Abstract
β-Thalassemia and sickle cell disease (SCD) are the world’s two most widely disseminated hereditary hemoglobinopathies. β-Thalassemia originated in the Mediterranean, Middle Eastern, and Asian regions, and SCD originated in central Africa. However, subsequent population migration means that these two diseases are now global and thus constitute a growing health problem in many countries. Despite remarkable improvements in medical care for patients with β-hemoglobinopathies, there is still only one definitive treatment option: allogeneic hematopoietic stem cell (HSC) transplantation. The development of gene therapy for β-hemoglobinopathies has been justified by (1) the limited availability of human leukocyte antigen (HLA)-identical donors, (2) the narrow window of application of HSC transplantation to the youngest patients, and (3) recent advances in HSC-based gene therapy. The huge ongoing efforts in translational medicine and the high number of related publications show that gene therapy has the potential to become the treatment of choice for patients who lack either an HLA genoidentical sibling or an alternative, medically acceptable donor. In this dynamic scientific context, we first summarize the main steps toward clinical translation of this therapeutic approach and then discuss novel lentiviral- and genome editing-based treatment strategies for β-hemoglobinopathies.
Keywords: gene therapy, hemoglobinopathies, hematopoietic stem cell, sickle cell disease, thalassemias
In this review, Cavazzana et al. summarize the main steps toward clinical translation of gene addition approaches for β-thalassemia and sickle cell disease and discuss novel lentiviral- and genome editing-based treatment strategies for β-hemoglobinopathies.
Main Text
Globin Gene Regulation during Development
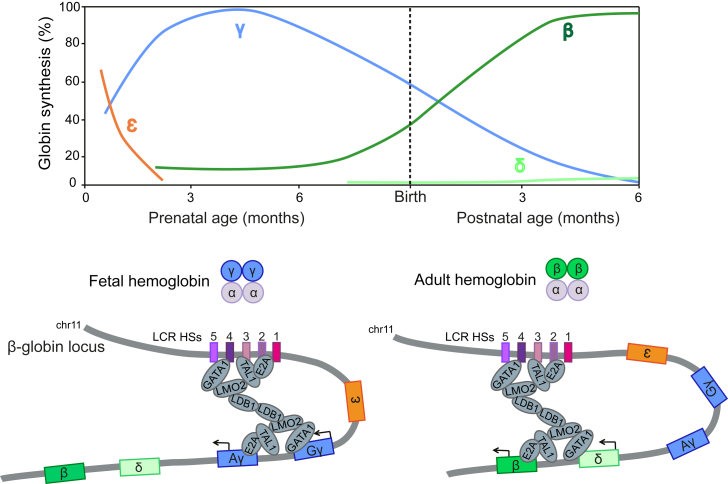
Hemoglobin (Hb), the protein that carries oxygen from the lungs to the tissues, is a tetramer composed of two α-like globin chains and two β-like globin chains. In humans, β-like globin genes are sequentially expressed throughout development (Figure 1). Around the time of birth, fetal Aγ- and Gγ-globin expression is silenced, and the adult β-globin gene is predominantly expressed; this process is referred as γ-to-β Hb switching.1 A 16-kb-long locus control region (LCR; located ∼40–60 kb upstream of the β-globin genes) contains powerful chromatin opening and DNA enhancer elements (DNase hypersensitive sites [HSs]), which are necessary for high-level globin gene expression. The LCR is thought to regulate the expression of the β-globin-like genes by direct interaction with their promoters. The LCR loops to the β-like globin promoters in a developmentally dynamic manner and is juxtaposed to the fetal γ-globin and adult β-globin promoters in fetal and adult erythroblasts, respectively. A pentameric complex (including GATA1, TAL1, E2A, LMO2, and LDB1) is thought to mediate formation of the loop between the LCR and the globin promoters and, as a consequence, β-like globin gene expression (Figure 1).
Figure 1.
Developmental Regulation of the β-Globin-like Genes
The human β-like globin gene locus contains five β-like globin genes. The ε-globin is expressed during the embryonic stage and replaced by γ-globin during fetal life. Around time of birth, a γ-to-β-globin switch occurs and the β-globin is predominantly expressed in the adult life. The adult δ-globin gene is poorly expressed. A pentameric complex mediates long-range interactions between the LCR and γ- and β-globin promoters in fetal and adult erythroblasts, respectively.
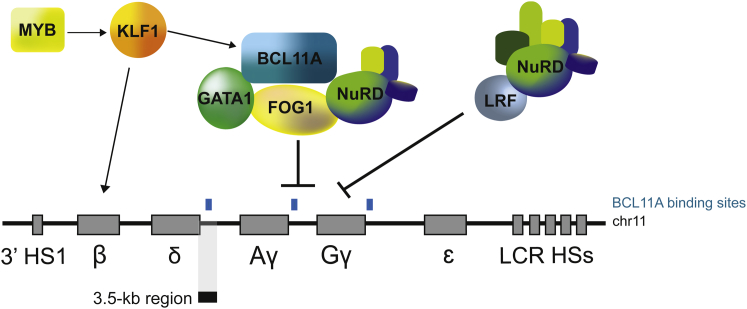
Several nuclear factors are thought to contribute to the Hb switching process, including the erythroid Kruppel-like factor (KLF1),2 MYB,3 the stage selector protein,4 and the nuclear receptors TR2/TR45, 6 and COUP-TFII.7 The zinc-finger transcription factor BCL11A has a major role in the silencing of γ-globin expression in human cells.8, 9 BCL11A is thought to exert this function by interacting with the erythroid master regulator GATA1, SOX6, FOG-1, and the NuRD repressor complex10 (Figure 2). BCL11A is essential for the proper development of B lymphocytes, and murine BCL11A-deficient hematopoietic stem cells (HSCs) show defects in engraftment, cell cycle, and multi-lineage differentiation.11, 12 However, BCL11A’s role in erythroid differentiation and the mechanism (direct or indirect) through which it represses fetal hemoglobin (HbF) expression are currently unknown. Masuda et al.13 recently identified LRF/ZBTB7A transcription factor as a potent repressor of fetal β-like globin expression in adult human erythroid cells. LRF exerts its repressive activity through the NuRD repressor complex,13 independently of the HbF repressor BCL11A (Figure 2). Lastly, chromatin modifiers and remodeling factors (e.g., EHMT1/2,14 CHD4,15 DNMT1,16 and LSD116) and the LIN28B-let7 microRNA pathway17 are also involved in γ-globin repression.
Figure 2.
Major Players in Fetal-to-Adult Hb Switching
BCL11A interacts with SOX6, GATA1, FOG1, and the NuRD repressor complex to repress the expression of γ-globin genes in adult erythroblasts. BCL11A binding sites (indicated with light blue boxes) were mapped in the γ-globin promoters and in a putative 3.5-kb HbF silencer (depicted as a black box). The expression of BCL11A is positively regulated by KLF1, which in turn is upregulated by MYB. Additionally, KLF1 favors fetal-to-adult Hb switching by directly activating β-globin gene expression. The transcription factor LRF silences γ-globin expression through the NuRD complex.
By integrating mutational and epigenetic analyses, researchers have characterized a number of cis-regulatory genomic regions in the β-globin locus with a potential role in Hb switching. Large, naturally occurring deletions encompassing β- and δ-globin genes in the β-globin gene cluster and point mutations in the Aγ- and Gγ-globin promoters result in increased HbF expression and a benign syndrome called hereditary persistence of fetal Hb (HPFH).18 Large HPFH deletions might eliminate HbF inhibitory sequences, whereas HPFH point mutations may disrupt binding sites for γ-globin silencers (e.g., BCL11A) or generate new binding sites for γ-globin activators.18
Mutational analyses of the β-globin gene cluster have identified a 3.5-kb δγ-intergenic region that is specifically deleted in HPFH individuals,9 thus representing a potential HbF silencer (Figure 2). The region contains a 250-bp polypyrimidine-rich sequence, the deletion of which resulted in delayed human γ-to-β-globin switching in transgenic mice,19 and SNPs associated with elevated HbF levels.20, 21 Importantly, the 3.5-kb δγ-intergenic region is occupied by BCL11A in human adult primary erythroblasts, suggesting a direct role of BCL11A in γ-globin repression.9 This region is included in the 7.2-kb δβ-Corfu region, the deletion of which results in a high HbF blood content in thalassemic patients.22
Cross-comparisons of point mutations carried by sickle cell disease (SCD) and thalassemic patients displaying HPFH traits have revealed a common T-to-C substitution at position −175 (−175T > C) in both the Aγ- and Gγ-globin promoters responsible for pancellular expression of high levels of γ-globin.23 In the murine adult MEL and human fetal erythroid K562 cell lines, nuclease-mediated insertion of the −175T > C substitution into the γ-globin promoter creates a de novo E-box binding site for TAL1, which recruits the E2A/LMO2/LDB1 complex, promotes loop formation between the LCR and γ-globin promoter, and thus increases HbF expression.23 Interestingly, a 13-nt deletion (−102 to −114) in the Aγ-globin promoter is associated with pancellular HbF expression, which is probably due to the loss of recruitment of the transcriptional repressor BCL11A24 (M.J. Weiss and J.K. Joung, 2016, American Society of Hematology, conference) (Figure 2). These data suggest that BCL11A binding sites in the β-globin locus (e.g., in the 3.5-kb δγ-intergenic region and in the γ-globin promoter) may be responsible for HbF silencing in a redundant fashion.
