. Furthermore, because chronic inflammation itself in certain autoimmune diseases (e.g. RA) is pathogenic, the ability of GC to attenuate inflammation speaks to the disease-modifying properties of these drugs (54, 55). The molecular basis for GC action has been reviewed in detail (56) and can be broadly classified into the following major mechanisms. First, liganded GR can interfere with the DNA binding of transcription factors, notably NF-κB and AP1, at proinflammatory gene promoters (57, 58). Second, GR engages in protein-protein interactions with transcriptional regulators on DNA (tethering GRE) and actively represses their activity by preventing the recruitment of key coactivators, chromatin modifiers, or components of basal transcriptional machinery (59–63). Third, activated GR was shown to antagonize cytokine gene transcription by sequestering common coregulators, such as CBP (64), although this observation was later debated, when GR was shown to repress the activity of NF-κB and AP1, irrespective of the amount of CBP in the cell (65, 66).
In addition to attenuating the activities of transcription factors driving proinflammatory cytokine gene expression, GR can activate certain genes encoding inhibitors of inflammation. For example, in thymocytes or HeLa cells, GC treatment increased the expression of I-κBα, an inhibitor of NF-κB signaling that sequesters NF-κB dimers in the cytoplasm and prevents their nuclear translocation (67, 68). This mechanism, however, was not operational in other cell types (69–71); thus, its physiological significance was later debated (reviewed in Ref. 72). GR was also shown to augment the levels of dual-specificity phosphatase-1 (also known as MAPK phosphatase-1), which inactivates several MAPK, including p38, a critical kinase for AP1 and NF-κB activation (73, 74). The importance of this mechanism is exemplified in dual-specificity phosphatase-1-deficient MΦ, which are partially resistant to Dex-mediated down-regulation of a subset of proinflammatory cytokines, including TNFα and IL-1β (75). Another GR target, GC-inducible leucine zipper, inhibits AP1 and NF-κB activity by direct protein-protein interactions, precluding their binding to DNA (76, 77). Collectively, these reports highlight the multifarious ability of GR to affect proinflammatory cytokine gene expression.
Type I IFN-Driven Autoimmunity
Although most autoimmune diseases involve an inflammatory component, not all are initiated by the classic proinflammatory cytokines such as TNFα, IL-6, or IL-1β. Indeed, initiation and progression of a subset of immune disorders, including autoimmune thyroiditis and SLE, have been linked to dysregulated type I IFN. Unlike IFNγ, type I IFN (IFNα/β) function primarily as antiviral cytokines in the innate arm of immunity. The recently identified type III IFN (IFNλ also known as IL-28A/B and IL-29) is similar to IFNα/β in its antiviral function but signals through a different receptor complex whose expression is largely limited to cells of epithelial origin (78–80). A link between type I IFN and autoimmunity was suggested as early as 1971, when a high prevalence of the IFN-inducing Epstein-Barr virus was observed in SLE patient sera (81). Years later, Epstein-Barr virus was proposed to be an etiological cause for SLE in susceptible patients and, although a definitive link has not been established, a correlation between viral infections and incidence of SLE was noted (82). IFN therapy, common for the treatment of chronic myeloid leukemia, cutaneous T-cell lymphoma, and viral hepatitis, has also been causally linked to autoimmune side effects. Indeed, de novo appearance of autoantibodies against thyroid antigens, pancreatic islet cells, or the adrenal cortex have been reported in the serum of patients after IFN therapy (83–85). Similarly, autoimmune thrombocytopenia and anemia were observed in C57BL/6 mice injected repeatedly for 10 d with IFNβ (86).
A potentially fatal chronic disorder, SLE most commonly affects the skin (rash) and kidneys (nephritis) but can manifest anywhere in the body, including the heart (myocardial infarction), joints (arthritis), blood vessels (anemia, thrombocytopenia, and coronary artery disease), lungs (pulmonary hypertension), liver (serositis), and central nervous system (stroke, seizure, and psychosis). The course of the disease is variable, with periods of active disease (flares) alternating with remissions. SLE incidence is nine times higher in women (ages 15–50) than men, affecting approximately 1.5 million people in the United States alone (87). The underlying causes for this gender- and age-specific prevalence are unknown. Furthermore, SLE still lacks a well-defined diagnostic signature. The early studies of disease pathogenesis date back to 1948, when a Mayo Clinic hematologist cultured bone marrow preparations from healthy subjects with serum from lupus patients and observed the formation of polymorphonuclear leukocyte clusters around amorphous masses of disrupted nuclei (88). This phenomenon was later attributed to γ-globulins in the lupus serum reacting with DNA-histone complexes in the nuclear material. These antinuclear antibodies (89), however, are not clearly pathological and fail to correlate with disease convincingly enough to establish them as a diagnostic tool.
Beginning in the late 1970s, multiple groups reported high serum IFN levels and IFN-stimulated gene (ISG) expression (“IFN signature”) in peripheral blood mononuclear cells of lupus patients (90–94), which was found to correlate with SLE severity (95–97). Remarkably, patients with active lupus displayed enhanced ISG expression even when serum IFN levels were normal, perhaps suggesting that aberrant IFN-dependent transcription could contribute to disease (94). This promising correlation, however, also fails to meet the criteria necessary to successfully diagnose SLE. Indeed, the IFN signature appears to be an early event in disease pathogenesis, and many patients display neither the IFN signature nor abnormal cytokine levels.
