Abstract
Introduction
The authors describe the medications for treatment-resistant depression (TRD) in phase II/III of clinical development in the EU and USA and provide an opinion on how current treatment can be improved in the near future.
Areas covered
Sixty-two trials were identified in US and EU clinical trial registries that included six investigational compounds in recent phase III development and 12 others in recent phase II clinical trials. Glutamatergic agents have been the focus of many studies. A single intravenous dose of the glutamatergic modulator ketamine produces a robust and rapid antidepressant effect in persons with TRD; this effect continues to remain significant for 1 week. This observation was a turning point that opened the way for other, more selective glutamatergic modulators (intranasal esketamine, AVP-786, AVP-923, AV-101, and rapastinel). Of the remaining compounds, monoclonal antibodies open highly innovative therapeutic options, based on new pathophysiological approaches to depression.
Expert commentary
Promising new agents are emerging for TRD treatment. Glutamatergic modulators likely represent a very promising alternative to monoaminergic antidepressant monotherapy. We could see the arrival of the first robust and rapid acting antidepressant drug in the near future, which would strongly facilitate the ultimate goal of recovery in persons with TRD.
Keywords: AV-101, AVP-786, brexpiprazole, buprenorphine, cariprazine, clinical trials, esketamine, ketamine, rapastinel, treatment-resistant depression
1. Introduction
Although exact definitions and stringency levels vary, treatment-resistant depression (TRD) is commonly defined as a clinical entity grouping cases of major depressive disorder (MDD) that do not respond adequately to two successive trials of antidepressant treatment at an adequate dose and duration [1–3]. TRD is associated with severe impairment in cognitive functioning, increased risk of developing/being associated with comorbid illnesses, decreased workplace performance, increased risk of suicide, and increased cost of care [4]. TRD is becoming a major threat to public health because TRD prevalence represents at least 15–30% of MDD cases [1,2,5–7], and MDD itself is one of the most commonly encountered mental disorders with a worldwide point prevalence rate of 4.7% [8].
It is commonly presumed that the variability in antidepressant treatment response reflects biological heterogeneity [9]. TRD is a complex, multifactorial, and heterogeneous group of disorders, integrating neurobiological and environmental factors, which reduces the sensitivity to selective serotonin reuptake inhibitors (SSRIs) and second-line antidepressants [10–12]. Among these factors, converging lines of evidence indicate an important pathophysiological role of the glutamatergic system [13,14]. Recent evidence is suggesting that TRD may involve a maladaptive response to stress in early life, leading to disturbances characterized by a complex crosstalk within the extrasynaptic glutamatergic receptor signal pathway, involving glutamate reuptake and release by glial cells [14].
Extensive evidence supports the use of atypical antipsychotics as possible adjunctive therapy for the management of TRD [1,7]. Four atypical antipsychotics have been approved by the FDA as treatments for MDD: aripiprazole, quetiapine extended-release, and brexpiprazole, in combination with antidepressants, and olanzapine combined with fluoxetine specifically [1,15–17]. Of these four choices, only olanzapinefluoxetine combination has the indication for TRD, while the others are indicated for MDD insufficiently responding to first-line antidepressant treatment [15]. Use of adjunctive atypical antipsychotics for MDD can be associated with significant side effects such as weight gain, akathisia, and sedation [18]. Other combination therapies for TRD include lithium [19], thyroid hormone [20], buspirone [21], mirtazapine [22], and bupropion [23]. Of note, the STAR*D study found no clear-cut ‘winner’ for improving clinical outcomes in real-world patients with TRD [24]. Moreover, the NICE Appraisal Committee recommended the new multimodal antidepressant, vortioxetine, as an option for treating TRD [25].
Cognitive behavioral therapy and other psychotherapies remain a treatment option, alone or in combination with pharmacotherapy [1,26]. Neurostimulation and neuromodulation strategies (electroconvulsive therapy, repetitive transcranial magnetic stimulation, vagus nerve stimulation) are also available for subjects insufficiently responding to pharmacotherapy or psychosocial interventions [1,27–30].
We aimed to evaluate how the pharmacotherapy of TRD may evolve in the near future by using a previously developed search strategy [31–33]. For this purpose, we used the US and EU clinical trial registries to obtain information about recent, still unpublished clinical trials on TRD. Among others, these registers inform about the characteristics and status of exploratory phase II studies (recruiting, ongoing, completed and results), the names of drugs entering pivotal phase III studies, and the rational and expected results of the trials, as expressed by the investigators themselves. This information was supplemented with data officially reported, either in publications or in meetings. Regular follow-up reviews of this kind could provide a fresh look of current progress in the field, identify emerging problems, and anticipate the arrival of potentially effective drugs.
2. Methods
As done for other conditions before [31,32], the medications in phase II and III trials of clinical development for the treatment of TRD, have been appraised using the clinical trial registries of the (National Institutes of Health (NIH), USA and the European Medicines Agency (EMA) and conducting systematic reviews of electronic data bases on identified compounds.
2.1. Identification of recent phase II/III clinical trials in TRD
Clinical trial registries operated and maintained by the US NIH (ClinicalTrials.gov, www.clinicaltrials.gov) and the EMA (EU Clinical Trials Register, www.clinicaltrialsregister.eu) were accessed to identify recent phase II and phase III clinical pharmacological trials in TRD. Clinical trials investigating psychosocial interventions or neurostimulation strategies were not assessed, but the interested reader is referred elsewhere [1,26–29]. Regulatory guidelines, such as the EMA’s draft guideline for the clinical investigation of medicines in depression (including TRD) are also available elsewhere [34]. Identified clinical trials were given with their designated name, a literature reference, or the database identifier number, in decreasing order of priority.
2.1.1. Selection criteria
In order to be retained for this review, pharmacologic agents needed to be in phase II and/or III clinical trials for TRD and have at least one trial satisfying the following criteria:
Trial updated on 1 January 2014 or later,
Trial not terminated (does not apply for new compounds),
Trial status known,
Completion date by 1 January 2013 or later.
In case that data were not posted in clinical trial registries or in case of unpublished data, we requested information from the principal or lead investigator of the study in question.
2.1.2. Search of clinical trials
Clinical trials with the above characteristics were searched in the ClinicalTrials.gov database, using the following search terms: ‘treatment-resistant depression,’ ‘treatment-refractory depression’ or ‘difficult-to-treat depression’ (for ‘conditions’); ‘drug’ (for ‘interventions’); ‘phase II,’ or ‘phase III’ (for ‘phase’). Similar search terms were used for the EMA database. Trials in MDD (for ‘conditions’) that were conducted in TRD patients were also retained for the analysis.
2.2. Additional sources of information
We searched electronically for articles related to the compounds identified in the clinical trial registries, particularly those published from 2012 to the present, using the name of the compound AND ‘treatment-resistant depression,’ OR ‘depression.’ The following biomedical literature search resources were used: PubMed (www.ncbi.nlm.nih.gov/pubmed), Science Direct (www.sciencedirect.com), Google Scholar (https://scholar.google.com), and Cochrane Library (www.cochranelibrary.com).
The above information was supplemented by publically available information from the EMA (www.ema.europa.eu) and FDA (www.fda.gov), presentations at international congresses, references cited in articles and books, and clinical practice recommendations (CPR). We also consulted the websites of pharmaceutical and biotech companies active in the field of psychiatry.
2.3. Extraction of investigational compounds and relevant trial data
We extracted the names of compounds and trials sponsors, together with their associated data regarding efficacy and tolerability/side effects, the duration of the study, number of patients and results (if available). Unless otherwise indicated, compounds were administered orally.