β-Hemoglobinopathies: Clinical Presentation and Conventional Treatments
SCD and β-thalassemia are the most common monogenic disorders worldwide, with ∼317,000 affected neonates born each year.12
β-Thalassemia is caused by more than 200 different β-globin gene mutations that reduce or abrogate production of the β-globin chains (the β+ and β0 genotypes, respectively). The excess of α-globin chains results in ineffective erythropoiesis, intramedullary apoptosis of erythroid precursors, and hemolytic anemia. Patients with β-thalassemia intermedia and minor can survive without regular blood transfusions. Conversely, patients affected by β-thalassemia major are transfusion dependent while they suffer from severe anemia, chronic hemolysis, iron overload, hepatosplenomegaly, and complications such as cardiopathies and endocrine disorders. The age at which these complications appear is tightly linked to the adequacy of supportive care.
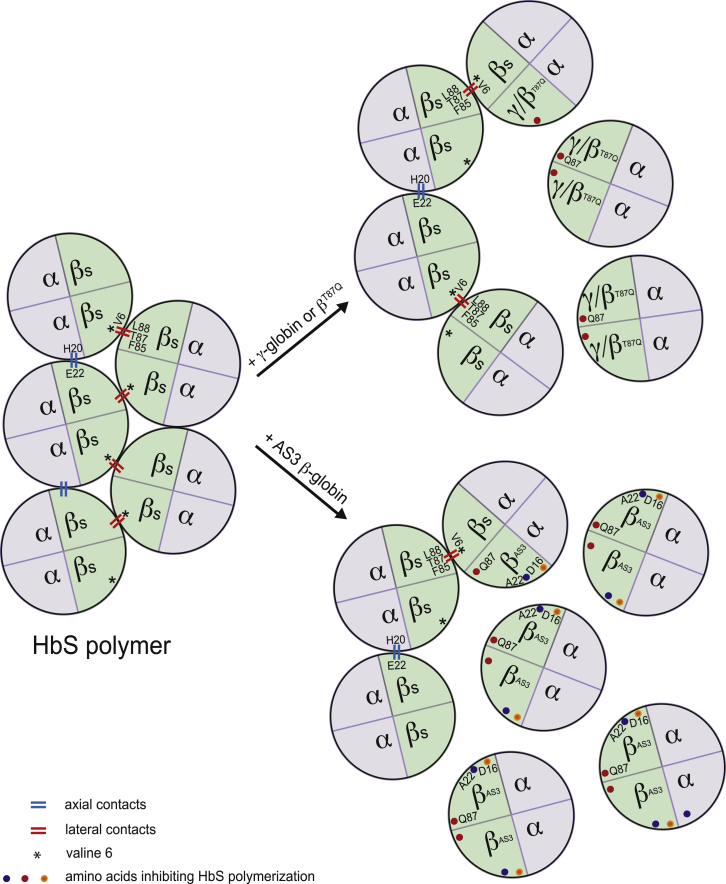
SCD is caused by an A-to-T point mutation in the sixth codon of the β-globin gene, which leads to an E6V substitution in the β-globin polypeptide. The resulting defective β-globin (βS) polymerizes in the deoxygenated state, and the normally flexible, doughnut-shaped red blood cells (RBCs) take on an inflexible hook/sickle shape. Sickled RBCs obstruct microvessels and have a short half-life due to repeated sickling-unsickling cycles. The pathophysiology of SCD differs from that of β-thalassemia. In fact, SCD is a chronic disease that dramatically impairs the function of many organs, independently of the transfusion and chelation regimens adopted. SCD is associated with substantial morbidity, poor quality of life, and a shortened life expectancy, even in high-income countries like France.25 SCD-associated morbidity and mortality in young adults is largely due to currently unpreventable complications, such as priapism, avascular necrosis, chronic pulmonary impairment, hypertension, stroke, and recurrent veno-occlusive crises.26 Survival has improved significantly over the last two decades, and 94% of children with SCD now survive until the age of 18 years thanks to better monitoring, Pneumococcus vaccination, penicillin prophylaxis, and treatment with hydroxyurea, an anti-cancer drug, which increases blood HbF levels.13 Indeed, the clinical course of SCD is improved when fetal γ-globin genes are highly expressed,27 as seen in patients with HPFH traits. In SCD, γ-globin exerts a potent anti-sickling function by competing with the sickle β-globin for incorporation in the Hb tetramers and by inhibiting sickle hemoglobin (HbS) polymerization (Figure 3). However, pharmacological treatments increasing HbF levels are not equally effective in all patients, and toxicity remains a problem. In fact, mortality is still high once patients reach adulthood.14
Figure 3.
Inhibition of HbS Polymerization by Anti-sickling β-like Globins
HbS tetramers polymerize upon de-oxygenation. In HbS polymers, the valine at position 6 (V6) of the βS-chain forms a lateral contact with the phenylalanine and leucine residues at positions 85 and 88 (F85 and L88) of the βS-chain of the adjacent tetramer. Additionally, a glutamic acid (at position 22) of the β-chain interacts with a histidine residue (at position 20) of the α-globin of a neighboring tetramer (axial contact). The incorporation of γ- or βT87Q-globin into the Hb prevents HbS polymerization. βT87Q-Globin harbors an amino acid substitution at position 87 (threonine [T87] to glutamine [Q87]). The glutamine residue derived from the γ-globin chain inhibits the formation of lateral contacts between the Hb tetramers. In addition to the T87Q amino acid substitution, in AS3 β-globin, the glutamic acid at position 22 is replaced by an alanine (A22), which disrupts the axial contacts between the Hb tetramers, and the glycine at position 16 is replaced by an aspartic acid (D16), thus increasing the affinity of the AS3 β-chain to the α-globin polypeptide.
The ever-increasing demand for lifelong transfusions is impossible to satisfy (for safety reasons in developing countries and for availability reasons in high-income countries). Allogenic transplantation of HSCs (HSCT) is currently the only definitive treatment for β-hemoglobinopathies. However, only a small percentage of patients can undergo this procedure, because (1) it has to be performed in specialized units and (2) the majority of the populations affected by these diseases are poorly represented in the international registry of HSC donors. Hence, in the absence of an HLA-geno-compatible sibling, the vast majority of patients lack a well-matched, unrelated donor.
Novel Therapeutic Approaches to β-Hemoglobinopathies
Gene therapy using autologous, genetically modified HSCs is an alternative to allogenic HSCT for treating β-hemoglobinopathies. It circumvents the need for a matched donor and thus avoids the risk of graft versus host disease and graft rejection after HSCT. Furthermore, the conditioning regimen required for the engraftment of genetically modified cells, because of their autologous origin, does not include immunosuppressive drugs. Hence, the infusion of the genetically modified cells and patient follow-up can potentially be performed in many pediatric and adult hematopoietic cell transplantation units, even those with limited expertise in allogenic HSCT. The rapid worldwide application of this therapeutic approach may now be possible, thanks to better safety and the absence of treatment-related mortality in gene therapy trials to date.