GC Regulation of Type I IFN Production
IFN gene expression is induced by viral components, such as double-stranded RNA (dsRNA), which bind pattern recognition receptors, specifically TLR (e.g. TLR3) on the cell surface or endosomal membranes (Fig. 1, middle panel). Receptor ligation initiates a signaling cascade that through a series of adapters ultimately leads to the activation of IFN regulatory factor (IRF)3, NF-κB, and AP1, which cooperate to induce the transcription of IFN (98). IRF3 binding sites, IFN-stimulated response elements (ISRE), are tandem repeats of GAAA sequences (GAAANNGAAA), which also serve as binding sites for other IRF family members. Type I IFN β and α1 (murine α4) are considered to be “immediate-early” cytokines, their transcription being induced directly via the IRF3/NF-κB pathways and not requiring prior synthesis of protein intermediates, such as IRF7 (98, 99). Transcriptional control of the IFNβ gene involves a coordinate action of three families of factors, IRF3, NF-κB, and AP1, which form an enhanceosome at the IFNβ gene promoter (Fig. 1, middle panel); all three are required for the preinitiation complex assembly and efficient IFNβ gene induction (100–102). Newly synthesized early IFN molecules signal in an auto- or paracrine manner (reviewed in Refs. 98, 103) by binding their cognate receptor, IFN-α receptor, triggering the recruitment, phosphorylation, heterodimerization, and nuclear translocation of the signal transducer and activator of transcription (STAT) proteins 1 and 2 (Fig. 1, left panel). The association of a third transcription factor, IRF9 (p48/ISGF3γ), with STAT2 completes the formation of a heterotrimeric complex, known as ISGF3, with the ISRE-binding specificity, which initiates a secondary wave of ISG transcription (104). Similar to IFNβ, many ISGF3-driven genes contain binding elements for other transcription factors, including NF-κB and AP1, whereas others are regulated exclusively via the ISRE. The majority of these ISGF3 target genes encode antiviral proteins, among them ISG56, ISG54, 2',5'-oligoadenylate synthetase-1 (OASL-1), and myxovirus resistance-1 (Mx1), which are also part of the SLE IFN signature (95, 97).
Along with alleviating the symptoms of SLE, GC treatment suppresses ISG expression, thereby eradicating the IFN signature (96). Although the mechanistic basis of this suppression is not well understood, it could conceivably be attributed to the ability of GC to attenuate, directly or indirectly, the transcriptional activity of factors that regulate IFN gene expression. Notably, in addition to AP1 and NF-κB, recent evidence points to the IRF family of transcriptional regulators as previously unrecognized targets for GR-mediated inhibition. Indeed, GC were shown to inhibit the activity of TANK-binding kinase (TBK)1 that activates IRF3 and IRF7, key components of the IFNβ enhanceosome (101, 105). Specifically, Dex treatment of U373 astrocytoma cells abolished LPS- or dsRNA-induced phosphorylation of TBK1 at S172, required for TBK1 kinase activity (106). The exact contribution of this mechanism to GC inhibition of IFN gene transcription, however, remains to be determined, because residual IRF3 activity, ISG induction by dsRNA, and to a lesser extent, viral infection persisted in TBK1-deficient MΦ (107).
In 2005, studies from our laboratory and the Glass group described two distinct mechanisms targeting IRF3 transcriptional activity by the activated GR. Specifically, in an unbiased yeast two-hybrid screen, we isolated IRF3 as an interacting partner for GRIP1, a member of the p160 family of coregulators and a known cofactor for GR and other NR (Fig. 1, center panel) (108). The GRIP1-IRF3 interaction was also observed in vitro and in murine MΦ and was disrupted by Dex-activated GR. Furthermore, depletion of GRIP1 from IRF3 by small interfering RNA knockdown or liganded GR severely compromised the dsRNA-dependent induction of IFNβ and other ISG, whereas GRIP1 overexpression relieved inhibition. These studies implicated GRIP1 as a bona fide IRF3 coactivator, whose sequestration by hormone-activated GR attenuated the transcription of IRF3 target genes.
In parallel, Ogawa et al. (109) have shown that the induction of IRF3 target genes by bacterial LPS in MΦ can be inhibited by GC through a distinct mechanism. Specifically, they observed that LPS treatment induced corecruitment of IRF3 and the p65 subunit of NF-κB to both ISRE- or NF-κB-containing promoters and that p65 served as an IRF3 coactivator in this context. In response to GC, GR sequestered p65 from IRF3, thereby antagonizing the expression of ISRE-regulated ISG (109). Although IFNβ gene expression was not specifically examined, this report further corroborates the emergence of IRF as a previously unrecognized family of transcription factors under indirect GC control.
Recently, a link has been established between sustained TLR7/9 signaling and resistance to GC treatment in SLE (110). Specifically, it was shown that GC-induced apoptosis of plasmacytoid DC (pDC), critical IFN-producing cells contributing to the IFN signature, was attenuated by persistent TLR7/9 activation, whereas pharmacological blockade of TLR7/9 restored pDC sensitivity to GC and normalized ISG expression (110, 111). GC resistance of pDC was cell type specific and appeared to result from TLR7/9-induced escape of the NF-κB pathway from GC-mediated inhibition. Although the mechanistic basis of these latter observations remains unclear, they further suggest extensive cross talk between GR, NF-κB, and IRF-associated signaling pathways ultimately regulating IFN gene expression.