3. Compounds undergoing phase III clinical development for TRD
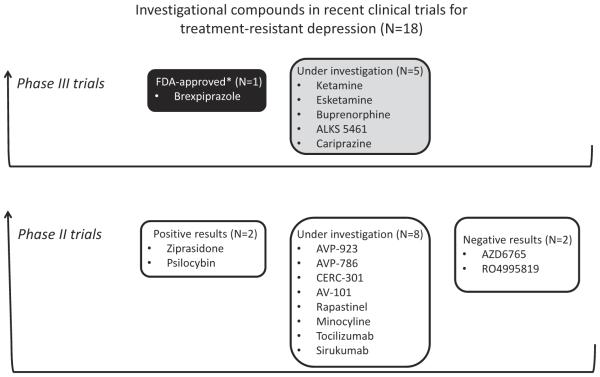
The clinical trial registries of the NIH (www.clinicaltrials.gov) and the EMA (www.clinicaltrialsregister.eu) were accessed from 18 March 2016 to 21 December 2016. These databases included 18 phase III clinical trials on TRD updated on 1 January 2014 or later. Three clinical trials were excluded from the present analysis for the following reasons: primary completion date before 2013 (bupropion), terminated trials (olanzapine–fluoxetine combination) and no investigational drug (pulsating electromagnetic fields). The remaining 15 phase III clinical trials that fulfilled the inclusion criteria, concerned the following six investigational compounds (Table 1 and Figure 1): ketamine [35–60], esketamine [43,60–65], buprenorphine [66–70], ALKS 5461 [71,72], brexpiprazole [17,73,74], and cariprazine [75–77] (trials with ALKS 5461, brexpiprazole, and cariprazine have indicated ‘MDD’ for ‘conditions,’ but were conducted in patients who meet some definitions of TRD).
Table 1.
Investigational drugs for treatment-resistant depression reviewed in this article.
| Phase | Family | Compound | Main pharmacological action | Main medication uses | References |
|---|---|---|---|---|---|
| Phase III | Glutamatergic agents | Ketamine | NMDAR antagonistb | Anesthetic, sedative | [35–65] |
| Esketamine | NMDAR antagonistb | Investigational new drug | |||
| Opioids | Buprenorphine | OR modulatorb | Opioid addiction | [66–72] | |
| ALKS 5461 a | OR modulatorb | Investigational new drug | |||
| Atypical antipsychotics | Brexpiprazole c | D2R partial agonist | Schizophrenia, BD, MDD | [17,73–77] | |
| Cariprazine | D3R/D2R partial agonist | Schizophrenia, BD | |||
| Phase II | Glutamatergic agents | AVP-923 | NMDAR antagonistb | Pseudo-bulbar affect | [48,78–94] |
| AVP-786 | NMDAR antagonistb | Investigational new drug | |||
| CERC-301 | NMDAR antagonistb | Investigational new drug | |||
| AV-101 | NMDAR antagonistb | Investigational new drug | |||
| AZD6765 | NMDAR antagonistb | Investigational new drug | |||
| Rapastinel | NMDAR antagonistb | Investigational new drug | |||
| RO4995819 | mGlu2/3R antagonist | Investigational new drug | |||
| Atypical antipsychotics | Ziprasidone | D2R and 5-HT2A antagonist | Schizophrenia, BD | [95–98] | |
| Psychedelics | Psilocybin | 5-HT2AR partial agonistb | Investigational new drug | [99,100] | |
| Tetracycline antibiotics | Minocycline | Bacteriostatic | Skin infections | [101–104] | |
| Monoclonal antibodies | Tocilizumab | IL-6R antagonist | Rheumatoid arthritis | [105,106] | |
| Sirukumab | IL-6R antagonist | Investigational new drug |
Buprenorphine + samidorphan.
See text.
FDA-approved compound for MDD treatment, currently in phase III/IV development (see text).
5-HT2AR, serotonin 2A receptor. BD: bipolar disorder; D2R: dopamine D2 receptor; MDD: major depressive disorder; mGlu2/3R: metabotropic glutamate 2/3 receptor; NMDAR: N-methyl-D-aspartate receptor antagonists; OR: opioid receptor; IL-6R: interleukin-6 receptor.
Figure 1.
Status of investigational drugs in recent phase II/III clinical trials for TRD. *FDA-approved as an adjunctive therapy to antidepressants for the treatment of major depressive disorder.
3.1. Ketamine
3.1.1. Background
Ketamine acts primarily as a glutamatergic modulator, but also as a partial agonist at dopamine D2 receptors and as a dopamine reuptake inhibitor [43,44,47,57]. As glutamatergic agent, ketamine is a N-methyl-D-aspartate receptor (NMDAR) antagonist [43,44]. A recent study in mice showed that a ketamine metabolite is essential for its antidepressant effects [57,59]. The antidepressant action of this metabolite is independent of NMDAR inhibition, but involves early and sustained activation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) [57].
Ketamine is a general anesthetic, which has been used for over 40 years because of its short half-life (180 min), the lack of respiratory depression, and its good safety profile [40]. Unfortunately, a significant first-pass hepatic metabolism limits the available routes of administration for non-anesthetic uses.
Early clinical studies suggested that sub-anesthetic doses of intravenous ketamine were effective and rapid acting in major depression [35–37]. In 2006, a randomized controlled trial (RCT) by Zarate et al. [39] demonstrated that a single intravenous dose of ketamine produced a robust and rapid antidepressant effect in persons with TRD, which continued to remain significant for 1 week. Ketamine efficacy was confirmed and extended in several randomized trials involving more than 200 persons with depression [41,42,46]. The limited duration of the antidepressant effect after a single dose (not exceeding 72 h to 1 week) was confirmed by most studies [41,42,46].
Ketamine is a generic medication, and it was being studied at academic medical centers in eight out of nine recent clinical trials (Table 2).
Table 2.
Recent clinical trials with ketamine in treatment-resistant depression (2014 and later).
| Phase | Sponsor | Identifier | Primary outcome | Time frame | N a | Results or status |
|---|---|---|---|---|---|---|
| III | VA Office of R&D (USA) | NCT02556606 | Time to relapse | 4 W | 72 | March 2020b |
| II/III | University of Ottawa (Canada) | NCT01945047 | Safety & HDRSi | 2 W | 63 | December 2015b |
| II | Janssen R&D (USA) | Singh et al, 2016 [38] | MADRSj | 15 D | 68 | KET = −17.7d, c, PL = −3.1 |
| Massachusetts General Hospital (USA) | NCT01920555 | HDRSi | 24 H | 100 | January 2016b | |
| Veteran’s Affairs Office R&D (USA) | NCT02360280 | MADRSj | 13 D | 56 | January 2018b | |
| Paul J. Lamothee (Mexico) | NCT01868802 | HDRSi | 1–7 D | 60 | May 2016b | |
| Magdeburg Universityg (Germany) | EU 2010–023414-31 | HDRSi | 24 H | 40 | NC | |
| University of Minnesotaf (USA) | NCT02078817 | CGI | 1 W | 20 | April 2016b | |
| Mayo Clinic (USA) | NCT02094898 | MADRSj | 8Wh | 30 | June 2016b |
Enrolment (final or estimated).
Primary completion date (past or estimated).
Intravenous ketamine at 0.5 mg/kg, thrice-weekly.
Statistically significant.
American British Cowdray Medical Center.
Clinical and Translational Science Institute.
Department of Psychiatry, School of Medicine.
Maintenance treatment (4 weeks) + follow up after treatment ends for four weeks.
Hamilton Depression Rating Scale.
Montgomery–Asberg Depression Rating Scale.
CGI: Clinical Global Impression; D: days; EU: EudraCT number; H: hours; KET: ketamine; NC: not communicated; PL: placebo; R&D: research development; W: weeks.
3.1.2. Phase II clinical trials
The clinical trial registries of the NIH and the EMA included seven recent phase II clinical trials of ketamine in TRD (Table 2). NCT01627782 showed that administering intravenous ketamine two or three times weekly (0.5 mg/kg) similarly maintained antidepressant efficacy over 15 days [38]. NCT01920555 is a dose-finding, multisite NIMH-sponsored study (there is still concern about the optimal dose for efficacy and safety). Three other RCTs (NCT02360280, NCT01868802, and EudraCT 2010–023414-31) are currently recruiting participants to investigate the efficacy, safety, tolerability, dosage, and/or durability of sub-anesthetic intravenous ketamine in TRD (see Table 2 for details).