The source of autologous CD34+ hematopoietic stem/progenitor cells (HSPCs) is critical for obtaining an adequate dose of cells retaining stem cell properties. The conventional collection of autologous bone marrow (via multiple punctures of the iliac crests under general anesthesia) is invasive and does not yield enough CD34+ HSPCs, particularly in β-thalassemic and SCD patients. In β-thalassemic bone marrow, ineffective erythropoiesis is characterized by (1) accelerated erythroid differentiation, (2) a maturation block at the polychromatophilic stage, and (3) elevated death of erythroid precursors.28 The consequence of these alterations is the accumulation of five to six times more erythroid precursors than in normal bone marrow; this contributed to the initial failure to harvest an adequate dose of HSPCs from β-thalassemic patient bone marrow.29 Therefore, in these patients, mobilization of HSPCs is recommended for the gene therapy procedure.30, 31, 32 The current gold standard procedure for β-thalassemic patients involves a combination of granulocyte-colony stimulating factor (G-CSF) and plerixafor (Mozobil, formerly AMD3100), a bicyclam molecule that antagonizes the binding of stromal cell derived factor-1 (SDF-1) expressed by bone marrow stromal cells to the chemokine CXC-receptor 4 (CXCR4) located on the surface of HSPCs. G-CSF plus plerixafor has been shown to provide very high numbers of CD34+ cells by single apheresis in mobilized thalassemic patients,32 despite the recently reported highest enrichment in HSCs using plerixafor alone.33
Similarly, two or three bone marrow harvests are required to collect an adequate dose of HSPCs from SCD patients; this is mainly because the HSC harvest is negatively influenced by (1) the inflammatory SCD bone marrow microenvironment34 and (2) the formation of cell aggregates during the isolation of bone marrow mononuclear cells (M.C., unpublished data). In individuals with SCD, the administration of G-CSF led to severe adverse events35 and is therefore contraindicated. We recently suggested that plerixafor is potentially a safer mobilizing agent for SCD patients (http://www.clinicaltrials.gov identifier NCT02212535).
Lastly, the choice of the optimal conditioning regimen is particularly important, since the intake of a high proportion of transduced HSCs is required to ameliorate the clinical phenotype of β-thalassemia and SCD; the selective advantage is at best limited to the preferential survival of corrected erythroblasts.
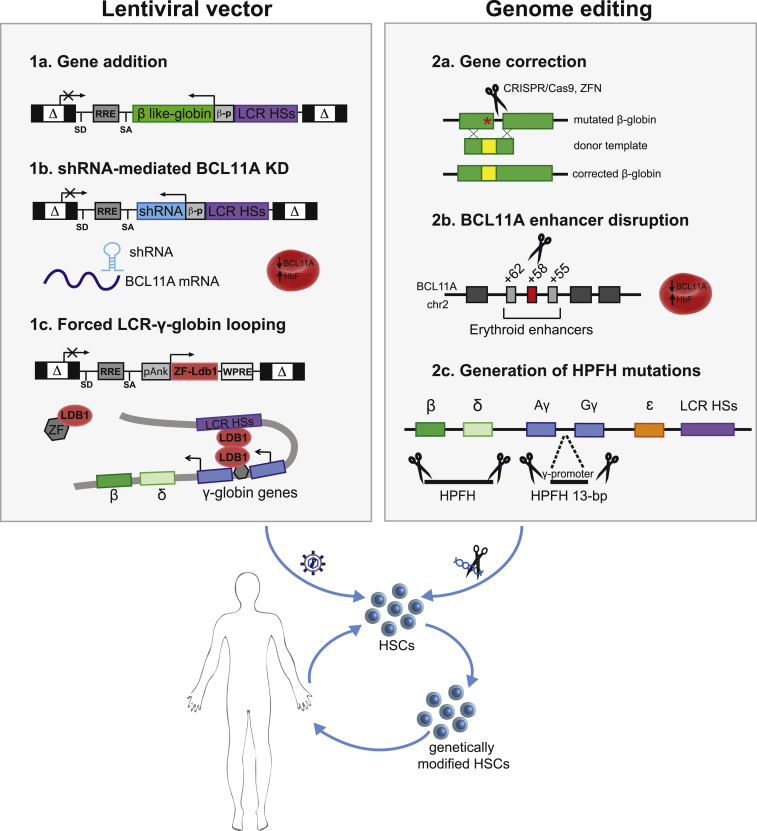
Several approaches can be used to genetically manipulate HSCs and correct the genetic defect underlying β-hemoglobinopathies. A number of ongoing clinical trials (based on the transplantation of autologous β-thalassemic and SCD HSCs) are investigating the use of lentiviral vectors that express β-like globin transgenes (G. Ferrari, 2016, Mol Ther., conference).29, 36, 37, 38 Novel therapeutic strategies are based on the use of lentiviral vectors and/or genome editing tools to re-activate endogenous HbF expression. Lastly, several research groups have explored genome editing-based approaches for correcting the SCD mutation (Figure 4).
Figure 4.
Novel Therapeutic Approaches for β-Hemoglobinopathies
Overview of lentiviral (1a–1c) and genome editing (2a and 2b) approaches for correcting HSCs from SCD and β-thalassemic patients. (1a) Gene addition: therapeutic, erythroid-specific expression of β-like globin transgenes using lentiviral vectors. (1b) shRNA for BCL11A knockdown (KD): HbF reactivation by lentiviral-mediated erythroid-specific expression of an shRNA against BCL11A. (1c) Forced LCR-γ-globin looping: HbF induction by lentiviral expression of a ZF-LDB1 fusion protein forcing the juxtaposition of the LCR to the γ-globin genes. (2a) Gene correction: correction of SCD and β-thalassemic mutations via nuclease-induced HDR. (2b) BCL11A enhancer disruption: HbF reactivation induced by nuclease-mediated targeting of the BCL11A erythroid-specific enhancer, located at +58 kb from BCL11A transcription start site. (2c) Generation of HPFH mutations: HbF induction by reproducing a 13-bp HPFH deletion in the γ-globin promoters via MMEJ or deletional HPFH mutations (encompassing the β- and δ-globin genes) by NHEJ.
Lentiviral Vector-Based Strategies
Gene Addition Strategies
The correction of β-hemoglobinopathies by gene addition (i.e., the efficient introduction of a fully functional β-like globin transgene into HSCs) has required more than 20 years of research.
Two major breakthroughs have enabled efficient HSC transduction and the therapeutic expression of β-like globin transgenes in HSC-derived RBCs: (1) the development of HIV-1-derived lentiviral vectors39 and (2) the discovery of the LCR HSs capable of boosting β-like globin expression.40, 41 The introduction of critical LCR HS enhancers into the vector and the optimization of the vector titer (e.g., by deleting a portion of the second β-globin intron and removing multiple cryptic polyadenylation and splicing signals42, 43) led to proof-of-concept studies in murine models of β-thalassemia44, 45 and SCD.46 Since then, several laboratories have shown that LVs carrying the human β- or γ-globin gene under the control of β-globin promoter and LCR elements are able to rescue murine and human β-thalassemic and SCD phenotypes47, 48, 49 (Figure 5)
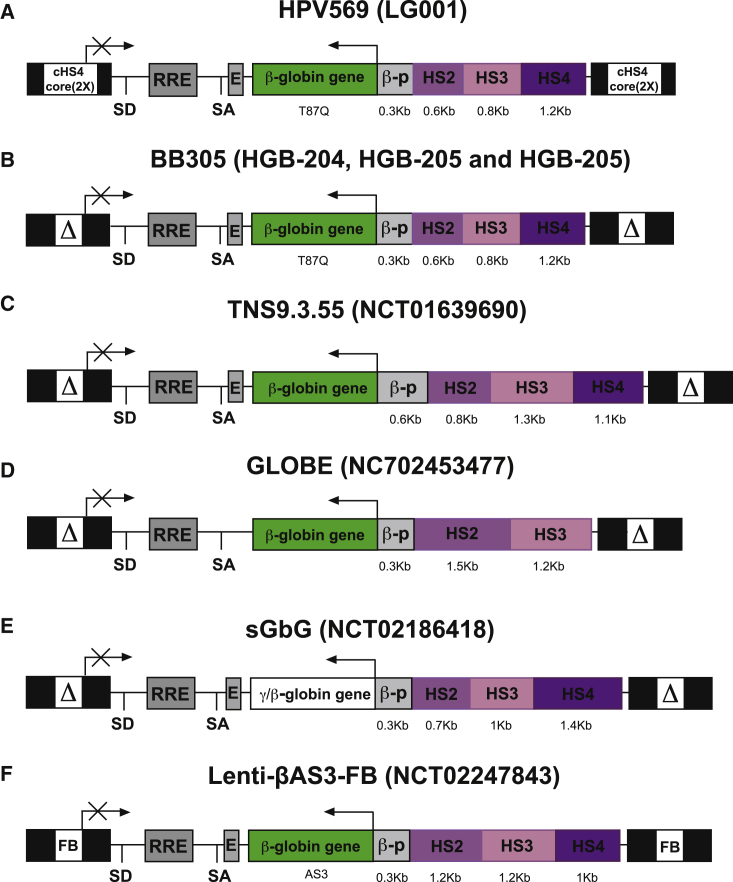
Figure 5.