GC Regulation of the IFN Signaling Pathway Through a Shared Cofactor
Despite the wealth of evidence that points to GC-mediated inhibition of cytokine production, little is known about the effects of GC on Janus kinase (Jak)/STAT signaling pathways initiated by cytokines at the cell surface. GR has been shown to interfere with IL-2 signaling, although via an indirect mechanism whereby GR blocks the expression of the common receptor IL-2Rβ and the signaling intermediate Jak3 (112). In stark contrast, GR synergizes with prolactin-activated STAT5 and with IL-6-activated STAT3 (113–116), although the mechanisms of synergy remain unclear. In fact, most reports reveal little effect of GR on cytokine signaling via Jak/STAT. Unexpectedly, however, we found that the type I IFN-initiated Jak/STAT pathway is under GR control and that the target of GC inhibition is the effector complex, ISGF3 (45). Unlike other STAT complexes, the ISGF3 heterotrimer contains a non-STAT subunit, IRF9, which is thought to dictate the ability of ISGF3 to recognize ISRE, rather than the TTCCNGGAA palindromic sequences targeted by STAT homo- or heterodimers. We found that GRIP1 physically interacted with IRF9, resembling its interactions with IRF3, and served as a coactivator for the IFN-inducible ISGF3 transcription complex. Furthermore, GR activation by coadministration of Dex in MΦ antagonized IFN-induced ISGF3 promoter occupancy, histone acetylation, and RNA polymerase II recruitment to IFN target genes and, similar to IRF3, effected an ISG expression profile identical to that observed upon GRIP1 knockdown or genetic disruption (45). Notably, this regulation was specific to MΦ, in which GRIP1 protein level is exceptionally low; IFN signaling was refractory to Dex in “GRIP1-high” fibroblasts. Supporting these observations, GRIP1 overexpression in MΦ-like RAW264.7 cells relieved GC control of ISG induction.
The fact that two fundamentally different transcriptional regulators (IRF3, responsible for IFN production; and ISGF3, controlling IFN signaling) both required GRIP1 for optimal target gene induction not only suggests a pivotal role of this coregulator in the innate immune response but also reveals a previously unrecognized ability of GC to target the IFN network at two distinct steps. Remarkably, GRIP1 knockout mice display a hepatic expression profile with a disproportionately high number of down-regulated immune-related genes, a pattern not shared by mice deficient in other p160 family members (117, 118). Interestingly, the domain of GRIP1 responsible for binding IRF family members is not conserved among other p160, highlighting its unique role in mediating GC effects on the immune system. Conversely, the GRIP1-binding IRF association domain of IRF3 and IRF9 shares significant sequence homology with that of the other IRF proteins, and at least in vitro, GRIP1 interacts with IRF1, IRF5, and IRF7 (45, 108, 119), suggesting that a similar paradigm may hold true for other IRF family members. Unfortunately, due to their reproductive, metabolic, and endocrine phenotypes (120–123), GRIP1-null mice do not represent an appropriate model for studying autoimmunity. Conditional depletion of GRIP1 in specific immune cell compartments in the adult animal will further our understanding of the role of this protein, and its potential interactions with IRF, in autoimmune pathogenesis.
Conclusions
GC are a standard therapeutic approach in many diseases ranging from mild skin rashes to life-threatening syndromes; autoimmune processes, in particular, have been managed with GC for decades. The common thread connecting all these disorders is an exaggerated proinflammatory cytokine response. Not surprisingly, the clinical efficacy of GC is often attributed to GR-mediated suppression of cytokine gene expression. However, our recent studies revealed that the Jak/STAT signaling pathway triggered by at least one cytokine, type I IFN, is directly controlled by GC. Although the presence of the IRF subunit in the IFN-inducible transcription complex, ISGF3, makes this signaling pathway, perhaps uniquely, susceptible to GC regulation, accumulating evidence suggests that the molecular mechanism of regulation is not specific to ISGF3. Indeed, with several IRF family members relying on GRIP1 coactivator properties, one envisions the implications of such interactions for other IRF-regulated pathways. For example, multiple association studies describing genetic polymorphisms in the IRF5 gene and its regulatory region as risk alleles for SLE (124, 125) warrant an examination of the potential role of GRIP1 in this process. IRF4, a critical regulator of Th17 differentiation and pathogenesis of autoimmunity (126–129), is also of particular interest. It is tempting to speculate that by antagonizing IRF4-dependent transcription through a GRIP1-dependent mechanism, GC inhibit Th17 lineage commitment and autoimmune effects associated with Th cell dysregulation. As appealing as this model might be mechanistically, however, critical consideration needs to be given to the unique cell type-specific environment (developmental and epigenetic restrictions, relative expression levels of individual regulatory components and their posttranslational modifications, or the particular signaling inputs to which a given tissue is exposed in vivo) that would determine the balance between the individual signaling networks. Because cofactors such as GRIP1 are beginning to emerge as rheostats that determine the “current” through a given signaling pathway, understanding their physical and functional interfaces with specific regulators in the context of a disease-relevant cell type could reap therapeutic benefits.
Acknowledgments
We thank Dr. L. Ivashkiv for critical comments on the manuscript and Dr. Y. Chinenov for preparation of illustrations.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: GR;
Coregulators: SRC-1 | GRIP1 | AIB1 | CBP | p300;
Ligands: Dexamethasone | Hydrocortisone.
