Abstract
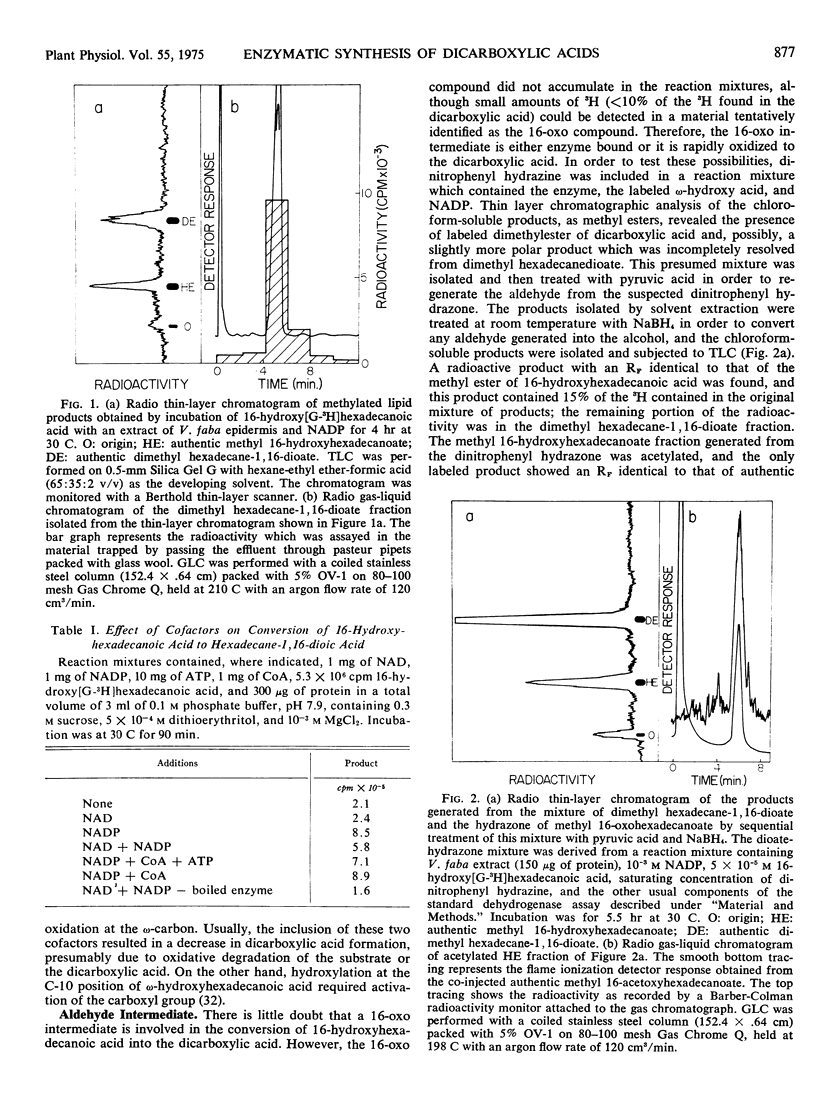
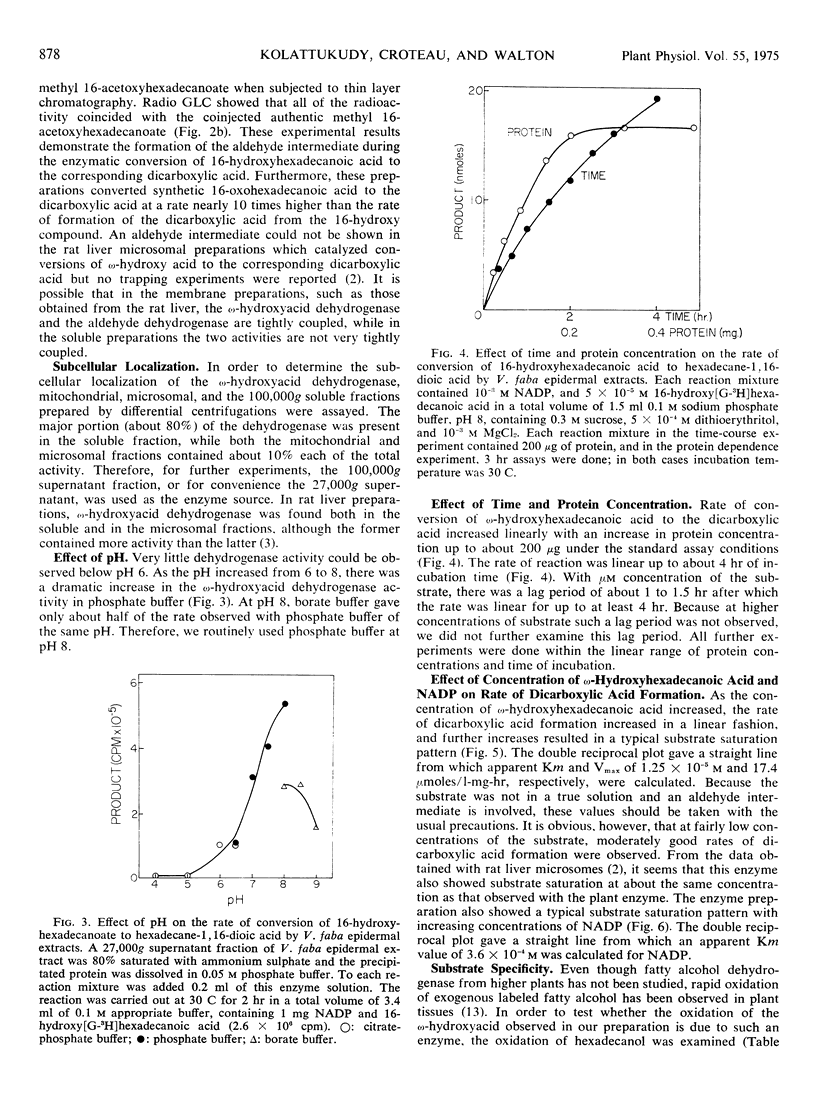
Long chain dicarboxylic acids are constituents of the protective biopolymers cutin and suberin of plants. Cell-free extracts from the excised epidermis of Vicia faba leaves catalyzed conversion of 16-hydroxy[G-3H]hexadecanoic acid to the corresponding dicarboxylic acid with nicotinamide-adenine dinucleotide phosphate as the preferred cofactor. This enzymatic activity, located largely in the 100,000g supernatant fraction, had a pH optimum near 8. This dehydrogenase showed an apparent Km of 1.25 × 10−5m and 3.6 × 10−4m for 16-hydroxyhexadecanoic acid and NADP, respectively. Modification of the substrate, either by esterification of the carboxyl group or by introduction of another hydroxyl group at C-10, resulted in a substantial (two-thirds) decrease in the rate of reaction, and hexadecanol was not a good substrate. The enzyme was inhibited by thiol reagents such as N-ethylmaleimide and p-chloromercuribenzoate. The aldehyde intermediate was trapped by the inclusion of dinitrophenyl hydrazine in the reaction mixture, and the 16-oxo compound was regenerated and identified. Furthermore, synthetic 16-oxo-[G-3H] hexadecanoic acid was readily converted to the dicarboxylic acid by the cell-free preparation. These results demonstrate that epidermis of Vicia faba contains an ω-hydroxyacid dehydrogenase and an ω-oxoacid dehydrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Hamberg M. -Oxidation of fatty acids. II. Enzymatic oxido-reduction of 17-hydroxystearic acid. J Biol Chem. 1971 Dec 25;246(24):7417–7420. [PubMed] [Google Scholar]
- KESTER A. S., FOSTER J. W. DITERMINAL OXIDATION OF LONG-CHAIN ALKANES BY BACTERIA. J Bacteriol. 1963 Apr;85:859–869. doi: 10.1128/jb.85.4.859-869.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSUNOSE M., KUSUNOSE E., COON M. J. ENZYMATIC OMEGA-OXIDATION OF FATTY ACIDS. I. PRODUCTS OF OCTANOATE, DECONATE, AND LAURATE OXIDATION. J Biol Chem. 1964 May;239:1374–1380. [PubMed] [Google Scholar]