β-Globin-like Expressing Lentiviral Vectors Used in Clinical Trials
Schematic representation of β-like globin expressing lentiviral vectors in their proviral forms. The clinical trial number is indicated in brackets. LTRs deleted of 400 bp in the HIV U3 region (ΔLTR), rev-responsive element (RRE), splicing donor (SD), and splicing acceptor (SA) sites, human β-like globin genes, β-globin promoter (bp), the 3′ β-globin enhancer (E), and Dnase-I hypersensitive sites HS2, HS3, and HS4 from β-globin LCR are shown. Two copies of the cHS4 core and the FB insulator were inserted into the LTRs of HPV569 and Lenti-βAS3-FB lentiviral vectors, respectively.
Several clinical trials for β-hemoglobinopathies have been completed and/or recently initiated (Figure 5). In the first phase 1/2 clinical trial (LG001, initiated in France in 2006), we tested the HPV569 lentiviral vector (Figure 5A). This vector expresses a β-globin transgene harboring a critical amino acid derived from the γ-globin chain (βT87Q), which is responsible for inhibiting HbS polymerization by blocking the lateral contacts required for the formation of Hb polymers (Figure 3).50, 51 This vector carries a minimal β-globin promoter and the HS2, HS3, and HS4 elements from the LCR. Two copies of the core of the chicken β-globin HS4 (cHS4) chromatin insulator were inserted in the viral long terminal repeats (LTRs). cHS4 displays enhancer-blocking activity52, 53, 54 and therefore might increase the safety of the lentiviral-based gene therapy approach by protecting the neighboring genes from the LCR’s potent enhancer influence. The first β-thalassemic patient to become transfusion independent following gene therapy was reported some years ago.17 The patient has a βE/β0 genotype and expresses low levels of a slightly unstable but functional βE chain. The patient received a myeloablative conditioning regimen with intravenous busulfan at a starting dose of 3.2 mg/kg per day. Pharmacokinetic monitoring enabled an exposure of 4,500–5,000 (μM⋅min)/day to be maintained for 4 days. After transplantation of bone-marrow-derived HSPCs, we observed a gradual increase in gene marking. The patient became transfusion independent 1 year after gene therapy. Over time, the total Hb level ranged between 8 and 9 g/dL, with equal proportions of HbAT87Q (containing the transgene product), endogenous HbF, and hemoglobin E (HbE) containing the βE-globin. Integration site analysis revealed a partial clonal dominance upon vector integration within the high mobility group AT hook 2 (HMGA2) gene. This gene was activated both transcriptionally and post-transcriptionally by vector-induced abnormal splicing and premature transcript termination; the resulting truncated mRNA was insensitive to degradation by let7 microRNA. The abnormal splicing was due to the presence of a cryptic splice acceptor site in the cHS4 chromatin insulator within the lentiviral 5′ LTR. This benign clonal dominance declined after several years (M.C., unpublished data). Moreover, insertion of the cHS4 insulator into the lentiviral LTRs resulted in low titers, poor transduction efficiency, and vector re-arrangements, with the loss of insulator elements.17
For these reasons, we designed a new vector (BB305; Figure 5B). After genomic integration, BB305 was identical to HPV569, except for the absence of the cHS4 insulator.55 Further optimization of vector production (including replacement of the 5′ LTR promoter with the cytomegalovirus promoter, to drive viral RNA production) raised the vector titers and increased transduction efficiency.55 In two multicenter international studies (HGB-204 and HGB206) and one single-center French study (HGB-205), the BB305 vector was used to transduce HSPCs either mobilized from β-thalassemic patients using G-CSF and plerixafor or purified from the bone marrow of SCD patients. The HGB-204 and HGB-205 studies were based on the myeloablative, busulfan-based conditioning regimen used in the first clinical study (LG001). All the non-β0/β0 patients (n = 5 and 4 in the HGB-204 and HGB-205 studies, respectively) became transfusion-independent ≥12 months after gene therapy.36, 38 In all 5 β0/β0patients included in the HGB-204 trial, the annual transfusion volumes were significantly lower than before gene therapy.36, 38 Importantly, all the patients showed a highly polyclonal integration profile, with no clonal dominance.36, 38
In 2012, a phase 1 clinical study in β-thalassemic patients in the United States (NCT01639690) used the TNS9.3.55 vector expressing the wild-type β-globin transgene (Figure 5C) and a reduced intensity busulfan-based conditioning regimen. Gene marking was limited, and the four treated patients did not show sufficient clinical benefit.56 A second trial is planned using a TNS9.3.55 variant vector,56 incorporating an insulator selected by a systematic high-throughput screening of human insulators.57
A phase 1/2 clinical trial for thalassemia major patients started in Italy in 2015 (NC702453477). It is based on the GLOBE vector, which expresses the wild-type β-globin transgene under the control of the β-globin promoter and only two elements from the LCR (Figure 5D).49, 58 A myeloablative conditioning regimen based on treosulfan and thiotepa was used, as it is considered to be less toxic than busulfan and to significantly decrease extramedullary erythropoiesis.59 Transduced CD34+ cells were injected directly into the bone marrow cavity to avoid their trapping in the spleen or liver of patients with hepatosplenomegaly and thus to increase the level of engraftment. The preliminary results are extremely encouraging, although a longer follow-up period will be required for firm conclusions (G. Ferrari, 2016, American Society of Hematology, conference).
The first SCD patient to have undergone gene therapy was treated in the HGB-205 study.60 Bone marrow CD34+ cells were harvested twice from the patient, who then underwent myeloablation with intravenous busulfan. 15 months after gene therapy, the total Hb level was 12 g/dL, with therapeutic Hb and HbS accounting for 48% and 46% of the Hb tetramers, respectively. A stable, high level of gene marking was observed in all lineages except for T cells, given the absence of immunosuppression during conditioning. The clinical phenotype and the biological hallmarks of SCD were corrected. Hence, the HGB-205 study proved that gene therapy via gene addition can be a powerful curative tool for this devastating disease. In the HGB-206 multicenter phase 1/2 study, however, peripheral blood levels of the BB305 vector were low in all treated SCD subjects, with no evidence of clinical benefit.37 The adverse impact of sickle marrow pathology on HSCs, the adequacy of myeloablation, and the magnitude of the transduced cell dose are currently being explored as parameters that influence the clinical outcome of the BB305 lentivirus-based gene therapy approach.37
Two other clinical trials of gene therapy for SCD have been initiated in the United States (NCT02186418 and NCT02247843, led respectively by Punam Malik and Donald Kohn), although the results have not yet been published. In the first trial, a lentiviral vector is used to express an anti-sickling γ-globin transgene (sGbG; Figure 5E).61 In the second trial, a lentiviral-derived β-globin transgene harbors three anti-sickling point mutations (Lenti-βAS3-FB; Figure 5F): T87Q for blocking the lateral contact with valine 6 of the βS chain, E22A for disrupting axial contacts, and G16D for conferring to the transgene a competitive advantage over the βS in terms of the interaction with the α-globin polypeptide (Figure 3). Functional analysis showed that the purified recombinant AS3 β-globin strongly inhibits the polymerization of HbS tetramers.62 Moreover, the Lenti-βAS3-FB vector includes the FB insulator element, which contains enhancer-blocking components of the cHS4 and the analogous region of the human T cell receptor δ/α BEAD-1 insulator. It has been shown that this vector ameliorates the SCD cell phenotype of RBCs differentiated in vitro from patient HSPCs.63
Despite this undeniable progress in the optimization of gene addition strategies, there is room for further improvement to increase transgene expression in HSC-derived RBCs and boost the efficiency of HSC transduction. Indeed, severe β0-thalassemia and SCD require high levels of globin expression. With this objective in mind, the choice of regulatory elements from the LCR (i.e., those best able to drive high levels of globin expression without compromising the vector titer) is still a critical issue. Moreover, the expression of a potent anti-sickling globin transgene is essential for inhibiting HbS polymerization and rescuing the SCD phenotype. However, we still lack a side-by-side comparison of the anti-sickling activities of γ-globin and β-globin harboring either one or three anti-sickling mutations. Moreover, obtaining a sufficient number of genetically modified patient HSCs and correcting the most severe forms of β-hemoglobinopathies will require the optimization of vector infectivity and HSC transduction. Lastly, the inclusion of novel insulator elements57, 64 might (1) protect transgene expression from silencing and (2) avoid the LCR-mediated transactivation of neighboring genes without affecting the vector titer or the post-transcriptional processing of endogenous RNAs.