Footnotes
- AP
- Activating protein
- APC
- antigen-presenting cell
- CBP
- cAMP response element-binding protein-binding protein
- DC
- dendritic cell
- Dex
- dexamethasone
- dsRNA
- double-stranded RNA
- GC
- glucocorticoid
- GR
- GC receptor
- GRE
- GC-response element
- GRIP
- glucocorticoid receptor-interacting protein
- IFN
- interferon
- IRF
- IFN regulatory factor
- ISG
- IFN-stimulated gene
- ISRE
- IFN-stimulated response element
- Jak
- Janus kinase
- LPS
- lipopolysaccharide
- MΦ
- macrophage
- MS
- multiple sclerosis
- NCoA
- nuclear receptor coactivator
- NF
- nuclear factor
- NR
- nuclear receptor
- pDC
- plasmacytoid DC
- RA
- rheumatoid arthritis
- SLE
- systemic lupus erythematosus
- STAT
- signal transducer and activator of transcription
- TBK
- TANK-binding kinase
- TLR
- Toll-like receptor.
References
- 1. Ward E , Slocumb CH , Polley HF , Kendall EC , Hench PS. 1951. Clinical effects of cortisone administered orally to 100 patients with rheumatoid arthritis. Ann Rheum Dis 10:477–484 [PubMed] [Google Scholar]
- 2. Hench PS , Kendall EC , Slocumb CH , Polley HF. 1949. Adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis 8:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schappert SM , Rechtsteiner EA. 2008. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Report:1–29 [PubMed] [Google Scholar]
- 4. Tarcic N , Ovadia H , Weiss DW , Weidenfeld J. 1998. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol 82:40–46 [DOI] [PubMed] [Google Scholar]
- 5. Pratt WB , Galigniana MD , Harrell JM , DeFranco DB. 2004. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 16:857–872 [DOI] [PubMed] [Google Scholar]
- 6. Lefstin JA , Yamamoto KR. 1998. Allosteric effects of DNA on transcriptional regulators. Nature 392:885–888 [DOI] [PubMed] [Google Scholar]
- 7. Margolis RN , Evans RM , O'Malley BW. 2005. The nuclear receptor signaling atlas: development of a functional atlas of nuclear receptors. Mol Endocrinol 19:2433–2436 [DOI] [PubMed] [Google Scholar]
- 8. Stallcup MR , Kim JH , Teyssier C , Lee YH , Ma H , Chen D. 2003. The roles of protein-protein interactions and protein methylation in transcriptional activation by nuclear receptors and their coactivators. J Steroid Biochem Mol Biol 85:139–145 [DOI] [PubMed] [Google Scholar]
- 9. Xu J , Li Q. 2003. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- 10. Rogatsky I , Luecke HF , Leitman DC , Yamamoto KR. 2002. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci USA 99:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogatsky I , Zarember KA , Yamamoto KR. 2001. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 20:6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tveita AA. 2010. The danger model in deciphering autoimmunity. Rheumatology 49:632–639 [DOI] [PubMed] [Google Scholar]
- 13. Sundrud MS , Nolan MA. 2010. Synergistic and combinatorial control of T cell activation and differentiation by transcription factors. Curr Opin Immunol 22:286–292 [DOI] [PubMed] [Google Scholar]
- 14. Hsieh CS , Macatonia SE , Tripp CS , Wolf SF , O'Garra A , Murphy KM. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547–549 [DOI] [PubMed] [Google Scholar]
- 15. Rogge L , D'Ambrosio D , Biffi M , Penna G , Minetti LJ , Presky DH , Adorini L , Sinigaglia F. 1998. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol 161:6567–6574 [PubMed] [Google Scholar]
- 16. Harrington LE , Hatton RD , Mangan PR , Turner H , Murphy TL , Murphy KM , Weaver CT. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- 17. Manel N , Unutmaz D , Littman DR. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol 9:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damsker JM , Hansen AM , Caspi RR. 2010. Th1 and Th17 cells: adversaries and collaborators. Ann NY Acad Sci 1183:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terato K , Hasty KA , Reife RA , Cremer MA , Kang AH , Stuart JM. 1992. Induction of arthritis with monoclonal antibodies to collagen. J Immunol 148:2103–2108 [PubMed] [Google Scholar]
- 20. Steinman L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13:139–145 [DOI] [PubMed] [Google Scholar]
- 21. Takahashi S , Fossati L , Iwamoto M , Merino R , Motta R , Kobayakawa T , Izui S. 1996. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest 97:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang B , André I , Gonzalez A , Katz JD , Aguet M , Benoist C , Mathis D. 1997. Interferon-γ impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA 94:13844–13849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hölttä V , Klemetti P , Sipponen T , Westerholm-Ormio M , Kociubinski G , Salo H , Räsänen L , Kolho KL , Färkkilä M , Savilahti E , Vaarala O. 2008. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis 14:1175–1184 [DOI] [PubMed] [Google Scholar]
- 24. Zheng Y , Danilenko DM , Valdez P , Kasman I , Eastham-Anderson J , Wu J , Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648–651 [DOI] [PubMed] [Google Scholar]
- 25. Matusevicius D , Kivisäkk P , He B , Kostulas N , Ozenci V , Fredrikson S , Link H. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 5:101–104 [DOI] [PubMed] [Google Scholar]
- 26. Chabaud M , Durand JM , Buchs N , Fossiez F , Page G , Frappart L , Miossec P. 1999. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42:963–970 [DOI] [PubMed] [Google Scholar]