Two open-label phase II trials will further study ketamine in TRD. NCT02078817 will investigate intravenous sub-anesthetic ketamine for adolescents with TRD. NCT02094898 will investigate if low-dose ketamine infusions reduce depression severity and suicide risk
3.1.3. Phase III clinical trials
Two phase III clinical trials have been designed to investigate ketamine in TRD. NCT02556606 will compare the effectiveness of a single infusion of ketamine or midazolam (a short-acting benzodiazepine) in persons with late-life TRD (nonresponders to two or more adequate trials of FDA-approved antidepressants, as determined by the Antidepressant Treatment Response Questionnaire (ATRQ) criteria). As of this writing, this study was not yet open for patient recruitment. NCT01945047 (phase II/III study) is recruiting participants to investigate the beneficial effects of a single ketamine injection as a rapid intervention for TRD (failure to respond adequately to at least two antidepressants and two augmentation strategies), and to investigate the possibility of obtaining a prolonged antidepressant effect with repeated injections (N ≤ 3). Midazolam will be used for comparison.
3.2. Intranasal esketamine
3.2.1. Background
Esketamine (Janssen R&D, USA) is the S-enantiomer of ketamine, which possesses a 3–4-fold higher affinity for NMDA receptors and greater anesthetic potency than the R-ketamine enantiomer (arketamine) [43,60,64,65]. The availability of an intranasal formulation led Janssen R&D to launch a development program, including three phase II and five phase III clinical trials (Table 3).
Table 3.
Recent clinical trials with esketamine in treatment-resistant depression (2014 and later).
| Phase | Sponsor | Identifier | Primary outcome | Time frame | N a | Results or status |
|---|---|---|---|---|---|---|
| III | Janssen R&D (USA) | TRANSFORM-1 | MADRSd | 4 W | 348 | October 2017b |
| Janssen R&D (USA) | TRANSFORM-2 | MADRSd | 4 W | 196 | March 2017b | |
| Janssen R&D (USA) | TRANSFORM-3 | MADRSd | 4 W | 230 | July 2017b | |
| Janssen R&D (USA) | SUSTAIN-1 | Time to relapsee | 104 W | 333 | August 2017b | |
| Janssen R&D (USA) | SUSTAIN-2 | Safety | 56 W | 1071 | January 2018b | |
| II | Janssen R&D (USA) | PeRSEVERe | MADRSf | 12 W | 68 | ΔScoreg: −5.3 ± 2.1c |
| Janssen R&D (USA) | SYNAPSE | MADRSd | 2 W | 108 | July 2015b | |
| Janssen R&D (USA) | Singh et al, 2015 [64] | MADRSd | 1 D | 30 | ESKh = −16.9c, PL = −3.8 |
Enrolment (final or estimated).
Primary completion date (past or estimated).
Statistically significant.
Change from baseline in Montgomery±Asberg Depression Rating Scale (MADRS).
Time to relapse in patients with stable remission.
Change from baseline in MADRS total score at day 1 (4 h post-dose).
Least-square mean difference ± SE, two-sided p = 0.015.
Intravenous esketamine at 0.4 mg/kg.
D: days; W: weeks.
3.2.2. Phase II clinical trials
A phase II RCT (NCT01640080, Table 3 and ref. [64]) assessed the efficacy of intravenous esketamine in improving symptoms of depression in patients with TRD. A rapid (within 2 h) and robust antidepressant effect was observed after a 40-min infusion of either 0.20 mg/kg or 0.40 mg/kg of esketamine as compared with placebo (Table 3 and ref. [64]). Another phase II RCT (SYNAPSE, NCT01998958, Table 3 and ref. [61]) evaluated the efficacy and dose response of intranasal esketamine in improving depressive symptoms in persons with TRD. Results from one of two panels of the multicenter study were reported at the American Society of Clinical Psychopharmacology (ASCP) Annual Meeting (Miami, USA, June 22–25, 2015) [61]. Over a one-week period, changes in MADRS total scores on Day 8 in all three esketamine treatment groups (28 mg, 56 mg, and 84 mg) were statistically superior to placebo. Transient elevations in blood pressure (maximum mean changes = 19 mmHg for systolic and 10 mmHg for diastolic blood pressure, respectively) and heart rates were observed in a majority of patients receiving esketamine on dosing days. Dissociative symptoms abated with repeated dosing [61]. Preliminary data from a third phase II RCT (PeRSEVERe) showed that intranasal esketamine reduced depression symptoms at day 1 (4 h post-dose; primary outcome criterion) and thoughts of suicide in high-risk depressed patients (Table 3 and ref. [63]).
Following the above positive results, the FDA granted a Breakthrough Therapy Designation (BTD) for intranasal esketamine in MDD with imminent risk for suicide [107] (break-through therapy is an FDA designation that expedites drug development), and esketamine entered phase III development as add-on therapy to oral antidepressants in TRD.
3.2.3. Phase III clinical trials
TRANSFORM-1 (NCT02417064) and TRANSFORM-2 (NCT02418585) are recruiting participants to evaluate the efficacy, safety, and tolerability of fixed and flexible doses of intranasal esketamine, respectively, in TRD (defined by nonresponse to 2 or more oral antidepressant treatments taken at adequate dosage and for adequate duration, assessed using the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (MGH-ATRQ) (Table 3)). TRANSFORM-3 (NCT02422186) will evaluate intranasal esketamine in the elderly (Table 3). SUSTAIN-1 (NCT02493868) will evaluate intranasal esketamine for relapse prevention and SUSTAIN-2 (NCT02497287) is a long-term safety and efficacy study (Table 3).
3.3. Buprenorphine
3.3.1. Background
Buprenorphine, a semisynthetic opioid derivative of thebaine, is a partial agonist at μ-opiate receptors, an antagonist of κ-receptors, and also displays affinity for δ-receptors (Table 1 and reference [66]). Buprenorphine is used as an analgesic at lower dosages (0.2–1.2 mg/d, sublingual or parenteral) to control moderate-to-severe pain and at higher dosages (0.8–4 mg/d) to treat opioid addiction [66].
Opiates were used to treat major depression until the mid-1950s [69]. In the 1990s, the advent of buprenorphine and other opioids with reduced dependence and abuse liabilities has made their re-evaluation possible for this indication. Two open-label pilot studies suggested a possible role for buprenorphine in treating depressive symptoms in people with opioid addiction [108] and in patients with TRD [69]. In addition, successful treatment of severe non-suicidal self-injury with buprenorphine has been reported in six individuals [70].
3.3.2. Recent phase II clinical trials
Buprenorphine is a generic medication, which is being studied at academic medical centers (Table 4). Recently, Karp et al. [67] conducted a pilot, one-arm phase II trial (BUILD, NCT01071538) designed to investigate the safety and clinical effect of low-dose sublingual buprenorphine (mean dose = 0.4 mg/d) in older adults with TRD. The mean (±SD) MADRS score decreased from 27.0 ± 7.3 at baseline to 9.5 ± 9.5 at week 8, with a sharp decline in depression severity during the first three weeks of exposure (mean change = −15.0 ± 7.9). Buprenorphine was well tolerated (there were no sustained elevations of systolic or diastolic blood pressure) [67].
Table 4.
Recent clinical trials with buprenorphine, ALKS 5461, brexpiprazole and cariprazine in treatment-resistant depression (2014 and later).
| Phase | Sponsor | Identifier | Primary outcome | Time frame | N a | Results or status |
|---|---|---|---|---|---|---|
| Buprenorphine | ||||||
| III | University of Pittsburgh (USA) | NCT01407575 | MADRSe | 6 W | 10 | Negative? (see text) |
| II | University of Pittsburgh (USA) | Karp 2014 [67] | MADRSe | 8 W | 15 | −17.5 (No placebo) |
| I/II | CAMH (CAN)c | NCT02263248 | MADRSe | 32 W | 72 | December 2017b |
| Washington University (USA)d | NCT02181231 | MADRSe | 32 W | 100 | August 2018b | |
| ALKS 5461 | ||||||
| III | Alkermes (USA and Ireland) | FORWARD-3 | MADRSe | 10 W | 447 | NS |
| Alkermes (USA and Ireland) | FORWARD-4 | MADRSe | 11 W | 385 | NS | |
| Alkermes (USA and Ireland) | FORWARD-5 | MADRSe | 11 W | 350 | October 2016b | |
| Alkermes (USA and Ireland) | FORWARD-1 | Safety | 8 W | 66 | September 2014b | |
| Alkermes (USA and Ireland) | FORWARD-2 | Safety | 56 W | 1500 | December 2017b | |
| Brexpiprazole | ||||||
| III | Lundbeck (DK) | NCT01838681 | Remission | 36 W | 2193 | June 2016b |
| Cariprazine | ||||||
| III | Forest (USA) | NCT01715805 | MADRSe | 8 W | 1100 | Negative |
| II | Forest (USA) | Durgam 2016 [76] | MADRSe | 8 W | 819 | −2.2f |
Enrolment (final or estimated).