Re-activation of Fetal γ-Globin Gene Expression via the shRNA-Mediated Downregulation of BCL11A
The lentiviral-mediated expression of a β-globin transgene is able to provide clinical benefit in β+-thalassemic patients with residual, endogenous β-globin expression29, 36 (G. Ferrari, 2016, American Society of Hematology, conference) and in SCD patients.60 However, the treatment of these diseases requires further key improvements. First, higher Hb production levels per cell must be achieved, especially in severe forms of β-thalassemia (β0/β0 patients) and in SCD. Indeed, it is hard to achieve the physiological levels of endogenous β-globin expression with a lentiviral vector, because the latter cannot accommodate the entire LCR.65 Second, it is necessary to reduce expression of the sickle β-globin gene in SCD. In fact, the correction of SCD implies lower incorporation of the sickle β-globin chain into the Hb tetramer, since elevated HbS levels are associated with a higher risk of vaso-occlusive crises. In this context, a therapeutic strategy aimed at forcing the β-to-γ-globin switching favors high-level expression of the endogenous γ-globin at the expense of the mutated β-globin. These strategies are underpinned by the observation that elevated levels of fetal γ-globin ameliorate the clinical course of β-thalassemia and SCD,27 by reducing the α-β globin imbalance and the α-globin precipitation in β-thalassemia, and by inhibiting HbS polymerization in SCD (Figure 3).
A therapeutic approach aiming to increase HbF levels could rely on the downregulation of nuclear factors involved in γ-globin silencing. However, several of these factors (e.g., KLF1, MYB, and LRF) have an essential role in erythroid development and thus are not ideal targets to develop a safe approach for treating β-hemoglobinopathies. By way of an example, inefficient erythroid terminal differentiation causes mild macrocytic anemia in LRF knock out mice,66 and LRF knockdown delays human erythroid differentiation.13
In this context, BCL11A has been suggested as a molecular target for the therapeutic induction of γ-globin in β-globin disorders. Knockdown studies have demonstrated that BCL11A is required to maintain the silencing of HbF expression in human erythroid progenitors.8 γ-Globin gene re-activation was accompanied by a reduction in β-globin levels, probably as a result of preferential interaction between the LCR and the γ-globin promoters.10 In humanized mouse models of SCD, the loss of BCL11A produces pancellular HbF induction and reverses the characteristic end-organ damage.67 Interestingly, individuals with BCL11A haploinsufficiency showed persistent HbF expression and retained normal hematologic functions.68 However, there are several potential drawbacks in targeting BCL11A, given its essential role in the development of B-lymphocytes and in HSCs.11 One potential approach involves lentiviral-mediated erythroid-specific expression of a short hairpin RNA (shRNA) for BCL11A.12 Brendel et al.12 recently reported that LCR-driven erythroid-specific expression of an shRNA for BCL11A overcomes the toxicity associated with polymerase III (Pol-III)-dependent shRNAs and with ubiquitous knockdown of BCL11A in HSCs, while it reverses the sickle phenotype. This approach was associated with a 90% reduction in BCL11A levels in RBCs derived from normal and SCD HSCs, and HbF represented up to 70% of the total Hb content. The extensive expertise in the clinical translation of lentiviral-based strategies will facilitate the implementation of a clinical trial using this approach. However, complete inactivation of BCL11A in the erythroid lineage adversely affects human RBC enucleation; hence, finely controlled modulation of BCL11A expression levels is likely to be required to provide a safe therapeutic approach.69
Re-activation of Fetal γ-Globin Gene Expression by Manipulating the Chromatin Structure of the β-Globin Locus
In 2014, Deng et al.70 developed an interesting approach for derepressing γ-globin expression using lentiviral vectors expressing an artificial zinc finger (ZF) protein directed against the γ-globin promoter and fused to the looping factor LDB1. This approach forced the formation of a loop between the LCR and the γ-globin promoters, which eventually re-activated endogenous γ-globin expression and concomitantly reduced sickle β-globin levels in primary erythroid cells derived from adult HSPCs.62, 70 This strategy reduced the proportion of sickling RBCs derived from human SCD HSPCs in vitro.62 Interestingly, a low level of expression of the ZF-LDB1 fusion protein was enough to activate the γ-globin genes and allow sustained HbF production. The short ankirin promoter driving ZF-LDB1 expression will probably enable the production of a relatively high-titer lentiviral vector as compared with β-like globin-expressing vectors that require the inclusion of large fragments of the LCR region to achieve high levels of transgene expression (Figure 5). Preclinical in vivo studies addressing the safety and specificity of this strategy are necessary before this alternative lentiviral-based therapeutic approach can be considered in patients with β-hemoglobinopathies.
Other Strategies: Downregulation of α- and βS-Globin Expression
In β-thalassemia, the precipitation of α-globin chains is thought to cause the apoptosis of RBC precursors through several mechanisms.28 It has recently been suggested that the inclusion of a specific microRNA against one of the two α-globin alleles in a β-globin lentiviral vector might increase the efficacy of gene addition strategies in the treatment of β-thalassemia (P. Leboulch, 2016, Hemoglobin Switching, conference). Similarly, Samakoglu et al.71 incorporated an shRNA targeting βS-globin transcripts into a γ-globin expressing lentiviral vector. Downregulation of the βS-globin gene might benefit the lentiviral-based gene transfer of the anti-sickling γ-globin gene by decreasing inter-chain competition for incorporation into the Hb tetramer and thus reducing the need for high levels of globin transgene expression.
Genome Editing-Based Strategies
Genome-editing technologies have developed in a spectacular manner over the last couple of decades, allowing in vitro and in vivo genetic modification of many different cell types. Zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeat (CRISPR)-associated nuclease Cas9 enable precise genome editing by inducing DNA double-strand breaks (DSBs) at selected genomic loci. The DSBs are then repaired by the cellular repair machinery, via homology-directed repair (HDR) or non-homologous end-joining (NHEJ). HDR is a high-fidelity repair mechanism, which uses homologous DNA as repair template; therefore, by providing exogenous donor DNA (dDNA) flanked by sequences that are homologous to the nuclease target site, it is possible to achieve dDNA site-specific integration. One can thus insert a therapeutic gene into a preselected locus that allows safe, robust expression or downstream of the endogenous gene promoter to recreate physiological expression patterns. The NHEJ pathway has mainly been exploited to obtain permanent gene inactivation and disrupt cis-elements regulating gene expression. These genome-editing tools can potentially be applied to the development of safe therapeutic approaches for β-hemoglobinopathies.
Gene Correction Strategies
Correcting the mutations that cause β-hemoglobinopathies in the native β-globin locus can potentially increase the efficacy of gene therapy by overcoming the limitations associated with currently available gene transfer vectors.
Recently, several groups have developed genome-editing strategies to correct the SCD mutation via HDR. The combination of ZFNs or CRISPR/Cas9 nucleases with single-strand oligonucleotide donors or integrase-defective lentiviral vectors carrying the donor templates resulted in the HDR-mediated modification of up to 40% of the β-globin genes in HSPCs.72, 73, 74 However, this frequency was only 0.2% to 2.3% in severe combined immunodeficiency repopulating cells (i.e., bona fide HSCs), suggesting that HSCs might be less permissive to HDR and/or donor delivery than committed hematopoietic progenitors.72, 73, 74, 75
To overcome this potential limitation, Dever et al.76 developed a CRISPR/Cas9 gene-editing approach based on the use of serotype 6 adeno-associated viral vectors (rAAV6) to deliver a donor template containing a selectable marker (i.e., GFP and tNGFR) flanked by arms with homology to the β-globin gene. Selection of GFPhigh or tNGFRhigh HSPCs, followed by transplantation into immunodeficient mice, increased the engraftment of β-globin-gene-targeted HSCs from ∼3.5% (for unsorted populations) to over 97%. This work showed that the low frequency of HDR-mediated β-globin gene targeting in HSCs can be overcome by the selection of gene-targeted cells prior to transplantation. This approach could be used to correct the SCD mutation and (if optimized for knocking in a β-globin cDNA) several different β-thalassemia-causing mutations in the β-globin coding region.
However, this enrichment strategy, although it increased the absolute numbers of genetically modified cells in vivo, resulted in the overall recovery of fewer HSPCs than in conventional approaches; it will therefore be essential to optimize an ex vivo HSCs expansion phase prior to transplantation. Moreover, these gene correction and gene targeting strategies may be more laborious (from a manufacturing standpoint) than NHEJ-based approaches (discussed below), since they require the concomitant delivery of a donor template and a sequence-specific nuclease.