- 27. Tzartos JS , Friese MA , Craner MJ , Palace J , Newcombe J , Esiri MM , Fugger L. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kebir H , Kreymborg K , Ifergan I , Dodelet-Devillers A , Cayrol R , Bernard M , Giuliani F , Arbour N , Becher B , Prat A. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langrish CL , Chen Y , Blumenschein WM , Mattson J , Basham B , Sedgwick JD , McClanahan T , Kastelein RA , Cua DJ. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y , Langrish CL , McKenzie B , Joyce-Shaikh B , Stumhofer JS , McClanahan T , Blumenschein W , Churakovsa T , Low J , Presta L , Hunter CA , Kastelein RA , Cua DJ. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest 116:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park H , Li Z , Yang XO , Chang SH , Nurieva R , Wang YH , Wang Y , Hood L , Zhu Z , Tian Q , Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edgerton C , Crispín JC , Moratz CM , Bettelli E , Oukka M , Simovic M , Zacharia A , Egan R , Chen J , Dalle Lucca JJ , Juang YT , Tsokos GC. 2009. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol 130:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crispín JC , Oukka M , Bayliss G , Cohen RA , Van Beek CA , Stillman IE , Kyttaris VC , Juang YT , Tsokos GC. 2008. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181:8761–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong CK , Lit LC , Tam LS , Li EK , Wong PT , Lam CW. 2008. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol 127:385–393 [DOI] [PubMed] [Google Scholar]
- 35. Luger D , Silver PB , Tang J , Cua D , Chen Z , Iwakura Y , Bowman EP , Sgambellone NM , Chan CC , Caspi RR. 2008. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med 205:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elenkov IJ. 2004. Glucocorticoids and the Th1/Th2 balance. Ann NY Acad Sci 1024:138–146 [DOI] [PubMed] [Google Scholar]
- 37. Ramírez F , Fowell DJ , Puklavec M , Simmonds S , Mason D. 1996. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol 156:2406–2412 [PubMed] [Google Scholar]
- 38. DeKruyff RH , Fang Y , Umetsu DT. 1998. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 160:2231–2237 [PubMed] [Google Scholar]
- 39. Blotta MH , DeKruyff RH , Umetsu DT. 1997. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol 158:5589–5595 [PubMed] [Google Scholar]
- 40. Wu CY , Wang K , McDyer JF , Seder RA. 1998. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol 161:2723–2730 [PubMed] [Google Scholar]
- 41. Fahey AJ , Robins RA , Kindle KB , Heery DM , Constantinescu CS. 2006. Effects of glucocorticoids on STAT4 activation in human T cells are stimulus-dependent. J Leukoc Biol 80:133–144 [DOI] [PubMed] [Google Scholar]
- 42. Luther C , Adamopoulou E , Stoeckle C , Brucklacher-Waldert V , Rosenkranz D , Stoltze L , Lauer S , Poeschel S , Melms A , Tolosa E. 2009. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J Immunol 183:841–848 [DOI] [PubMed] [Google Scholar]
- 43. Zhou L , Ivanov II , Spolski R , Min R , Shenderov K , Egawa T , Levy DE , Leonard WJ , Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974 [DOI] [PubMed] [Google Scholar]
- 44. Waage A , Slupphaug G , Shalaby R. 1990. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol 20:2439–2443 [DOI] [PubMed] [Google Scholar]
- 45. Flammer JR , Dobrovolna J , Kennedy MA , Chinenov Y , Glass CK , Ivashkiv LB , Rogatsky I. 2010. Type I interferon signaling pathway is a target for glucocorticoid Inhibition. Mol Cell Biol 30:4564–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zitnik RJ , Whiting NL , Elias JA. 1994. Glucocorticoid inhibition of interleukin-1-induced interleukin-6 production by human lung fibroblasts: evidence for transcriptional and post-transcriptional regulatory mechanisms. Am J Respir Cell Mol Biol 10:643–650 [DOI] [PubMed] [Google Scholar]
- 47. Vieira PL , Kaliński P , Wierenga EA , Kapsenberg ML , de Jong EC. 1998. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol 161:5245–5251 [PubMed] [Google Scholar]
- 48. Monari C , Bevilacqua S , Piccioni M , Pericolini E , Perito S , Calvitti M , Bistoni F , Kozel TR , Vecchiarelli A. 2009. A microbial polysaccharide reduces the severity of rheumatoid arthritis by influencing Th17 differentiation and proinflammatory cytokines production. J Immunol 183:191–200 [DOI] [PubMed] [Google Scholar]
- 49. Momcilović M , Miljković Z , Popadić D , Marković M , Savić E , Ramić Z , Miljković D , Mostarica-Stojković M. 2008. Methylprednisolone inhibits interleukin-17 and interferon-γ expression by both naive and primed T cells. BMC Immunol 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang K , Wen J , Liu X , Kijlstra A , Chen L , Chi W , Zhou H , Huang X , Yang P. 2009. Inhibitory effect of rapamycin and dexamethasone on production of IL-17 and IFN-γ in Vogt-Koyanagi-Harada patients. Br J Ophthalmol 93:249–253 [DOI] [PubMed] [Google Scholar]
- 51. Velten FW , Duperrier K , Bohlender J , Metharom P , Goerdt S. 2004. A gene signature of inhibitory MHC receptors identifies a BDCA3(+) subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur J Immunol 34:2800–2811 [DOI] [PubMed] [Google Scholar]
- 52. Duperrier K , Velten FW , Bohlender J , Demory A , Metharom P , Goerdt S. 2005. Immunosuppressive agents mediate reduced allostimulatory properties of myeloid-derived dendritic cells despite induction of divergent molecular phenotypes. Mol Immunol 42:1531–1540 [DOI] [PubMed] [Google Scholar]