Primary completion date (past or estimated).
Centre for Addiction and Mental Health (Toronto, Ontario, Canada).
Washington University School of Medicine (USA).
Change from baseline in Montgomery–Asberg Depression Rating Scale (MADRS).
Statistically significant.
NS: non-statistically significant; W: weeks.
Two phase I/II RCT (IRLGREY-B and IRL Grey B, or NCT02263248 and NCT02181231) have been designed to investigate the feasibility, safety, and tolerability of adjunctive buprenorphine (0.2–2 mg/d) in patients (≥50 years) in whom venlafaxine XR did not relieve the depressive symptoms (‘difficult to treat depression,’ Table 4). NCT02263248 is currently recruiting participants, and NCT02181231 is not yet open for participant recruitment.
3.3.3. Phase III clinical trials
BUP-TRD (NCT01407575) was a phase III RCT comparing the safety and efficacy of buprenorphine with placebo for adults with TRD (Table 4). The trial was completed in January 2014, with only 13 included patients. Blood pressure and heart rate were higher in patients treated on buprenorphine compared with those on placebo.
3.4. ALKS 5461
3.4.1. Background
ALKS 5461 (Alkermes, USA and Ireland) is a combination drug formulation of buprenorphine and the μ-opioid antagonist samidorphan [71,72]. Samidorphan was added to counteract the μ-opioid agonist activity of buprenorphine and reduce its addictive potential [71]. Following positive phase II studies [72], ALKS-5461 entered phase III development (Table 4).
3.4.2. Phase III clinical trials
ALKS 5461 failed to meet prespecified primary efficacy end point (change from baseline on the MADRS) in two of three core phase III trials (FORWARD-3, NCT02158546 and FORWARD-4, NCT02158533) [109] (Table 4). The third core phase III trial (FORWARD-5, NCT02218008) is currently ongoing, with completion date expected in October 2016. Two other phase III clinical trials (FORWARD-1, NCT02085135 and NCT02141399) are investigating safety and tolerability of ALKS 5461.
3.5. Brexpiprazole
3.5.1. Background
Brexpiprazole (Rexulti®, Otsuka, Japan) is an atypical antipsychotic, acting as a partial agonist at dopamine D2 receptors and as an antagonist at 5-HT2A receptors [73,74]. On July 2015, the FDA approved brexpiprazole as an add-on treatment to antidepressant medications for adults with MDD [73], based on a clinical development program that included two acute phase pivotal Phase III placebo-controlled trials [110,111].
3.5.2. Phase III/IV clinical trials in TRD
NCT01838681 is a phase III/IV trial designed to investigate the maintenance of efficacy and safety during long-term treatment with adjunctive brexpiprazole in a large number of adult subjects with MDD and an inadequate response to antidepressant treatment (insufficient response to at least one and no more than three adequate antidepressant treatments) (Table 4). The study was recently completed, but results were not posted to ClinicalTrials.gov.
3.5.3. Other development studies
Additional phase III studies with brexpiprazole for agitation in dementia of the Alzheimer type are underway (NCT01862640 and NCT01922258) [32].
3.6. Cariprazine
3.6.1. Background
Cariprazine is a dopamine D2 and D3 partial agonist, with preferential affinity for dopamine D3 receptors [31,75,76]. Animal studies revealed anti-anhedonic-like effects with cariprazine, suggesting potential utility to treat MDD [75].
3.6.2. Phase II clinical trials in TRD
One phase II RCT [76] evaluated the efficacy and safety of cariprazine as adjunctive therapy in patients with MDD who have inadequate response to standard antidepressant therapy (NCT01469377). Compared with placebo, reduction in MADRS total score at week 8 was significantly greater with adjunctive cariprazine 2–4.5 mg/d [76]. Cariprazine was generally well tolerated [76].
3.6.3. Phase III clinical trials in TRD
One phase III RCT evaluated the efficacy and safety of flexible doses of cariprazine as adjunctive therapy in patients with TRD (NCT01715805). Allergan and Gedeon Richter recently communicated that cariprazine did not have significant efficacy compared with placebo [77].
3.6.4. Regulatory aspects
In 2015, the FDA approved cariprazine to treat schizophrenia and manic or mixed episodes associated with bipolar I disorder [112].
4. Compounds in recent phase II clinical trials
The clinical trial registry of the NIH (www.clinicaltrials.gov; accessed from 22 March 2016 to 15 June 2016) included 41 recent phase II clinical trials on TRD (trials last updated in 1 January 2014 or later). Three additional phase II trials were found in the clinical trial registry of the EMA (www.clinicaltrials register.eu; accessed from 23 March 2016 to 15 June 2016). Seventeen clinical trials were excluded from the present analysis for the following reasons: primary completion date before 2014 (SAMe), terminated trials (CERC-501, EVT 101), unknown recruitment status (riluzole), discontinued development (BMS 820836), compound in phase III clinical development (ketamine, esketamine, buprenorphine), no investigational drug (trans-cranial magnetic stimulation, pulsating electromagnetic fields, psychotherapy), other condition (obsessive-compulsive disorder, pediatric bipolar disorder, postsurgical abdominal pain, chronic hepatitis C, alcoholism).
A total of 26 phase II clinical trials fulfilled the inclusion criteria and were included in the analysis. 13 of these trials concerned compounds in phase III development, and were described in Section 3. The remaining 13 trials concerned seven glutamatergic agents (AVP-923 [78–80], AVP-786 [81], CERC-301 [48,82–84], AV-101 [85,86], AZD6765 [87–89], rapastinel [90,91] and RO4995819 [92–94]), two other psychotropic drugs (ziprasidone [95–98] and psilocybin [99,100]) and three somatic drugs (minocycline [101–104], tocilizumab [105], and sirukumab [106]) (Table 1 and Figure 1). Some new glutamatergic agents were included in the analysis although they did not fully satisfy the inclusion criteria, i.e.: ‘condition’ was MDD and not TRD (rapastinel, AV-101, and CERC-301) and RO4995819 development was terminated.
4.1. Glutamatergic agents
4.1.1. Avp-923
4.1.1.1. Background
AVP-923 (Nuedexta®, Avanir, USA) is the first treatment for pseudo-bulbar affect (PBA) which was approved by the FDA [78] and the EMA [79]. PBA is characterized by uncontrollable episodes of crying and/or laughing/ crying that occurs in patients with types of CNS lesions (e.g. multiple sclerosis, amyotrophic lateral sclerosis).
AVP-923 is a fixed combination of dextromethorphan and quinidine [78,79]. Dextromethorphan is a noncompetitive NMDA receptor antagonist and σ1-receptor agonist, commonly used antitussive compound [113,114] (for σ1-receptors in depression, see references [115,116]). Quinidine is a CYP2D6 enzyme inhibitor, which serves to increase plasma concentrations of dextromethorphan and prolong its plasma half-life [78,79].
4.1.1.2
Recent phase II clinical trials. NCT01882829 was a small one-arm phase II study designed to evaluate the efficacy, safety, and tolerability of AVP-923 in patients with TRD (Table 5). The primary outcome measure was the change in MADRS total score from baseline to week 10. The study was recently completed, but results are not yet posted on ClinicalTrials.com.
Table 5.
