Abstract
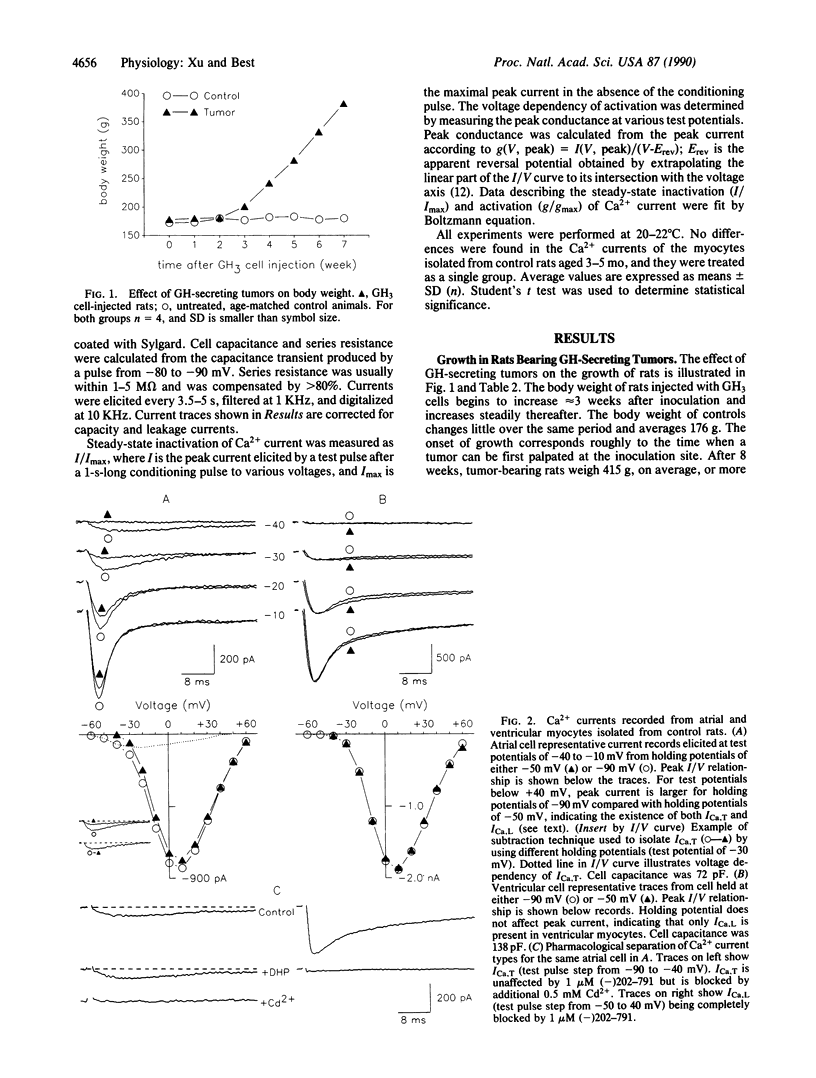
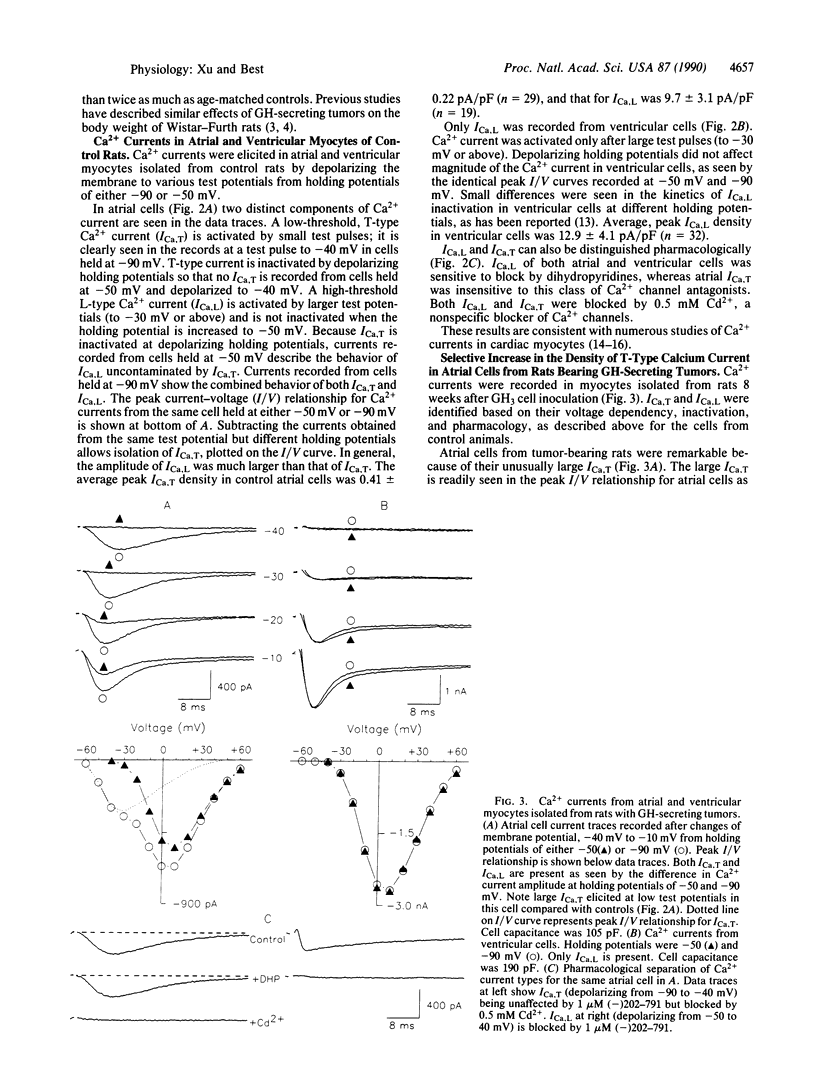
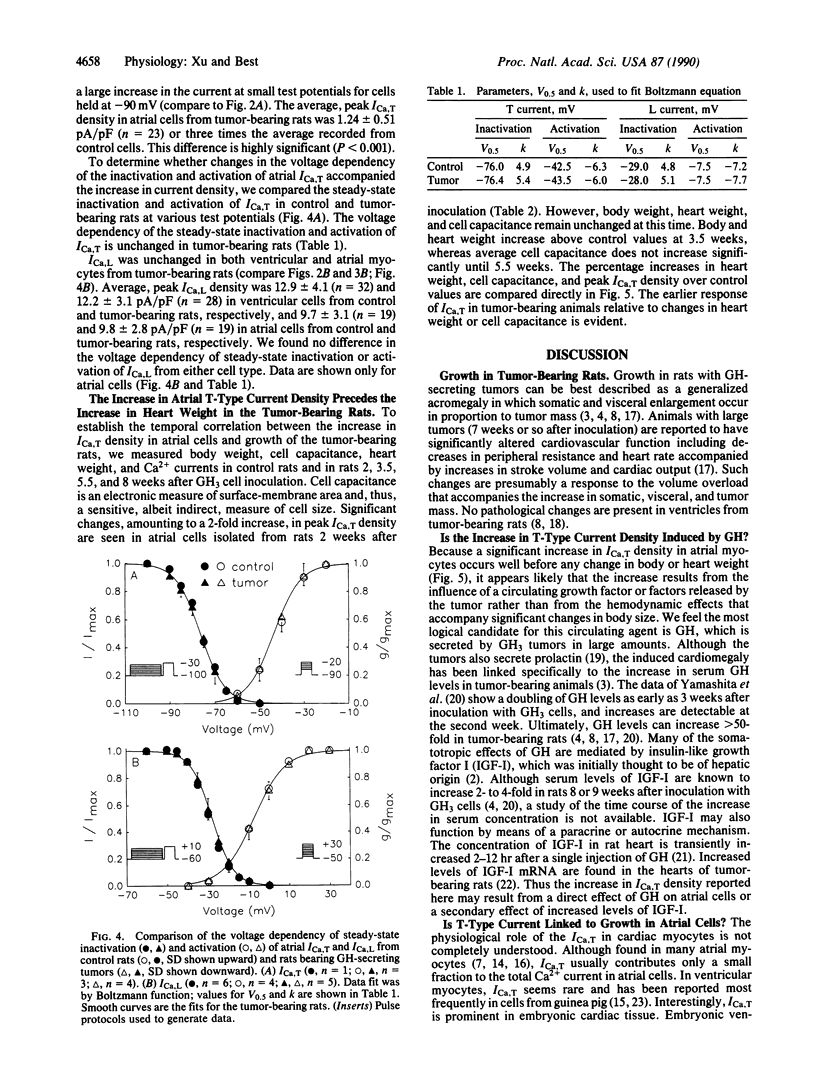
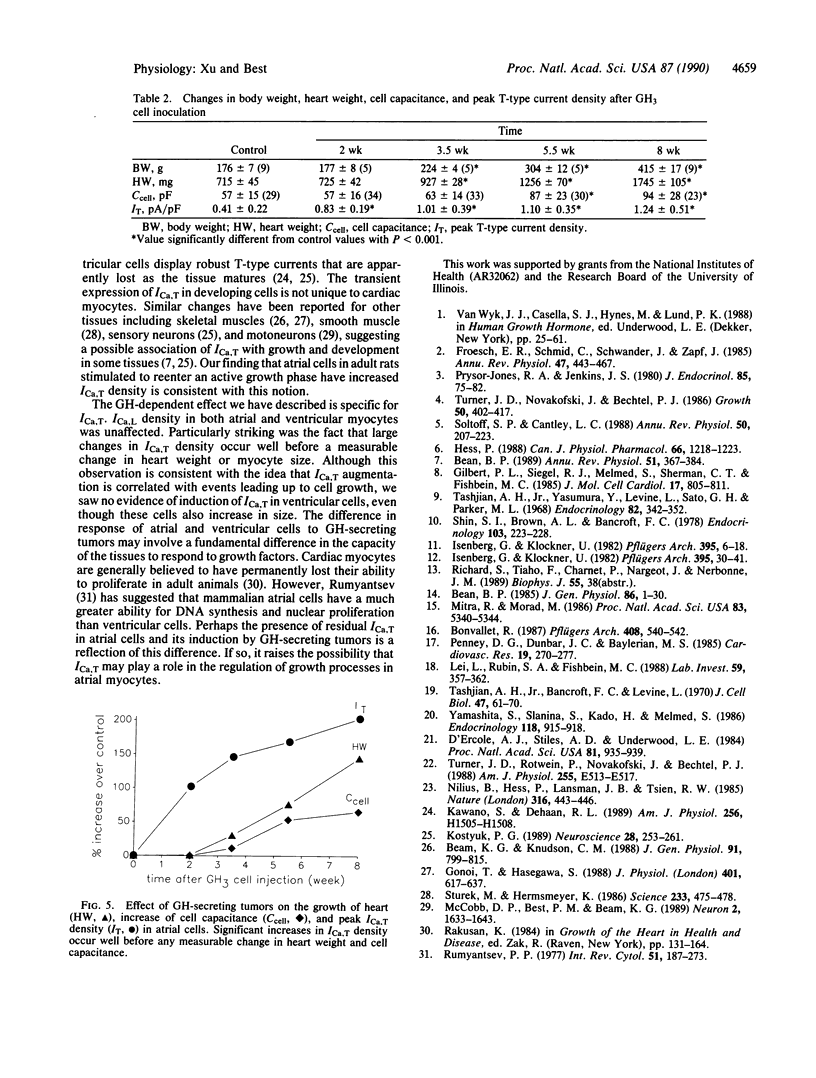
Growth hormone (GH) has pronounced effects on protein synthesis and cell growth in cardiac muscle from adult animals, although the mechanism of its action is not understood. Because Ca2+ has been implicated as a regulator of mitogenic processes in a number of tissues, we investigated whether GH affects the transmembrane movement of Ca2+ through voltage-activated channels of cardiac myocytes. Atrial and ventricular myocytes were isolated from adult rats with GH-secreting tumors and studied electrophysiologically by using patch-clamp techniques. Tumor-bearing rats re-enter an active growth phase and double their body weight over age-matched controls 8 weeks after introduction of the tumor. Atrial myocytes from tumor-bearing animals showed a 3-fold increase in the density of T-type Ca2+ current compared with cells from control animals, although the voltage dependency of activation and inactivation of T-type current was not altered. The increase in T-current density of atrial myocytes preceded by at least a week any measurable change in heart weight, body