- 53. Bornstein SR , Zacharowski P , Schumann RR , Barthel A , Tran N , Papewalis C , Rettori V , McCann SM , Schulze-Osthoff K , Scherbaum WA , Tarnow J , Zacharowski K. 2004. Impaired adrenal stress response in toll-like receptor 2-deficient mice. Proc Natl Acad Sci USA 101:16695–16700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Graudal N , Jürgens G. 2010. Similar effects of disease-modifying antirheumatic drugs, glucocorticoids, and biologic agents on radiographic progression in rheumatoid arthritis: meta-analysis of 70 randomized placebo-controlled or drug-controlled studies, including 112 comparisons. Arthritis Rheum 62:2852–2863 [DOI] [PubMed] [Google Scholar]
- 55. Gorter SL , Bijlsma JW , Cutolo M , Gomez-Reino J , Kouloumas M , Smolen JS , Landewé R. 2010. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 69:1010–1014 [DOI] [PubMed] [Google Scholar]
- 56. De Bosscher K , Haegeman G. 2009. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol 23:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jonat C , Rahmsdorf HJ , Park KK , Cato AC , Gebel S , Ponta H , Herrlich P. 1990. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62:1189–1204 [DOI] [PubMed] [Google Scholar]
- 58. Yang-Yen HF , Chambard JC , Sun YL , Smeal T , Schmidt TJ , Drouin J , Karin M. 1990. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62:1205–1215 [DOI] [PubMed] [Google Scholar]
- 59. Nissen RM , Yamamoto KR. 2000. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14:2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luecke HF , Yamamoto KR. 2005. The glucocorticoid receptor blocks P-TEFb recruitment by NFκB to effect promoter-specific transcriptional repression. Genes Dev 19:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. König H , Ponta H , Rahmsdorf HJ , Herrlich P. 1992. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J 11:2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamamoto KR , Darimont BD , Wagner RL , Iñiguez-Lluhí JA. 1998. Building transcriptional regulatory complexes: signals and surfaces. Cold Spring Harb Symp Quant Biol 63:587–598 [DOI] [PubMed] [Google Scholar]
- 63. Ray A , Prefontaine KE. 1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κB and the glucocorticoid receptor. Proc Natl Acad Sci USA 91:752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamei Y , Xu L , Heinzel T , Torchia J , Kurokawa R , Gloss B , Lin SC , Heyman RA , Rose DW , Glass CK , Rosenfeld MG. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403–414 [DOI] [PubMed] [Google Scholar]
- 65. De Bosscher K , Vanden Berghe W , Haegeman G. 2001. Glucocorticoid repression of AP-1 is not mediated by competition for nuclear coactivators. Mol Endocrinol 15:219–227 [DOI] [PubMed] [Google Scholar]
- 66. De Bosscher K , Vanden Berghe W , Vermeulen L , Plaisance S , Boone E , Haegeman G. 2000. Glucocorticoids repress NF-κB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc Natl Acad Sci USA 97:3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scheinman RI , Cogswell PC , Lofquist AK , Baldwin AS. 1995. Role of transcriptional activation of I κ B α in mediation of immunosuppression by glucocorticoids. Science 270:283–286 [DOI] [PubMed] [Google Scholar]
- 68. Auphan N , DiDonato JA , Rosette C , Helmberg A , Karin M. 1995. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of I κB synthesis. Science 270:286–290 [DOI] [PubMed] [Google Scholar]
- 69. Brostjan C , Anrather J , Csizmadia V , Stroka D , Soares M , Bach FH , Winkler H. 1996. Glucocorticoid-mediated repression of NFκB activity in endothelial cells does not involve induction of IκBα synthesis. J Biol Chem 271:19612–19616 [DOI] [PubMed] [Google Scholar]
- 70. Pérez P , Page A , Bravo A , Del Río M , Giménez-Conti I , Budunova I , Slaga TJ , Jorcano JL. 2001. Altered skin development and impaired proliferative and inflammatory responses in transgenic mice overexpressing the glucocorticoid receptor. FASEB J 15:2030–2032 [DOI] [PubMed] [Google Scholar]
- 71. De Bosscher K , Schmitz ML , Vanden Berghe W , Plaisance S , Fiers W , Haegeman G. 1997. Glucocorticoid-mediated repression of nuclear factor-κB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA 94:13504–13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cato A , Schaecke H , Asadullah K. 2002. Recent advances in glucocorticoid receptor action. Berlin: Springer [Google Scholar]
- 73. Kassel O , Sancono A , Krätzschmar J , Kreft B , Stassen M , Cato AC. 2001. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 20:7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lasa M , Abraham SM , Boucheron C , Saklatvala J , Clark AR. 2002. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22:7802–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abraham SM , Lawrence T , Kleiman A , Warden P , Medghalchi M , Tuckermann J , Saklatvala J , Clark AR. 2006. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203:1883–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mittelstadt PR , Ashwell JD. 2001. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J Biol Chem 276:29603–29610 [DOI] [PubMed] [Google Scholar]
- 77. Ayroldi E , Migliorati G , Bruscoli S , Marchetti C , Zollo O , Cannarile L , D'Adamio F , Riccardi C. 2001. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor κB. Blood 98:743–753 [DOI] [PubMed] [Google Scholar]
- 78. Ank N , West H , Paludan SR. 2006. IFN-λ: novel antiviral cytokines. J Interferon Cytokine Res 26:373–379 [DOI] [PubMed] [Google Scholar]
