Abstract
The separation of various organelles from cotton cotyledon (Gossypium hirsutum L.), cucumber cotyledon (Cucumis sativus L.), peanut cotyledon (Archis hypogaea L.), pine megagametophyte (Pinus ponderosa Laws), and watermelon cotyledon (Citrullus vulgaris Schrad.) by sucrose density gradient centrifugation was found to be similar to that described for castor bean endosperm (Ricinus communis L.). Equilibrium densities were 1.12 to 1.13 g cm3 for endoplasmic reticulum, 1.17 to 1.19 g/cm3 for mitochondria, and 1.25 g/cm3 for glyoxysomes. Isolated glyoxysomes from different fatty seedlings have striking similar specific activities of individual enzymes. The only exception is alkaline lipase activity which, when assayed with an artificial substrate, varies some 10-fold in glyoxysomes from different fatty seedlings. The properties of individual enzymes in glyoxysomes from different fatty seedlings are qualitatively similar as regard to sub-organelle localization and behavior in the presence of KCl and Triton X-100. In pine megagametophyte, the glyoxysomes and not the mitochondria are the intracellular site for the breakdown of stored lipid.
Full text
PDF

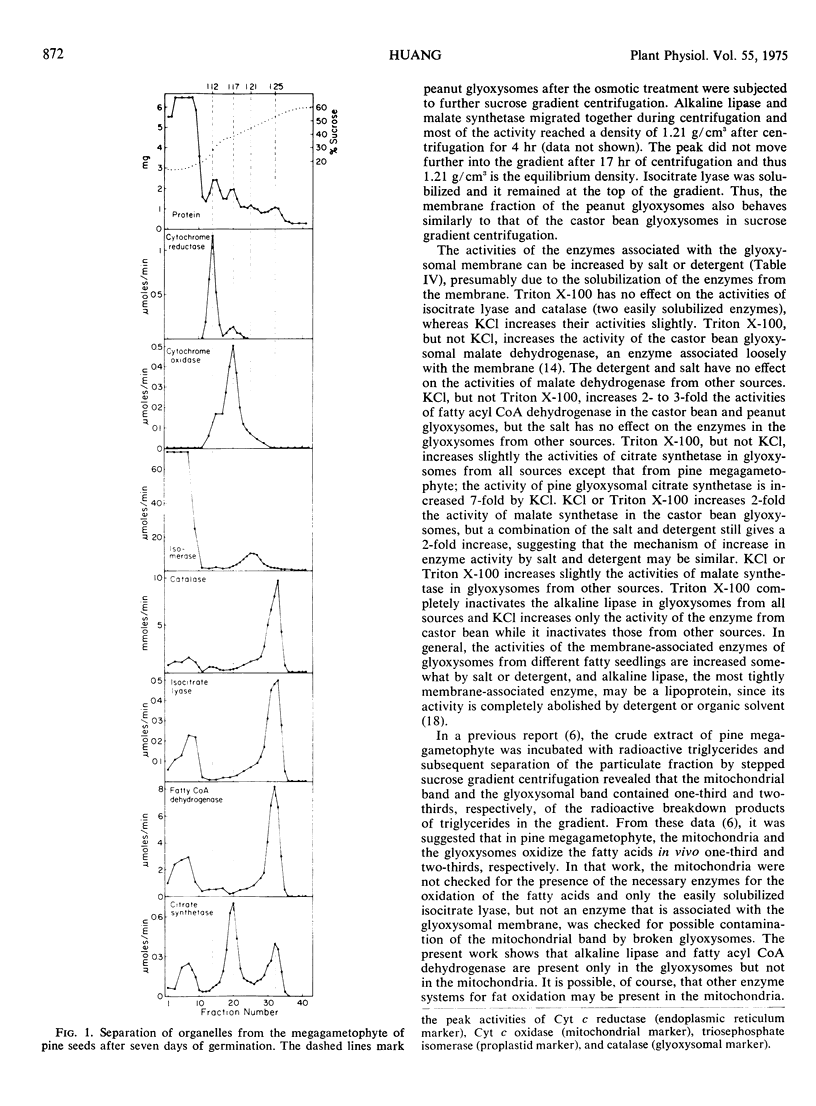