weight, or myocyte size. L-type Ca2+ currents in atrial and ventricular cells were not affected. The results suggest that a tumor-derived growth factor, most likely GH, can cause a specific enhancement of T-type Ca2+ current in atrial myocytes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvallet R. A low threshold calcium current recorded at physiological Ca concentrations in single frog atrial cells. Pflugers Arch. 1987 May;408(5):540–542. doi: 10.1007/BF00585084. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gilbert P. L., Siegel R. J., Melmed S., Sherman C. T., Fishbein M. C. Cardiac morphology in rats with growth hormone-producing tumours. J Mol Cell Cardiol. 1985 Aug;17(8):805–811. doi: 10.1016/s0022-2828(85)80042-0. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P. Elementary properties of cardiac calcium channels: a brief review. Can J Physiol Pharmacol. 1988 Sep;66(9):1218–1223. doi: 10.1139/y88-201. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Kawano S., DeHaan R. L. Low-threshold current is major calcium current in chick ventricle cells. Am J Physiol. 1989 May;256(5 Pt 2):H1505–H1508. doi: 10.1152/ajpheart.1989.256.5.H1505. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Lei L. Q., Rubin S. A., Fishbein M. C. Cardiac architectural changes with hypertrophy induced by excess growth hormone in rats. Lab Invest. 1988 Sep;59(3):357–362. [PubMed] [Google Scholar]