- 79. Sheppard P , Kindsvogel W , Xu W , Henderson K , Schlutsmeyer S , Whitmore TE , Kuestner R , Garrigues U , Birks C , Roraback J , Ostrander C , Dong D , Shin J , Presnell S , Fox B , Haldeman B , Cooper E , Taft D , Gilbert T , Grant FJ , Tackett M , Krivan W , McKnight G , Clegg C , Foster D , Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4:63–68 [DOI] [PubMed] [Google Scholar]
- 80. Kotenko SV , Gallagher G , Baurin VV , Lewis-Antes A , Shen M , Shah NK , Langer JA , Sheikh F , Dickensheets H , Donnelly RP. 2003. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4:69–77 [DOI] [PubMed] [Google Scholar]
- 81. Evans AS , Rothfield NF , Niederman JC. 1971. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet 1:167–168 [DOI] [PubMed] [Google Scholar]
- 82. James JA , Kaufman KM , Farris AD , Taylor-Albert E , Lehman TJ , Harley JB. 1997. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest 100:3019–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rönnblom LE , Alm GV , Oberg KE. 1990. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J Intern Med 227:207–210 [DOI] [PubMed] [Google Scholar]
- 84. Wesche B , Jaeckel E , Trautwein C , Wedemeyer H , Falorni A , Frank H , von zur Mühlen A , Manns MP , Brabant G. 2001. Induction of autoantibodies to the adrenal cortex and pancreatic islet cells by interferon α therapy for chronic hepatitis C. Gut 48:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kiehne K , Kloehn S , Hinrichsen H , Gallwitz B , Mönig H. 1997. Thyroid autoantibodies and thyroid dysfunction during treatment with interferon-α for chronic hepatitis C. Endocrine 6:231–234 [DOI] [PubMed] [Google Scholar]
- 86. Rosenthal GJ , Stranahan RP , Thompson M , Blair P , Germolec DR , Comment CE , Schwab K , Luster MI. 1990. Organ-specific hematopoietic changes induced by a recombinant human interferon-α in mice. Fundam Appl Toxicol 14:666–675 [DOI] [PubMed] [Google Scholar]
- 87. Rahman A , Isenberg DA. 2008. Systemic lupus erythematosus. N Engl J Med 358:929–939 [DOI] [PubMed] [Google Scholar]
- 88. Hargraves MM. 1969. Discovery of the LE cell and its morphology. Mayo Clin Proc 44:579–599 [PubMed] [Google Scholar]
- 89. Friou GJ. 1967. Antinuclear antibodies: diagnostic significance and methods. Arthritis Rheum 10:151–159 [DOI] [PubMed] [Google Scholar]
- 90. Preble OT , Black RJ , Friedman RM , Klippel JH , Vilcek J. 1982. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 216:429–431 [DOI] [PubMed] [Google Scholar]
- 91. Hooks JJ , Moutsopoulos HM , Geis SA , Stahl NI , Decker JL , Notkins AL. 1979. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 301:5–8 [DOI] [PubMed] [Google Scholar]
- 92. Bengtsson AA , Sturfelt G , Truedsson L , Blomberg J , Alm G , Vallin H , Rönnblom L. 2000. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9:664–671 [DOI] [PubMed] [Google Scholar]
- 93. Ytterberg SR , Schnitzer TJ. 1982. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum 25:401–406 [DOI] [PubMed] [Google Scholar]
- 94. von Wussow P , Jakschies D , Hochkeppel H , Horisberger M , Hartung K , Deicher H. 1989. MX homologous protein in mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum 32:914–918 [PubMed] [Google Scholar]
- 95. Crow MK , Wohlgemuth J. 2003. Microarray analysis of gene expression in lupus. Arthritis Res Ther 5:279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bennett L , Palucka AK , Arce E , Cantrell V , Borvak J , Banchereau J , Pascual V. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Baechler EC , Batliwalla FM , Karypis G , Gaffney PM , Ortmann WA , Espe KJ , Shark KB , Grande WJ , Hughes KM , Kapur V , Gregersen PK , Behrens TW. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 100:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Levy DE , Marié I , Prakash A. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr Opin Immunol 15:52–58 [DOI] [PubMed] [Google Scholar]
- 99. Marié I , Durbin JE , Levy DE. 1998. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J 17:6660–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Thanos D , Maniatis T. 1995. Identification of the rel family members required for virus induction of the human β interferon gene. Mol Cell Biol 15:152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sharma S , tenOever BR , Grandvaux N , Zhou GP , Lin R , Hiscott J. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151 [DOI] [PubMed] [Google Scholar]
- 102. Geiss G , Jin G , Guo J , Bumgarner R , Katze MG , Sen GC. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J Biol Chem 276:30178–30182 [DOI] [PubMed] [Google Scholar]
- 103. Shuai K , Liu B. 2003. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3:900–911 [DOI] [PubMed] [Google Scholar]
- 104. Martinez-Moczygemba M , Gutch MJ , French DL , Reich NC. 1997. Distinct STAT structure promotes interaction of STAT2 with the p48 subunit of the interferon-α-stimulated transcription factor ISGF3. J Biol Chem 272:20070–20076 [DOI] [PubMed] [Google Scholar]
- 105. McCoy CE , Carpenter S , Pålsson-McDermott EM , Gearing LJ , O'Neill LA. 2008. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J Biol Chem 283:14277–14285 [DOI] [PubMed] [Google Scholar]