- KUSUNOSE M., KUSUNOSE E., COON M. J. ENZYMATIC OMEGA-OXIDATION OF FATTY ACIDS. II. SUBSTRATE SPECIFICITY AND OTHER PROPERTIES OF THE ENZYME SYSTEM. J Biol Chem. 1964 Jul;239:2135–2139. [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. Metabolism of alkyl glyceryl ethers and their noninvolvement in alkane biosynthesis in plants. Arch Biochem Biophys. 1972 May;150(1):310–317. doi: 10.1016/0003-9861(72)90040-9. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry. 1972 May 9;11(10):1897–1907. doi: 10.1021/bi00760a026. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. The biochemistry of plant cuticular lipids. Prog Chem Fats Other Lipids. 1972;13(3):119–175. doi: 10.1016/0079-6832(73)90006-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebeault J. M., Meyer F., Roche B., Azoulay E. Oxydation des alcools supérieurs chez Candida tropicalis cultivé sur hydrocarbures. Biochim Biophys Acta. 1970 Dec 16;220(3):386–395. doi: 10.1016/0005-2744(70)90270-6. [DOI] [PubMed] [Google Scholar]
- MITZ M. A., HEINRIKSON R. L. Omega hydroxy fatty acid dehydrogenase. Biochim Biophys Acta. 1961 Jan 1;46:45–50. doi: 10.1016/0006-3002(61)90644-8. [DOI] [PubMed] [Google Scholar]
- PREISS B., BLOCH K. OMEGA-OXIDATION OF LONG CHAIN FATTY ACIDS IN RAT LIVER. J Biol Chem. 1964 Jan;239:85–88. [PubMed] [Google Scholar]
- Pettersen J. E., Jellum E., Eldjarn L. The occurrence of adipic and suberic acid in urine from ketotic patients. Clin Chim Acta. 1972 Apr;38(1):17–24. doi: 10.1016/0009-8981(72)90202-1. [DOI] [PubMed] [Google Scholar]
- Pettersen J. E., Stokke O. Branched short-chain dicarboxylic acids in human urine. Biochim Biophys Acta. 1973 Apr 28;304(2):316–325. doi: 10.1016/0304-4165(73)90250-x. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. The purification and properties of benzylalcohol dehydrogenase from Pseudomonas sp. Arch Biochem Biophys. 1969 Mar;130(1):422–429. doi: 10.1016/0003-9861(69)90054-x. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI K., SHIMAZONO N. Studies on omega-oxidation of fatty acids in vitro. I. Overall reaction and intermediate. Biochim Biophys Acta. 1963 Apr 23;70:132–142. doi: 10.1016/0006-3002(63)90733-9. [DOI] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry. 1972 May 9;11(10):1885–1896. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Enzymatic conversion of 16-hydroxypalmitic acid into 10,16-dihydroxypalmitic acid in Vicia faba epidermal extracts. Biochem Biophys Res Commun. 1972 Jan 14;46(1):16–21. doi: 10.1016/0006-291x(72)90623-7. [DOI] [PubMed] [Google Scholar]