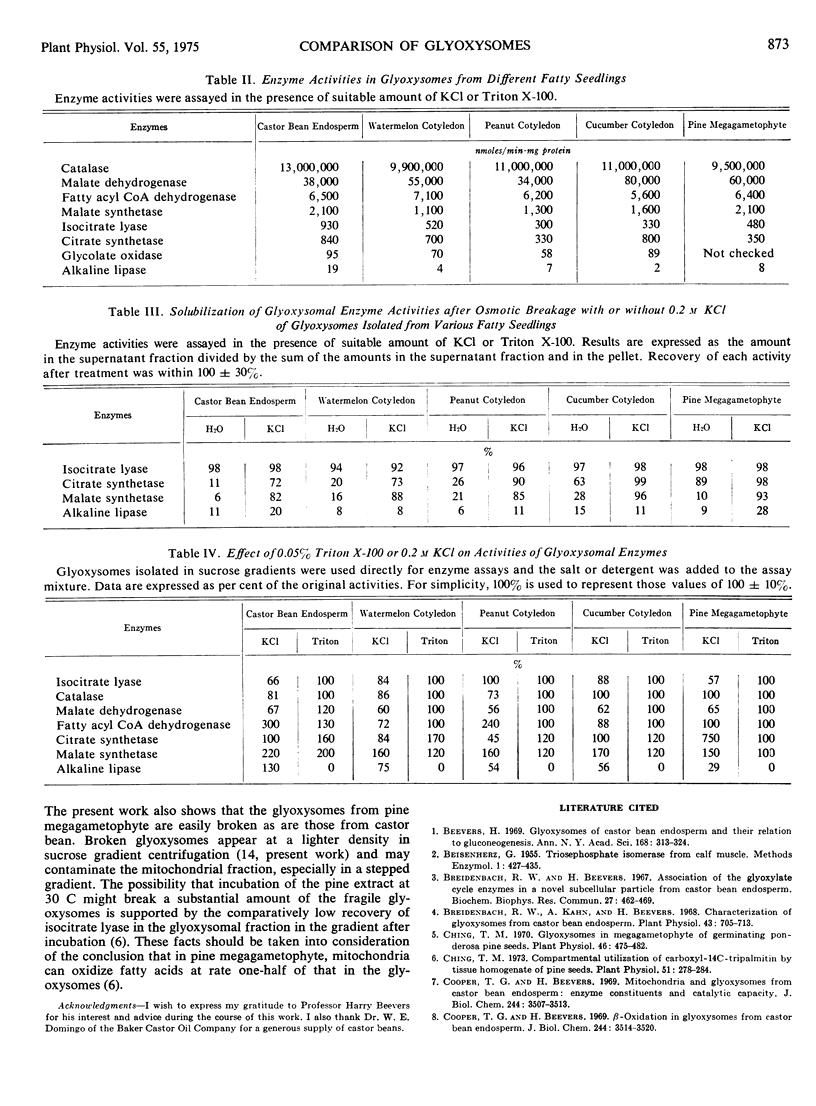

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Compartmental utilization of carboxyl-C-tripalmitin by tissue homogenate of pine seeds. Plant Physiol. 1973 Feb;51(2):278–284. doi: 10.1104/pp.51.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Douglass S. A., Criddle R. S., Breidenbach R. W. Characterization of deoxyribonucleic Acid species from castor bean endosperm: inability to detect a unique deoxyribonucleic Acid species associated with glyoxysomes. Plant Physiol. 1973 May;51(5):902–906. doi: 10.1104/pp.51.5.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Microbody enzymes and carboxylases in sequential extracts from c(4) and c(3) leaves. Plant Physiol. 1972 Aug;50(2):242–248. doi: 10.1104/pp.50.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theimer R. R., Beevers H. Uricase and allantoinase in glyoxysomes. Plant Physiol. 1971 Feb;47(2):246–251. doi: 10.1104/pp.47.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Burke J. J. Cytochemical localization of malate synthase in glyoxysomes. J Cell Biol. 1974 Feb;60(2):483–495. doi: 10.1083/jcb.60.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