- McCobb D. P., Best P. M., Beam K. G. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989 Jun;2(6):1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Penney D. G., Dunbar J. C., Jr, Baylerian M. S. Cardiomegaly and haemodynamics in rats with a transplantable growth hormone-secreting tumour. Cardiovasc Res. 1985 May;19(5):270–277. doi: 10.1093/cvr/19.5.270. [DOI] [PubMed] [Google Scholar]
- Prysor-Jones R. A., Jenkins J. S. Effect of excessive secretion of growth hormone on tissues of the rat, with particular reference to the heart and skeletal muscle. J Endocrinol. 1980 Apr;85(1):75–82. doi: 10.1677/joe.0.0850075. [DOI] [PubMed] [Google Scholar]
- Rumyantsev P. P. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186–273. [PubMed] [Google Scholar]
- Shin S. I., Brown A. L., Bancroft F. C. Growth response in athymic nude mice to transplanted growth hormone-secreting rat pituitary tumor cells. Endocrinology. 1978 Jul;103(1):223–228. doi: 10.1210/endo-103-1-223. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Cantley L. C. Mitogens and ion fluxes. Annu Rev Physiol. 1988;50:207–223. doi: 10.1146/annurev.ph.50.030188.001231. [DOI] [PubMed] [Google Scholar]
- Sturek M., Hermsmeyer K. Calcium and sodium channels in spontaneously contracting vascular muscle cells. Science. 1986 Jul 25;233(4762):475–478. doi: 10.1126/science.2425434. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Levine L. Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol. 1970 Oct;47(1):61–70. doi: 10.1083/jcb.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Turner J. D., Novakofski J., Bechtel P. J. Interaction between hypersomatotropism and age in the Wistar-Furth rat. Growth. 1986 Autumn;50(3):402–417. [PubMed] [Google Scholar]
- Turner J. D., Rotwein P., Novakofski J., Bechtel P. J. Induction of mRNA for IGF-I and -II during growth hormone-stimulated muscle hypertrophy. Am J Physiol. 1988 Oct;255(4 Pt 1):E513–E517. doi: 10.1152/ajpendo.1988.255.4.E513. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Slanina S., Kado H., Melmed S. Autoregulation of pituitary growth hormone messenger ribonucleic acid levels in rats bearing transplantable mammosomatotrophic pituitary tumors. Endocrinology. 1986 Mar;118(3):915–918. doi: 10.1210/endo-118-3-915. [DOI] [PubMed] [Google Scholar]