Investigational drugs in phase II trials for treatment-resistant depression (2014 and later).
| Compound | Sponsor | Identifier | Primary outcome | Time frame | N a | Results or status |
|---|---|---|---|---|---|---|
| Glutamatergic agents | ||||||
| AVP-923 | James Murrough (NY, USA) | NCT01882829 | MADRSe | 10 W | 20 | March 2016b |
| AVP-786 | Avanir (USA) | NCT02153502 | MADRSe | 10 W | 200 | March 2016b |
| CERC-301 | Cerecor (USA) | Reference [84] | HDRS-17d | 5 W | 137 | NS |
| Cerecor (USA) | NCT02459236 | Bech-6f | 4 D | 104 | September 2016b | |
| AV-101 | National Institute of Mental Health (USA) | NCT02484456 | HDRSd | Multiple | 25 | December 2019b |
| AZD6765 | Astra-Zeneca (UK) | Reference [89] | MADRSe | Multiple | 22 | Small, transient effectj |
| Rapastinel | Allergan (USA) | NCT01684163 | HDRSd | 16 W | 369 | April 2014b |
| RO4995819 | Hoffmann-La Roche (Switzerland) | 2011–002160-24g | MADRSe | 6 W | 340 | Terminated |
| Other psychotropic drugs | ||||||
| Ziprasidone | Massachusetts General Hospital (USA) | Reference [96] | HDRSd | 8 W | 139 | ZIP = 35% PL = 20%c,i |
| Psilocybin | Imperial College London (UK) | Reference [100] | Psychedelic | PDEh | 12 | Ongoing |
| Somatic drugs | ||||||
| Minocycline | Charite University (Germany) | NCT02456948 | MADRSe | 6 W | 160 | February 2019b |
| Tocilizumab | Brigham and Women’s Hospital (USA) | NCT02660528 | HDRSd | 8 W | 15 | June 2017b |
| Sirukumab | Janssen-Cilag International (Poland) | 2014–005206-3g | HDRSd | 12 W | 192 | Ongoing |
Enrolment (final or estimated).
Primary completion date (past or estimated).
Statistically significant.
Hamilton Depression Rating Scale.
Montgomery–Asberg Depression Rating Scale.
6-item unidimensional subset of the HDRS-17.
Eudra identifier.
Peak drug effects.
Percent of responders (see text).
See text.
D: days; NS: non-statistically significant; PL: placebo; W: weeks; ZIP: Ziprasidone.
4.1.1.3
Other development studies. A recent phase II RCT (NCT01584440) showed that AVP-923 had statistical and clinical relevant efficacy for treating agitation in patients with Alzheimer disease (AD) and was generally well tolerated [32,80].
4.1.2. AVP-786 (deuterated-dextromethorphan/quinidine combination)
4.1.2.1. Background
AVP-786 (Avanir, USA) is a new investigational compound consisting of a combination of deuterated (d6)-dextromethorphan and an ultra-low dose of quinidine [81]. The incorporation of deuterium serves to reduce dextromethorphan first-pass liver metabolism and quinidine dosage, potentially reducing the risk of drug interactions and cardiac effects [81].
4.1.2.2. Recent phase II clinical trials
NCT02153502 is a 10-week phase II RCT designed to evaluate the efficacy, safety, and tolerability of AVP 786 as an adjunctive therapy in patients with MDD and inadequate response to antidepressant treatment (less than 50% symptom reduction to at least 1 but no more than 3 adequate antidepressant trials during the current depressive episode) (Table 5). This study is currently recruiting participants.
4.1.2.3. Other development studies
Avanir Pharmaceuticals recently launched a phase III development program of AVP-786 for the treatment of agitation in patients with AD [32,81].
4.1.3. Cerc-301
4.1.3.1. Background
CERC-301 (MK-0657, Cerecor, USA) is a highly selective, orally bioavailable, NMDA receptor antagonist acting at the NMDA receptor 2B (NR2B) subunit [82–84]. CERC-301 showed acute antidepressant-like effects in the forced swim test (FST), a rat model of antidepressant efficacy [82]. A small RCT (N = 5) conducted by Ibrahim et al. [83] suggested antidepressant efficacy for CERC-301 in TRD
4.1.3.2 Recent phase II clinical trials
Paterson et al. [84] conducted a phase II RCT of adjunctive CERC-301 in subjects with severe depression and recent active suicidal ideation despite antidepressant treatment (NCT01941043, Table 5). CERC-301 was administered daily at a dose of 8 mg for 28 days and did not meet its primary end point (change in HDRS-17 at Day 7) [84].
NCT02459236 is another phase II RCT designed to evaluate the antidepressant effect of intermittent doses CERC-301 in TRD (Table 5). This study is currently recruiting participants.
4.1.3.3 Regulatory aspects
In November 2013, CERC-301 received Fast Track Designation by the FDA for the treatment of MDD [117].
4.1.4. AV-101
4.1.4.1. Background
AV-101 (4-chlorokynurenine, 4-Cl-KYN, VistaGen, USA) is a prodrug of 7-chloro-kynurenic acid (7-Cl-KYNA), a potent and highly selective antagonist of NMDAR at the glycine-B co-agonist site [85]. In animal tests of ketamine-like antidepressant actions, 4-chlorokynurenine exhibited rapid, dose-dependent and persistent antidepressant effects following a single treatment [86]. 4-Cl-KYN administration was not associated with the negative side effects of ketamine (rewarding and psychotomimetic effects), and did not induce locomotor sensitization or stereotypic behaviors [86].
4.1.4.2. Recent phase II clinical trials
NCT02484456 is a phase II RCT investigating antidepressant efficacy of AV-101 in subjects with a current or past history of lack of response to one adequate antidepressant trial (Table 5). This study is currently recruiting participants.
4.1.5. AZD6765
4.1.5.1. Background
AZD6765 (AR-R15896AR, Lanicemine, AstraZeneca, UK) is a low-trapping NMDA channel blocker [87] (trapping channel blockade means that the blocking molecule is trapped inside the channel pore [118]). In preliminary RCTs, single or multiple infusions of AZD6765 exhibited antidepressant efficacy without psychotomimetic or dissociative side effects [88].
4.1.5.2 Recent phase II clinical trials
Zarate et al. [89] conducted a phase II RCT (NCT00986479) to evaluate whether a single infusion of AZD6765 could produce rapid antidepressant effects in subjects with TRD (Table 5). The primary outcome measure was the MADRS, which was used to rate overall depressive symptoms at baseline and 60, 80, 110, and 230 min post-infusion and on days 1, 2, 3, and 7 post-infusion. AZD6765 infusion was associated with a rapid, but short-lived improvement of antidepressant efficacy compared with placebo [89]. The drug × time interaction was not significant (p = 0.32) [89]. No dissociative side effects were noted.
4.1.5.3. Other development studies
Following the results of study NCT00986479, AstraZeneca discontinued the development of AZD6765 in MDD [119].
4.1.6. Rapastinel
4.1.6.1. Background
Rapastinel (GLYX-13, Allergan) is a NMDAR modulator, acting as functional glycine site weak partial agonist [48,90]. Preskorn et al. [91] conducted a pilot phase II RCT (NCT01234558) showing that a single intravenous dose of rapastinel reduced depressive symptoms within 2 h, and this effect was maintained for around 7 days (NCT02192099 is an open-label extension trial).
4.1.6.2. Recent phase II clinical trials
NCT01684163 is a phase II RCT trial investigating the efficacy and safety of rapastinel in subjects with inadequate or partial responses to antidepressants. The study was completed in 2014, but results have not yet been posted to ClinicalTrials.gov.
4.1.6.3. Regulatory aspects
In January 2016, Allergan announced that rapastinel received Breakthrough Therapy Designation from the FDA for adjunctive treatment of MDD [120].
4.1.6.4. Other development studies
Allergan is also developing NRX-1074, a novel antidepressant, with mechanism of action and effects similar to those of rapastinel, but with the advantage of being orally active [121].
4.1.7. RO4995819
4.1.7.1. Background
RO4995819 (RG1578, decoglurant, Roche) is a negative allosteric modulator of mGlu2 and mGlu3 receptors [92].
4.1.7.2. Recent phase II clinical trials
Eudra 2011–002160-24 was a phase II RCT evaluating the antidepressant efficacy of adjunctive RO4995819 in patients with MDD who exhibited inadequate responses to at least one antidepressant treatment (Table 5). The trial was completed in June 2014. Data obtained showed a lack of efficacy [94].
4.1.7.3. Further development studies
Roche terminated the development of RO4995819 in TRD due to a lack of efficacy [93,94].