Re-activation of Fetal γ-Globin Gene Expression by BCL11A Downregulation through Genome Editing
Recently, two groups of researchers have proposed genome-editing approaches for knocking down BCL11A solely in the erythroid progeny of HSCs.77, 78, 79 These strategies are based on the CRISPR/Cas9- or ZFN-mediated disruption of an enhancer located in the first intron of the BCL11A gene, which is targeted by the erythroid master regulator GATA1 and is essential for BCL11A expression only in erythroid cells.77, 78 In particular, disruption of the GATA1 motif led to beneficial HbF upregulation and reduction of β-globin expression levels in HSPC-derived RBCs. Importantly, Chang et al.69 showed that BCL11A gene disruption adversely affects human RBC enucleation, whereas ZFN-mediated targeting of the BCL11A erythroid-specific enhancer does not impair erythroid maturation. Transplantation studies have shown that the BCL11A enhancer-ZFN-edited HSCs can stably engraft in immunodeficient mice.69 Similar preclinical studies using the CRISPR/Cas9 system are required to validate the safety of this potential therapeutic approach to β-hemoglobinopathies. The NHEJ-based genome-editing strategy might be more successful than HDR-based approaches in HSCs,72, 74, 76 because (1) it does not require the delivery of a donor template and (2) the NHEJ repair pathway might be more active than HDR in these cells.72, 75, 80
Re-activation of Fetal γ-Globin Gene Expression by Reproducing HPFH Mutations
Currently available genome-editing tools may allow the generation of HPFH mutations in HSCs, as a therapeutic strategy to increase HbF levels in both β-thalassemic and SCD patients.
Introducing HPFH point mutations into the γ-globin promoters via HDR23 has not yet been explored in human HSCs, and might be limited by the low efficiency of HDR in these cells.72, 73, 74 Recently, Traxler et al.24 used the CRISPR/Cas9 system to generate a 13 bp HPFH deletion in the γ-globin promoters via microhomology-mediated end-joining (MMEJ). This region is thought to contain a binding site for the HbF repressor BCL11A (Figure 2) (M.J. Weiss and J.K. Joung, 2016, American Society of Hematology, conference). In this study, HbF re-activation was associated with reduced levels of sickle β-globin and ameliorated the SCD cell phenotype in vitro. Given that the MMEJ and HDR repair pathways both use the same initial DNA resection machinery81 and human HSCs might be poorly permissive to HDR, the efficiency of this MMEJ-based strategy must now be evaluated by transplanting modified HSPCs into immunodeficient mice.
Re-creating deletional HPFH requires the NHEJ-based excision of large genomic fragments containing the β- and δ-globin genes and the putative 3.5-kb δγ-intergenic HbF silencer targeted by BCL11A (Figure 2). This strategy takes advantage of the NHEJ repair pathway, which is highly active in HSCs;80 however, it requires the generation of two DSBs, which might decrease the overall efficiency of genome editing. Nevertheless, in proof-of-principle studies, this strategy reactivated HbF, permanently disrupted the β-globin gene, and ameliorated the SCD cell phenotype (A. Miccio, 2016, American Society of Hematology, conference).82 The efficiency of genome editing and the potential toxicity of this approach need to be further tested in bona fide HSCs.
Conclusions
Lentiviral-based gene addition strategies have proved to be effective and safe in the treatment of several genetic diseases.60, 83, 84, 85 Recent phase 1/2 studies have shown that this approach rendered β+-thalassemia major patients transfusion independent and at least one SCD patient disease-symptoms-free, although results need to be reproduced in larger cohorts of patients and over a longer follow-up period. For β0-thalassemia and possibly SCD, further improvements in manufacturing, cell processing, and/or protocol design are needed for clear clinical benefit.
Several groups have proposed alternative lentiviral- and genome-editing-based strategies for raising endogenous γ-globin expression or correcting the disease-causing mutations. Whereas lentiviral vectors are regularly and safely used to genetically modify patient HSCs, the clinical application of genome-editing strategies must now be validated in terms of editing efficiency of long-term HSCs, any possible toxicity associated with delivery, and the potential off-target activity of the specific genome-editing tools.
Studies of patients having undergone allogenic HSCT have shown that donor chimerism of ∼20% significantly improves the clinical phenotype of β-thalassemic and SCD patients as a result of the survival advantage of normal donor erythroblasts.86, 87 The results of studies in the mouse suggest that a similar proportion of genetically modified HSCs would be enough to ameliorate the β-thalassemic and SCD phenotypes.58, 88 However, this frequency should be determined for each specific approach, since differences in efficacy of the various strategies might influence the survival advantage of erythroblasts derived from genetically modified HSCs.
Ultimately, the different strategies must be compared in terms of efficiency, efficacy, and safety in order to provide patients suffering from β-hemoglobinopathies with the best therapeutic option.
Author Contributions
A.M., C.A., and M.C. wrote and edited the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported by grants from AFM-Telethon (17224), the European Research Council (ERC 2015-AdG, GENEFORCURE), and Agence nationale de la recherche (ANR-16-CE18-0004).
Contributor Information
Marina Cavazzana, Email: m.cavazzana@aphp.fr.
Annarita Miccio, Email: annarita.miccio@institutimagine.org.
References
- 1.Sankaran V.G., Orkin S.H. The switch from fetal to adult hemoglobin. Cold Spring Harb. Perspect. Med. 2013;3:a011643. doi: 10.1101/cshperspect.a011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg J., Papadopoulos P., Georgitsi M., Gutiérrez L., Grech G., Fanis P., Phylactides M., Verkerk A.J., van der Spek P.J., Scerri C.A. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat. Genet. 2010;42:801–805. doi: 10.1038/ng.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadhouders R., Aktuna S., Thongjuea S., Aghajanirefah A., Pourfarzad F., van Ijcken W., Lenhard B., Rooks H., Best S., Menzel S. HBS1L-MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. J. Clin. Invest. 2014;124:1699–1710. doi: 10.1172/JCI71520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou W., Zhao Q., Sutton R., Cumming H., Wang X., Cerruti L., Hall M., Wu R., Cunningham J.M., Jane S.M. The role of p22 NF-E4 in human globin gene switching. J. Biol. Chem. 2004;279:26227–26232. doi: 10.1074/jbc.M402191200. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe O., Shen Y., Liu Q., Campbell A.D., Kuroha T., Yamamoto M., Engel J.D. The TR2 and TR4 orphan nuclear receptors repress Gata1 transcription. Genes Dev. 2007;21:2832–2844. doi: 10.1101/gad.1593307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanabe O., McPhee D., Kobayashi S., Shen Y., Brandt W., Jiang X., Campbell A.D., Chen Y.T., Chang Cs., Yamamoto M. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 2007;26:2295–2306. doi: 10.1038/sj.emboj.7601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipe A., Li Q., Deveaux S., Godin I., Roméo P.H., Stamatoyannopoulos G., Mignotte V. Regulation of embryonic/fetal globin genes by nuclear hormone receptors: a novel perspective on hemoglobin switching. EMBO J. 1999;18:687–697. doi: 10.1093/emboj/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sankaran V.G., Menne T.F., Xu J., Akie T.E., Lettre G., Van Handel B., Mikkola H.K., Hirschhorn J.N., Cantor A.B., Orkin S.H. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 9.Sankaran V.G., Xu J., Byron R., Greisman H.A., Fisher C., Weatherall D.J., Sabath D.E., Groudine M., Orkin S.H., Premawardhena A., Bender M.A. A functional element necessary for fetal hemoglobin silencing. N. Engl. J. Med. 2011;365:807–814. doi: 10.1056/NEJMoa1103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Sankaran V.G., Ni M., Menne T.F., Puram R.V., Kim W., Orkin S.H. Transcriptional silencing of gamma-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang J.C., Yu Y., Burke S., Buettner F., Wang C., Kolodziejczyk A.A., Teichmann S.A., Lu L., Liu P. Single-cell transcriptomic reconstruction reveals cell cycle and multi-lineage differentiation defects in Bcl11a-deficient hematopoietic stem cells. Genome Biol. 2015;16:178. doi: 10.1186/s13059-015-0739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brendel C., Guda S., Renella R., Bauer D.E., Canver M.C., Kim Y.J., Heeney M.M., Klatt D., Fogel J., Milsom M.D. Lineage-specific BCL11A knockdown circumvents toxicities and reverses sickle phenotype. J. Clin. Invest. 2016;126:3868–3878. doi: 10.1172/JCI87885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda T., Wang X., Maeda M., Canver M.C., Sher F., Funnell A.P., Fisher C., Suciu M., Martyn G.E., Norton L.J. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science. 2016;351:285–289. doi: 10.1126/science.aad3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renneville A., Van Galen P., Canver M.C., McConkey M., Krill-Burger J.M., Dorfman D.M., Holson E.B., Bernstein B.E., Orkin S.H., Bauer D.E., Ebert B.L. EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood. 2015;126:1930–1939. doi: 10.1182/blood-2015-06-649087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa F.C., Fedosyuk H., Chazelle A.M., Neades R.Y., Peterson K.R. Mi2β is required for γ-globin gene silencing: temporal assembly of a GATA-1-FOG-1-Mi2 repressor complex in β-YAC transgenic mice. PLoS Genet. 2012;8:e1003155. doi: 10.1371/journal.pgen.1003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J., Bauer D.E., Kerenyi M.A., Vo T.D., Hou S., Hsu Y.J., Yao H., Trowbridge J.J., Mandel G., Orkin S.H. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc. Natl. Acad. Sci. USA. 2013;110:6518–6523. doi: 10.1073/pnas.1303976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y.T., de Vasconcellos J.F., Yuan J., Byrnes C., Noh S.J., Meier E.R., Kim K.S., Rabel A., Kaushal M., Muljo S.A., Miller J.L. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013;122:1034–1041. doi: 10.1182/blood-2012-12-472308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forget B.G. Molecular basis of hereditary persistence of fetal hemoglobin. Ann. N Y Acad. Sci. 1998;850:38–44. doi: 10.1111/j.1749-6632.1998.tb10460.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill D., Yang J., Erdjument-Bromage H., Bornschlegel K., Tempst P., Bank A. Tissue-specific and developmental stage-specific DNA binding by a mammalian SWI/SNF complex associated with human fetal-to-adult globin gene switching. Proc. Natl. Acad. Sci. USA. 1999;96:349–354. doi: 10.1073/pnas.96.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akinsheye I., Alsultan A., Solovieff N., Ngo D., Baldwin C.T., Sebastiani P., Chui D.H., Steinberg M.H. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118:19–27. doi: 10.1182/blood-2011-03-325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lessard S., Beaudoin M., Benkirane K., Lettre G. Comparison of DNA methylation profiles in human fetal and adult red blood cell progenitors. Genome Med. 2015;7:1. doi: 10.1186/s13073-014-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakalova L., Osborne C.S., Dai Y.F., Goyenechea B., Metaxotou-Mavromati A., Kattamis A., Kattamis C., Fraser P. The Corfu deltabeta thalassemia deletion disrupts gamma-globin gene silencing and reveals post-transcriptional regulation of HbF expression. Blood. 2005;105:2154–2160. doi: 10.1182/blood-2003-11-4069. [DOI] [PubMed] [Google Scholar]
- 23.Wienert B., Funnell A.P., Norton L.J., Pearson R.C., Wilkinson-White L.E., Lester K., Vadolas J., Porteus M.H., Matthews J.M., Quinlan K.G., Crossley M. Editing the genome to introduce a beneficial naturally occurring mutation associated with increased fetal globin. Nat. Commun. 2015;6:7085. doi: 10.1038/ncomms8085. [DOI] [PubMed] [Google Scholar]
- 24.Traxler E.A., Yao Y., Wang Y.D., Woodard K.J., Kurita R., Nakamura Y., Hughes J.R., Hardison R.C., Blobel G.A., Li C., Weiss M.J. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016;22:987–990. doi: 10.1038/nm.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngo S., Bartolucci P., Lobo D., Mekontso-Dessap A., Gellen-Dautremer J., Noizat-Pirenne F., Audard V., Godeau B., Galacteros F., Habibi A. Causes of death in sickle cell disease adult patients: old and new trends. Blood. 2014;124:2715. [Google Scholar]
- 26.Madigan C., Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Expert Rev. Mol. Med. 2006;8:1–23. doi: 10.1017/S1462399406010659. [DOI] [PubMed] [Google Scholar]
- 27.Powars D.R., Weiss J.N., Chan L.S., Schroeder W.A. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63:921–926. [PubMed] [Google Scholar]
- 28.Ribeil J.A., Arlet J.B., Dussiot M., Moura I.C., Courtois G., Hermine O. Ineffective erythropoiesis in β -thalassemia. Sci. World J. 2013;2013:394295. doi: 10.1155/2013/394295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karponi G., Psatha N., Lederer C.W., Adair J.E., Zervou F., Zogas N., Kleanthous M., Tsatalas C., Anagnostopoulos A., Sadelain M. Plerixafor+G-CSF-mobilized CD34+ cells represent an optimal graft source for thalassemia gene therapy. Blood. 2015;126:616–619. doi: 10.1182/blood-2015-03-629618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulad F., Wang X., Qu J., Taylor C., Ferro L., Karponi G., Bartido S., Giardina P., Heller G., Prockop S.E. Safe mobilization of CD34+ cells in adults with β-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014;123:1483–1486. doi: 10.1182/blood-2013-06-507178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yannaki E., Karponi G., Zervou F., Constantinou V., Bouinta A., Tachynopoulou V., Kotta K., Jonlin E., Papayannopoulou T., Anagnostopoulos A., Stamatoyannopoulos G. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum. Gene Ther. 2013;24:852–860. doi: 10.1089/hum.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lidonnici M.R., Aprile A., Frittoli M.C., Mandelli G., Paleari Y., Spinelli A., Gentner B., Zambelli M., Parisi C., Bellio L. Plerixafor and G-CSF combination mobilizes hematopoietic stem and progenitors cells with a distinct transcriptional profile and a reduced in vivo homing capacity compared to Plerixafor alone. Haematologica. 2016 doi: 10.3324/haematol.2016.154740. Published online December 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D., Xu C., Manwani D., Frenette P.S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127:801–809. doi: 10.1182/blood-2015-09-618538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzhugh C.D., Hsieh M.M., Bolan C.D., Saenz C., Tisdale J.F. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy. 2009;11:464–471. doi: 10.1080/14653240902849788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavazzana M. Gene therapy studies in hemoglobinopathies: successes and challenges. Blood. 2016;128 SCI–50. [Google Scholar]
- 37.Kanter J., Walters M.C., Hsieh M.M., Krishnamurti L., Kwiatkowski J., Kamble R.T., von Kalle C., Kuypers F.A., Cavazzana M., Leboulch P. Interim results from a phase 1/2 clinical study of lentiglobin gene therapy for severe sickle cell disease. Blood. 2016;128:1176. [Google Scholar]
- 38.Thompson A.A., Kwiatkowski J.L., Rasko J., Hongeng S., Schiller G.J., Anurathapan U., Cavazzana M., Ho P.J., von Kalle C., Kletzel M. Lentiglobin gene therapy for transfusion-dependent β-thalassemia: update from the Northstar Hgb-204 phase 1/2 clinical study. Blood. 2016;128:1175. [Google Scholar]
- 39.Naldini L., Blomer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 40.Grosveld F., van Assendelft G.B., Greaves D.R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 41.Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D.R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989;338:352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- 42.Sadelain M., Wang C.H., Antoniou M., Grosveld F., Mulligan R.C. Generation of a high-titer retroviral vector capable of expressing high levels of the human beta-globin gene. Proc. Natl. Acad. Sci. USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leboulch P., Huang G.M., Humphries R.K., Oh Y.H., Eaves C.J., Tuan D.Y., London I.M. Mutagenesis of retroviral vectors transducing human beta-globin gene and beta-globin locus control region derivatives results in stable transmission of an active transcriptional structure. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.May C., Rivella S., Chadburn A., Sadelain M. Successful treatment of murine beta-thalassemia intermedia by transfer of the human beta-globin gene. Blood. 2002;99:1902–1908. doi: 10.1182/blood.v99.6.1902. [DOI] [PubMed] [Google Scholar]
- 45.Rivella S., May C., Chadburn A., Rivière I., Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- 46.Pawliuk R., Westerman K.A., Fabry M.E., Payen E., Tighe R., Bouhassira E.E., Acharya S.A., Ellis J., London I.M., Eaves C.J. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 47.Puthenveetil G., Scholes J., Carbonell D., Qureshi N., Xia P., Zeng L., Li S., Yu Y., Hiti A.L., Yee J.K., Malik P. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- 48.Hanawa H., Hargrove P.W., Kepes S., Srivastava D.K., Nienhuis A.W., Persons D.A. Extended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- 49.Roselli E.A., Mezzadra R., Frittoli M.C., Maruggi G., Biral E., Mavilio F., Mastropietro F., Amato A., Tonon G., Refaldi C. Correction of beta-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patients. EMBO Mol. Med. 2010;2:315–328. doi: 10.1002/emmm.201000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negre O., Eggimann A.V., Beuzard Y., Ribeil J.A., Bourget P., Borwornpinyo S., Hongeng S., Hacein-Bey S., Cavazzana M., Leboulch P., Payen E. Gene therapy of the β-hemoglobinopathies by lentiviral transfer of the β(A(T87Q))-globin gene. Hum. Gene Ther. 2016;27:148–165. doi: 10.1089/hum.2016.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi K., Konitzer P., Surrey S. Role of gamma 87 Gln in the inhibition of hemoglobin S polymerization by hemoglobin F. J. Biol. Chem. 1994;269:9562–9567. [PubMed] [Google Scholar]