- 106. Kishore N , Huynh QK , Mathialagan S , Hall T , Rouw S , Creely D , Lange G , Caroll J , Reitz B , Donnelly A , Boddupalli H , Combs RG , Kretzmer K , Tripp CS. 2002. IKK-i and TBK-1 are enzymatically distinct from the homologous enzyme IKK-2: comparative analysis of recombinant human IKK-i, TBK-1, and IKK-2. J Biol Chem 277:13840–13847 [DOI] [PubMed] [Google Scholar]
- 107. Hemmi H , Takeuchi O , Sato S , Yamamoto M , Kaisho T , Sanjo H , Kawai T , Hoshino K , Takeda K , Akira S. 2004. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med 199:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Reily MM , Pantoja C , Hu X , Chinenov Y , Rogatsky I. 2006. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J 25:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ogawa S , Lozach J , Benner C , Pascual G , Tangirala RK , Westin S , Hoffmann A , Subramaniam S , David M , Rosenfeld MG , Glass CK. 2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guiducci C , Gong M , Xu Z , Gill M , Chaussabel D , Meeker T , Chan JH , Wright T , Punaro M , Bolland S , Soumelis V , Banchereau J , Coffman RL , Pascual V , Barrat FJ. 2010. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lepelletier Y , Zollinger R , Ghirelli C , Raynaud F , Hadj-Slimane R , Cappuccio A , Hermine O , Liu YJ , Soumelis V. 2010. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid predendritic cells (pDCs). Blood 116:3389–3397 [DOI] [PubMed] [Google Scholar]
- 112. Bianchi M , Meng C , Ivashkiv LB. 2000. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci USA 97:9573–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stöcklin E , Wissler M , Gouilleux F , Groner B. 1996. Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728 [DOI] [PubMed] [Google Scholar]
- 114. Lerner L , Henriksen MA , Zhang X , Darnell JE. 2003. STAT3-dependent enhanceosome assembly and disassembly: synergy with GR for full transcriptional increase of the α 2-macroglobulin gene. Genes Dev 17:2564–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lechner J , Welte T , Tomasi JK , Bruno P , Cairns C , Gustafsson J , Doppler W. 1997. Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of β-casein gene transcription. J Biol Chem 272:20954–20960 [DOI] [PubMed] [Google Scholar]
- 116. Cella N , Groner B , Hynes NE. 1998. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol Cell Biol 18:1783–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jeong JW , Kwak I , Lee KY , White LD , Wang XP , Brunicardi FC , O'Malley BW , DeMayo FJ. 2006. The genomic analysis of the impact of steroid receptor coactivators ablation on hepatic metabolism. Mol Endocrinol 20:1138–1152 [DOI] [PubMed] [Google Scholar]
- 118. Chopra AR , Louet JF , Saha P , An J , Demayo F , Xu J , York B , Karpen S , Finegold M , Moore D , Chan L , Newgard CB , O'Malley BW. 2008. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bhandare R , Damera G , Banerjee A , Flammer JR , Keslacy S , Rogatsky I , Panettieri RA , Amrani Y , Tliba O. 2010. Glucocorticoid receptor interacting protein-1 restores glucocorticoid responsiveness in steroid-resistant airway structural cells. Am J Respir Cell Mol Biol 42:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gehin M , Mark M , Dennefeld C , Dierich A , Gronemeyer H , Chambon P. 2002. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol 22:5923–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Picard F , Géhin M , Annicotte J , Rocchi S , Champy MF , O'Malley BW , Chambon P , Auwerx J. 2002. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111:931–941 [DOI] [PubMed] [Google Scholar]
- 122. Mukherjee A , Soyal SM , Fernandez-Valdivia R , Gehin M , Chambon P , Demayo FJ , Lydon JP , O'Malley BW. 2006. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 26:6571–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Patchev AV , Fischer D , Wolf SS , Herkenham M , Götz F , Gehin M , Chambon P , Patchev VK , Almeida OF. 2007. Insidious adrenocortical insufficiency underlies neuroendocrine dysregulation in TIF-2 deficient mice. FASEB J 21:231–238 [DOI] [PubMed] [Google Scholar]
- 124. Graham RR , Kozyrev SV , Baechler EC , Reddy MV , Plenge RM , Bauer JW , Ortmann WA , Koeuth T , González Escribano MF , Pons-Estel B , Petri M , Daly M , Gregersen PK , Martín J , Altshuler D , Behrens TW , Alarcón-Riquelme ME. 2006. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet 38:550–555 [DOI] [PubMed] [Google Scholar]
- 125. Sigurdsson S , Nordmark G , Göring HH , Lindroos K , Wiman AC , Sturfelt G , Jönsen A , Rantapää-Dahlqvist S , Möller B , Kere J , Koskenmies S , Widén E , Eloranta ML , Julkunen H , Kristjansdottir H , Steinsson K , Alm G , Rönnblom L , Syvänen AC. 2005. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet 76:528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fanzo JC , Yang W , Jang SY , Gupta S , Chen Q , Siddiq A , Greenberg S , Pernis AB. 2006. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest 116:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Biswas PS , Bhagat G , Pernis AB. 2010. IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev 233:79–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen Q , Yang W , Gupta S , Biswas P , Smith P , Bhagat G , Pernis AB. 2008. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity 29:899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Biswas PS , Gupta S , Chang E , Song L , Stirzaker RA , Liao JK , Bhagat G , Pernis AB. 2010. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest 120:3280–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]