4.2. Other psychotropic drugs
4.2.1. Ziprasidone
4.2.1.1. Background
Ziprasidone (Geodon®, Pfizer, USA) is an antipsychotic approved by the FDA for the treatment of schizophrenia and bipolar disorder (acute mania and mixed states) [122]. Its intramuscular injection form was approved for acute agitation in schizophrenia patients. Ziprasidone is now available as a generic medication.
Atypical antipsychotics represent one possible adjunctive therapy for the management of TRD [123]. In particular, ziprasidone has been found to inhibit the neuronal uptake of serotonin and norepinephrine in vitro, with potency comparable to that of the antidepressant imipramine [97]. Papakostas et al. [98] conducted a pilot, open-label trial showing that 10 of 20 patients with TRD (TRD criteria: patients nonresponders to an SSRI) had a positive antidepressant response after 6 weeks of ziprasidone augmentation.
4.2.1.2. Recent phase II clinical trials
Papakostas et al. ([96], NCT00633399, Table 5) recently reported a phase II RCT testing the efficacy of adjunctive ziprasidone in 139 adults with unipolar TRD (patients nonresponders to 8 weeks of open-label escitalopram). Rates of clinical response (≥50% reduction in HDRS score) were significantly greater for the escitalopram + ziprasidone group compared to placebo (Table 5). Adjunctive ziprasidone also exhibited anxiolytic efficacy (secondary outcome measure) [96]. Finally, a greater proportion of patients discontinued adjunctive ziprasidone than placebo (14% vs. 0%) due to intolerance (principally due to sedation or ‘activation’-type adverse events: anxiety, agitation, insomnia) [96].
4.2.2. Psilocybin
4.2.2.1. Background
Psilocybin is a mushroom alkaloid, which is rapidly converted by the body to psilocin, a partial agonist for several brain serotonergic receptors, particularly 5-HT2AR [99].
4.2.2.2. Recent phase II clinical trials
Carhart-Harris et al. [100] conducted an open-label trial of adjunctive psilocybin in 12 patients with MDD who had failed to respond to at least two courses of antidepressant treatment of different class (Eudra 2013–003196-35; Table 5). There was no control group and patients received psychological support. The acute psychedelic effects peaked 2–3 h after dosing and declined to negligible levels at least 6 h after dosing. Depressive symptoms were markedly reduced 1 week and 3 months after high-dose treatment [100].
4.3. Somatic drugs
4.3.1. Minocycline
4.3.1.1. Background
Minocycline is a broad-spectrum tetracycline antibiotic (generic medication) used for the treatment of acne vulgaris and other skin infections [101]. A large number of studies have reported pleiotropic effects of minocycline in animal models and humans, including anti-inflammatory and neuroprotective actions (for references, see [102]). A small, open-label study suggested that adjunctive minocycline can be effective and well tolerated in TRD [103].
4.3.1.2. Recent phase II clinical trials
NCT02456948 is an RCT evaluating the antidepressant efficacy of adjunctive minocycline in patients resistant to standard antidepressant treatment (AD-ST) with escitalopram, citalopram, venlafaxine, or mirtazapine monotherapy. Treatment resistance to AD-ST will be assessed with the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (MGH-ATRQ) [124]. Patients will have suffered from TRD for at least 6 weeks in the current episode and will have been at a stable regimen for at least 14 days prior to baseline. The dose and duration of AD-ST must be verifiable (Table 5). This study is currently recruiting participants.
4.3.1.3. Other development studies
An RCT (incorrectly designated as a phase IV study) is currently recruiting participants to evaluate the antidepressant efficacy of minocycline in patients with TRD (NCT02263872 [102]; see also [104]).
4.3.2. Tocilizumab
4.3.2.1. Background
Tocilizumab (Actemra®, Roche, Switzerland) is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6), used as immunosuppressive agent for the treatment of rheumatoid arthritis [105]. It has previously been reported that IL-6 and/or inflammatory mediators may play a role in the development of depression [125–127], and a recent meta-analysis [128] showed that anti-inflammatory treatment reduced depressive symptoms.
4.3.2.2. Recent phase II clinical trials
NCT02660528 is a phase II single-arm, open-label trial designed to evaluate the antidepressant effects of tocilizumab among patients with treatment-refractory major depression (TRD criteria: MDD after treatment for depression for a minimum of 8 weeks) (Table 5).
4.3.3. Sirukumab
4.3.3.1. Background
Sirukumab (Janssen Biotech, USA) is an anti-IL-6 human monoclonal antibody in development for the treatment of rheumatoid arthritis [106].
4.3.3.2. Phase II clinical trials
Eudra 2014–005206-37 is an RCT evaluating the antidepressant efficacy of subcutaneous sirukumab in patients with MDD nonresponders to monoaminergic antidepressants (<25% improvement in HDRS total score) from the screening to baseline visits. This study is ongoing in Poland (other trial sites are planned in Canada, Russia, UK, and the USA).
5. Conclusions
A substantial amount of effort is being expended in the area of TRD-targeted therapies, with 6 compounds in phase III clinical development and 12 other compounds in phase II clinical trials. A single intravenous dose of the glutamatergic modulator ketamine produces a robust and rapid antidepressant effect in persons with TRD; this effect continues to remain significant for 1 week. This observation was a turning point that opened the way for the development and testing of other, more selective NMDAR antagonists for the treatment of TRD. Of these, intranasal esketamine recently entered phase III development and others are in phase II clinical trials (AVP-786, AVP-923, AV-101, and rapastinel). In contrast, disappointing results have been obtained with the NMDA antagonists CERC-301 and AZD6765 (low-trapping), as well as with the mGlu2/3R antagonist RO4995819. These negative results can be explained by animal studies suggesting that the antide-pressant action of ketamine is independent of NMDAR inhibition but rather involve AMPAR activation via a ketamine metabolite.
Of the remaining compounds, the atypical antipsychotic ziprasidone demonstrated efficacy in a phase II trial with an adequate number of participants. Positive results in small pilot studies with the mushroom alkaloid psilocybin and the opioid buprenorphine deserve further investigation with larger sample sizes. Finally, monoclonal antibodies open the possibility of highly innovative therapeutic options, based on new pathophysiologic approaches to managing depression.
6. Expert commentary
6.1. Key findings and weakness of previous research with antidepressants
6.1.1. Currently available antidepressants
Monoaminergic drugs are the cornerstone of current MDD treatment [129,130]. Thus, all available antidepressants increase levels of serotonin, norepinephrine, and/or dopamine through different pharmacologic mechanisms [129]. First-generation antidepressants include monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs). MAOIs act by inhibiting monoamine oxidase activity, thus preventing the breakdown of monoamine neurotransmitters. However, because of their potentially lethal dietary and drug interaction potential, MAOIs are usually reserved as a last line of treatment [131]. Most TCAs act primarily by blocking serotonin and/or norepinephrine reuptake, thus increasing the synaptic concentrations of these neurotransmitters. However, many TCAs also have high affinity for several other brain receptors, including adrenergic, histaminergic, cholinergic, NMDA, and opioid receptors [132], some of which are responsible for their more pronounced adverse effects.
Due to frequent undesirable side effects and toxic effects in overdose, TCAs have been replaced by second-generation antidepressants, including SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs); however, the newer antidepressants themselves are not devoid of adverse effects either [133]. Moreover, currently available antidepressants often take weeks to months to exert their full therapeutic effects, commonly resulting in considerable morbidity and increased risk for suicidal behavior [134–136].
6.1.2. Current TRD treatment
According to the American Psychiatric Association (APA) guidelines [130], the prescriber should consider a change in treatment for patients who have not fully responded to an adequate acute-phase treatment for a sufficient time, generally 4–8 weeks. The treatment plan can be revised by implementing one of several therapeutic options, including optimizing the initial treatment, adding a drug that is not traditionally used as an antidepressant (augmentation), changing to a different treatment (switch), or combining two or more antidepressants [1,7,130,137–139].