- 52.Emery D.W., Yannaki E., Tubb J., Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl. Acad. Sci. USA. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arumugam P.I., Higashimoto T., Urbinati F., Modlich U., Nestheide S., Xia P., Fox C., Corsinotti A., Baum C., Malik P. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol. Ther. 2009;17:1929–1937. doi: 10.1038/mt.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C.L., Xiong D., Stamatoyannopoulos G., Emery D.W. Genomic and functional assays demonstrate reduced gammaretroviral vector genotoxicity associated with use of the cHS4 chromatin insulator. Mol. Ther. 2009;17:716–724. doi: 10.1038/mt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Negre O., Bartholomae C., Beuzard Y., Cavazzana M., Christiansen L., Courne C., Deichmann A., Denaro M., de Dreuzy E., Finer M. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of β-thalassemia and sickle cell disease. Curr. Gene Ther. 2015;15:64–81. doi: 10.2174/1566523214666141127095336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansilla-Soto J., Riviere I., Boulad F., Sadelain M. Cell and gene therapy for the beta-thalassemias: advances and prospects. Hum. Gene Ther. 2016;27:295–304. doi: 10.1089/hum.2016.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu M., Maurano M.T., Wang H., Qi H., Song C.Z., Navas P.A., Emery D.W., Stamatoyannopoulos J.A., Stamatoyannopoulos G. Genomic discovery of potent chromatin insulators for human gene therapy. Nat. Biotechnol. 2015;33:198–203. doi: 10.1038/nbt.3062. [DOI] [PubMed] [Google Scholar]
- 58.Miccio A., Cesari R., Lotti F., Rossi C., Sanvito F., Ponzoni M., Routledge S.J., Chow C.M., Antoniou M.N., Ferrari G. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemia. Proc. Natl. Acad. Sci. USA. 2008;105:10547–10552. doi: 10.1073/pnas.0711666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernardo M.E., Piras E., Vacca A., Giorgiani G., Zecca M., Bertaina A., Pagliara D., Contoli B., Pinto R.M., Caocci G. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- 60.Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 61.Perumbeti A., Higashimoto T., Urbinati F., Franco R., Meiselman H.J., Witte D., Malik P. A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correction. Blood. 2009;114:1174–1185. doi: 10.1182/blood-2009-01-201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breda L., Motta I., Lourenco S., Gemmo C., Deng W., Rupon J.W., Abdulmalik O.Y., Manwani D., Blobel G.A., Rivella S. Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood. 2016;128:1139–1143. doi: 10.1182/blood-2016-01-691089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero Z., Urbinati F., Geiger S., Cooper A.R., Wherley J., Kaufman M.L., Hollis R.P., de Assin R.R., Senadheera S., Sahagian A. β-globin gene transfer to human bone marrow for sickle cell disease. J. Clin. Invest. 2013 doi: 10.1172/JCI67930. Published online July 1, 2013. 10.1172/JCI67930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miccio A., Poletti V., Tiboni F., Rossi C., Antonelli A., Mavilio F., Ferrari G. The GATA1-HS2 enhancer allows persistent and position-independent expression of a β-globin transgene. PLoS ONE. 2011;6:e27955. doi: 10.1371/journal.pone.0027955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawado T., Halow J., Bender M.A., Groudine M. The beta -globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 2003;17:1009–1018. doi: 10.1101/gad.1072303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda T., Ito K., Merghoub T., Poliseno L., Hobbs R.M., Wang G., Dong L., Maeda M., Dore L.C., Zelent A. LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis. Dev. Cell. 2009;17:527–540. doi: 10.1016/j.devcel.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J., Peng C., Sankaran V.G., Shao Z., Esrick E.B., Chong B.G., Ippolito G.C., Fujiwara Y., Ebert B.L., Tucker P.W., Orkin S.H. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334:993–996. doi: 10.1126/science.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basak A., Hancarova M., Ulirsch J.C., Balci T.B., Trkova M., Pelisek M., Vlckova M., Muzikova K., Cermak J., Trka J. BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. J. Clin. Invest. 2015;125:2363–2368. doi: 10.1172/JCI81163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang K.-H., Smith S.E., Sullivan T., Chen K., Zhou Q., West J.A., Liu M., Liu Y., Viera B.F., Sun C. Long-term engraftment and fetal globin reactivation upon genome editing of BCL11A in bone marrow-derived CD34+ hematopoietic stem and progenitor cells. Mol. Ther. 2017;4:137–148. doi: 10.1016/j.omtm.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng W., Rupon J.W., Krivega I., Breda L., Motta I., Jahn K.S., Reik A., Gregory P.D., Rivella S., Dean A., Blobel G.A. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158:849–860. doi: 10.1016/j.cell.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samakoglu S., Lisowski L., Budak-Alpdogan T., Usachenko Y., Acuto S., Di Marzo R., Maggio A., Zhu P., Tisdale J.F., Rivière I., Sadelain M. A genetic strategy to treat sickle cell anemia by coregulating globin transgene expression and RNA interference. Nat. Biotechnol. 2006;24:89–94. doi: 10.1038/nbt1176. [DOI] [PubMed] [Google Scholar]
- 72.Hoban M.D., Cost G.J., Mendel M.C., Romero Z., Kaufman M.L., Joglekar A.V., Ho M., Lumaquin D., Gray D., Lill G.R. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125:2597–2604. doi: 10.1182/blood-2014-12-615948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoban M.D., Lumaquin D., Kuo C.Y., Romero Z., Long J., Ho M., Young C.S., Mojadidi M., Fitz-Gibbon S., Cooper A.R. CRISPR/Cas9-mediated correction of the sickle mutation in human CD34+ cells. Mol. Ther. 2016;24:1561–1569. doi: 10.1038/mt.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeWitt M.A., Magis W., Bray N.L., Wang T., Berman J.R., Urbinati F., Heo S.J., Mitros T., Muñoz D.P., Boffelli D. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl. Med. 2016;8:360ra134. doi: 10.1126/scitranslmed.aaf9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genovese P., Schiroli G., Escobar G., Di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bauer D.E., Kamran S.C., Lessard S., Xu J., Fujiwara Y., Lin C., Shao Z., Canver M.C., Smith E.C., Pinello L. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canver M.C., Smith E.C., Sher F., Pinello L., Sanjana N.E., Shalem O., Chen D.D., Schupp P.G., Vinjamur D.S., Garcia S.P. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192–197. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vierstra J., Reik A., Chang K.H., Stehling-Sun S., Zhou Y., Hinkley S.J., Paschon D.E., Zhang L., Psatha N., Bendana Y.R. Functional footprinting of regulatory DNA. Nat. Methods. 2015;12:927–930. doi: 10.1038/nmeth.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gundry M.C., Brunetti L., Lin A., Mayle A.E., Kitano A., Wagner D., Hsu J.I., Hoegenauer K.A., Rooney C.M., Goodell M.A., Nakada D. Highly efficient genome editing of murine and human hematopoietic progenitor cells by CRISPR/Cas9. Cell Rep. 2016;17:1453–1461. doi: 10.1016/j.celrep.2016.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Truong L.N., Li Y., Shi L.Z., Hwang P.Y., He J., Wang H., Razavian N., Berns M.W., Wu X. Microhomology-mediated end joining and homologous recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA. 2013;110:7720–7725. doi: 10.1073/pnas.1213431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye L., Wang J., Tan Y., Beyer A.I., Xie F., Muench M.O., Kan Y.W. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl. Acad. Sci. USA. 2016;113:10661–10665. doi: 10.1073/pnas.1612075113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 84.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walters M.C., Patience M., Leisenring W., Rogers Z.R., Aquino V.M., Buchanan G.R., Roberts I.A., Yeager A.M., Hsu L., Adamkiewicz T., Multicenter Investigation of Bone Marrow Transplantation for Sickle Cell Disease Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol. Blood Marrow Transplant. 2001;7:665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 87.Gaziev J., Lucarelli G. Stem cell transplantation for hemoglobinopathies. Curr. Opin. Pediatr. 2003;15:24–31. doi: 10.1097/00008480-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Altrock P.M., Brendel C., Renella R., Orkin S.H., Williams D.A., Michor F. Mathematical modeling of erythrocyte chimerism informs genetic intervention strategies for sickle cell disease. Am. J. Hematol. 2016;91:931–937. doi: 10.1002/ajh.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]