Generally speaking, optimization consists of increasing anti-depressant doses, as tolerated, at least to standard maximal doses for 6–12 weeks [7]. The addition of a non-antidepressant medication (augmentation) is a logical next strategy when a patient experiences at least a partial response to the initial agent [7]. First-line adjunctive medications are atypical anti-psychotics [1,7], lithium [19], and thyroid hormone [20]. Switching can be another option following optimization failure, in cases of poor tolerability of an initial antidepressant or in case of complete nonresponse [7]. The combination of two or more antidepressant treatments, typically with antidepressants from two different mechanistic classes (especially mirtazapine and bupropion), allows creating a broader-spectrum antidepressant regimen. Finally, neurostimulation and neuromodulation strategies are another option for subjects responding insufficiently to pharmacotherapy or psychosocial interventions [1,23–26].
Well-controlled recurrence prevention trials comparing the above strategies are largely unavailable [1]. Moreover, adding second-line agents to treatment regimens of nonresponders can create an additional burden on both patients and healthcare providers. In particular, antipsychotic use is associated with significant side effects such as weight gain, akathisia, and sedation [18].
In spite of the above strategies, many patients with MDD remain nonresponders [7]. These patients are at increased risk of severe cognitive impairment, low functioning, comorbid illnesses, suicide, and financial burden to the health system [4].
6.2. Recent clinical research with antidepressants
6.2.1. Ketamine for TRD treatment
A new era for antidepressant research has been recently opened up by the demonstration by Zarate et al. [39] of the robust and rapid antidepressant effect of a single intravenous administration of low-dose ketamine in persons with TRD. The exciting prospect of a rapid-acting antidepressant raised much interest, as shown by the numerous contributions to the medical literature and scientific meetings [35–38,41,42,46,48–52,54–57]. The antidepressant efficacy of single-dose ketamine was confirmed in a sufficient number of RCTs. The antidepressant action lasted for at least 3 days, in clear excess of what would be predicted by ketamine’s short-elimination half-life (2–3 h). Side effects, including increases in blood pressure and heart rate, confusion and dissociation were generally mild and transient.
One special issue was maintaining the antidepressant efficacy of ketamine over the longer term [35,36,38,41,51,53,54,140]. Repeated dosing with intravenous ketamine given over ≤2 weeks was associated with greater reductions in depressive symptoms and longer duration of the antidepressive action as compared to a single ketamine infusion [36,38,46,140]. Moreover, rapid and sustained reductions in suicidal ideation were observed following repeated doses of intravenous ketamine [51]. However, a number of major unresolved issues remain. First, there is a lack of safety data regarding the longterm ketamine dosing [41,46,53]. Indeed, the long-term antide-pressant and remission potential of ketamine is challenged by its addictive properties, the dissociative psychotomimetic side effects, and the elevation of blood pressure [41,46,52]. Second, it is also unclear whether the usual dose of 0.5 mg/kg is the optimal dose level [41]. Finally, there have been few investigations concerning the antidepressant response to ketamine when given via other administration routes [41]. There is evidence for efficacy of oral ketamine from one small open-label study [54] and one additional, small RCT [55]. Early controlled studies of intranasal or serial infusion ketamine therapy appear promising [46]. A few other studies have investigated the efficacy of ketamine by intramuscular or subcutaneous injection [41].
A large body of evidence indicates that glutamate home-ostasis and neurotransmission are disrupted in MDD [35,56,141–143]. The mechanism of action of ketamine was therefore expected to involve glutamate receptor function [37,49,56,141–143]. This hypothesis was supported by positron emission tomography/magnetic resonance imaging studies showing that the rapid antidepressant action of ketamine facilitates glutamatergic neurotransmission through blocking the NMDA receptors resulting in increased glutamate in the prefrontal cortex (PFC) [50,58].
Stress and depression are associated with neuronal atrophy and a loss of synaptic connections, particularly in the PFC and hippocampus (for review, see ref [143].). Animal studies showed that the rapid antidepressant action of ketamine is associated with synapse formation in the medial PFC, an effect likely due to an increase in brain-derived neurotrophic factor (BDNF) [143,144].
The perspective of ketamine as a novel treatment for the rapid reduction of symptoms in depressed patients has raised considerable interest among clinicians, patients, and the pharmaceutical industry, leading to the increasing off-label use of ketamine in TRD [41,46,53]. However, a controversy was raised based on the lack of well-controlled, long-term trials and the risk of potential harm [41,53]. Psychiatrists are raising ethical concerns in the UK [53]. Similarly, a Canadian agency did not recommend prescribing ketamine to treat depressive disorders [53], and recent actions have been taken by health authorities in Australia to curtail off-label use of ketamine in TRD [41]. In the American Journal of Psychiatry, its Editor-in-Chief, Robert Freedman, opined that, in addition to concerns over ketamine’s stimulant and opioid properties that make it susceptible to overuse, there is a need to design studies that will adequately assess clinical utility in the long term, including comparisons with currently available augmentation strategies [145]. Moreover, Freedman [145] recommended that more thought be given regarding the definition of TRD that should be used in ketamine trials; given the availability of safer treatments, too liberal a threshold for TRD can be problematic here.
6.2.2. Intranasal esketamine
Aside from ketamine, the most advanced NMDAR antagonist for TRD is esketamine (S-ketamine) [61,64]. Janssen R&D (USA) is studying intranasal esketamine as a therapy for TRD and also as a medication for patients in whom suicide is an imminent risk. Following positive phase II RCTs in TRD [61], Janssen launched a phase III development program for adjunctive intranasal esketamine in TRD (Table 3).
A recent study in mice showed that the antidepressant action of ketamine is independent of NMDAR inhibition, but involves early and sustained AMPARs activation via an R-ketamine metabolite [57]. Care should be taken to extrapolate these results to humans, because R-ketamine exhibited greater potency and longer-lasting antidepressant effects in comparison to S-ketamine in mice [62], which is different from humans. Therefore, the antidepressant role of ketamine enantiomers and metabolites in humans clearly deserves further investigation.
6.2.3. Other NMDAR antagonists
Ketamine is limited as a treatment for TRD by its dissociative and psychotomimetic effects and by the need for intravenous administration. There is an urgent need, therefore, to identify well-tolerated, orally available compounds that target the glutamatergic system/NMDA receptors as a novel treatment approach to TRD.
New NMDAR antagonists (modulators) are therefore continuously being introduced for use in TRD [13,48,49,82–84,88–91]. These show relatively modest antidepressant effects compared to ketamine, but some are orally bioavailable and/or have shown more favorable tolerability profiles (reduced dissociative and/or psychotomimetic effects) [13].
The orally bioavailable, highly selective NMDAR antagonist CERC-301 (acting at the NR2B subunit) is one compound that did not meet its primary end point in a phase II RCT [84]. According to Paterson et al. [84], the lack of efficacy may reflect suboptimal dosing (the low tested dose, daily rather than intermittent dosing) or the patient population selected for the study (depressed patients with recent suicidal ideation). Subsequently, another phase II RCT was launched to evaluate the antidepressant effect of intermittent doses CERC-301 (Table 5). However, another possibility is that the antidepressant action of ketamine itself may include actions at other receptors, which may explain why ketamine is efficacious, but why other related compounds are not. For instance, ketamine inhibits the reuptake of serotonin, dopamine, and norepinephrine [44]. Similar considerations could explain the negative results obtained with the low-trapping NMADR antagonist AZD6765 [89] and with the mGlu2/3R antagonist RO4995819 [93,94].
Remaining NMDAR antagonists currently tested in phase II RCTs include the orally available AVP-923 [78,79] and AVP 786 [81], AV-101 (a potent and highly selective antagonist of the NMDAR at the glycine-B co-agonist site) [85,86] and rapastinel, a weak partial allosteric agonist at the glycine site of the NMDA receptor complex [90].
6.2.4. Non-NMDA antagonists
6.2.4.1. Opioid receptor modulators
Researchers had a strong preclinical rationale for investigating buprenorphine and the buprenorphine-containing agent ALKS 5461 in the context of TRD [69–71], as well as positive results in proof-of-concept phase II studies [67,72], which largely justified phase III development. However, negative results were found in two out of three phase III RCTs with ALKS 5461 [109]. Results from a third phase III study with ALKS 5461, as well as from two phase II studies with buprenorphine (NCT02263248 and NCT NCT02181231), may help provide a more definitive answer. Finally, on a cautionary note, a recent study by Ray et al. [146] demonstrated that the prescription of long-acting opioids was associated with a significantly increased risk of all-cause mortality (including deaths from causes other than overdose).
6.2.4.2. Atypical antipsychotics
A recent phase II RCT reported that adjunctive ziprasidone reduces depression and anxiety in TRD [96]. Ziprasidone was approved by the FDA for the treatment of schizophrenia and bipolar disorder [122], but it is used off-label for depression. Interest in its use for TRD stems from its favorable side effect profile, consisting of less weight gain and metabolic adverse effects compared to other antipsychotics. However, its association with prolongation of the ECG QTc interval makes it a poor choice to combine with some antidepressants, such as citalopram that also prolong QTc [147,148].
Brexpiprazole is an atypical antipsychotic with a favorable side effect profile [17]. In contrast to ziprasidone, brexpiprazole has already been approved by the FDA as an adjunctive antidepressant treatment in MDD [73], and serves as an alternative to adjunctive aripiprazole, adjunctive quetiapine extended-release, or olanzapine-fluoxetine combination.
Cariprazine failed to demonstrate efficacy in a phase III RCT in patients with TRD [77]. However, Allergan plan to move forward with another phase III study of cariprazine in adjunctive MDD [77].
Similarly to ketamine, one important issue is maintaining the antidepressant efficacy of atypical antipsychotics over the longer term. This problem should be evaluated in maintenance treatment of patients with TRD.
6.2.4.3. Monoclonal antibodies
Two anti-IL6 monoclonal antibodies (tocilizumab and sirukumab) are in phase II clinical trials for TRD. However, the results of human studies concerning IL-6 are controversial, given the uncertain relationship between IL-6 and depression. In 2013, a meta-analysis of three studies found that raised plasma levels of IL-6 had a small and statistically borderline association with the subsequent development of depressive symptoms [125]. In another study, high IL-6 blood levels in childhood were found to be associated with an increased risk of developing depression in young adulthood [126], but not in a prospective analysis of 4,756 women (who were depression free up to 1996) over a follow-up of 6–18 years [95]. Finally, it is unclear whether high circulating IL-6 is causally associated with MDD (see for instance the unclear relation between elevated IL-6 and cancer [149]).
6.2.4.4. Psilocybin
The positive results of small pilot studies of the mushroom alkaloid psilocybin in TRD [100] deserve further investigation in larger samples.
7. Five-year view
NMDAR antagonists likely represent a very promising alternative treatment for TRD. A large amount of data is being generated through clinical trials of ketamine, esketamine and an increasing number of new non-ketamine NMDAR antagonist compounds and compounds with novel mechanisms not restricted to monoamine transmitter modulation.
Advances in the mechanism of action of investigational antidepressants, as well as the neurobiology of depressive subtypes, are important steps for the development of targeted therapies [7]. Aside from monoamines, glutamate and inflammatory targets, BDNF is a promising target for the development of new antidepressants, as suggested by numerous studies reporting reduced levels of neurotrophic factors in depression [28,143].
7.1. Methodological aspects
There are no universally accepted criteria for defining TRD and diagnostic tools are sometime lacking [3]. This is mirrored by the clinical trials reviewed here, which used a variety of clinical criteria to identify and include TRD patients, but only some of them verified that the TRD diagnosis was accurate for all enrolled subjects using a reliable and validated questionnaire/assessment. Notably, clinical researchers used many tools for identifying TRD, such as the Antidepressant Treatment History Form [150] and the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (ATRQ) [124] (see also reference [138]). A standardized approach would be preferable in order to be able to appropriately compare studies.
Specific tools for identifying TRD can be helpful in clinical practice. Indeed, accurately identifying TRD is a prerequisite for optimally changing treatment regimens to help patients achieve remission [139]. Evidence indicates that large numbers of depressed patients are undertreated, resulting in prolonged episodes and the appearance of ‘pseudo-resistance’ [2,150]. Therefore, the lack of response to previous antidepressant treatment should be assessed using specific instruments to precisely define the level of therapeutic resistance [2,150].
The development of biomarkers that could identify nonresponsive patients or predict antidepressant response may be one strategy to enhance TRD detection or signal detection of treatment efficacy in clinical trials. The CAN-BIND research program investigates and identifies neuroimaging, electrophysiological, molecular and clinical biomarkers to predict outcomes in patients with MDD treated with antidepressant medication [151]. The stratification of patients on the basis of genetic biomarkers may be facilitated by further developments in genetic testing.
The combination of pharmacological treatments with psychosocial/behavioral approaches to TRD has been insufficiently studied and deserves further investigation. Finally, clinical trials of long-term recurrence prevention in TRD are needed.
7.2. Social disability and functional recovery
In the context of clinical trials on TRD, symptom improvement remains the focus for the regulatory approval of new medications. However, patients with TRD frequently present with impaired social functioning because of sustained depressive symptoms [152] and functional outcomes do not always correspond to symptom-based outcomes [153]. Moreover, functional recovery is increasingly recognized as a priority in the treatment of MDD [153]. Therefore, functional outcomes are meaningful evaluation criteria to be included in future TRD trials.
In conclusion, we could see the arrival of the first robust and rapid antidepressant drug in the near future, which would strongly facilitate the ultimate goal of recovery in persons with TRD.
Key issues.
Treatment-resistant depression (TRD) represents at least 15% to 30% of major depressive disorder (MDD) cases and may cause severe impairment in cognitive functioning, increased risk of developing comorbid illnesses, decreased workplace performance, increased risk of suicide and personal suffering, and increased health care costs.
We evaluated how the pharmacotherapy of TRD is likely to evolve in the near future. For that purpose, we catalogued and appraised medications currently in phase II/III of clinical development, using the clinical trial registries of the NIH (National Institutes of Health, USA) and the EMA (European Medicines Agency).
Eighteen investigational compounds were identified. Among them, the NMDA receptor (NMDAR) antagonist ketamine produces a robust and rapid antidepressant effect in persons with TRD. This observation was a turning point that opened the way for other, more selective NMDAR antagonists, which are expected to be better tolerated and/or easier to administer. Of these, intranasal esketamine recently initiated phase III development, and AVP-786, AVP-923, AV-101, and rapastinel (an NMDAR modulator) are in phase II clinical trials.
Disappointing results have been obtained with the NMDA antagonists CERC-301 and AZD6765 (low-trapping), as well as with the mGlu2/3 R antagonist RO4995819. These negative results can be explained by animal studies showing that the antidepressant action of ketamine may well be independent of NMDAR inhibition, but rather involve AMPAR activation via a ketamine metabolite.
Of the remaining compounds, the atypical antipsychotic ziprasidone demonstrated efficacy in a phase II trial with an adequate number of participants.
Glutamatergic agents likely represent a very promising alternative to monoaminergic antidepressants. A large amount of data is being generated with ketamine, esketamine and an increasing number of new compounds. We could see the arrival of the first robust and rapidly-acting antidepressant drug in the near future, which would strongly facilitate the ultimate goal of recovery in persons with TRD.
Acknowledgments
The authors are grateful to Greg Panico (Janssen Research & Development, USA) for providing information about intranasal esketamine.
Funding
This paper was not funded.
Footnotes
Declaration of interest
R.P. Garay is President of a non-profit association for therapeutic innovation (Craven, Villemoisson-sur-Orge, France). C.A. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynor-ketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders. C.A. Zarate has assigned his patent rights to the U.S. government but will share a percent of royalties that may be received by the government. T. Charpeaud declares consultant activities and conferences for AstraZeneca, BMS, Janssen, Otsuka; invitation to Congress by Janssen, Lundbeck, Otsuka; and research activities for AMGEN, Janssen and Lilly. In the past 36 months L. Citrome has engaged in collaborative research with, or received consulting or speaking fees, from: Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, Vanda. C.U. Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Forum, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ProPhase, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck and Pfizer. He received grant support from Takeda. A Hameg is a member of a non-profit association for therapeutic innovation (Craven, Villemoissonsur-Orge, France). P.M. Llorca declares grant support in addition to support for consultancy, expertise and honoraria for conferences from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Janssen-Cilag, Lundbeck A/S, Otsuka, Roche, and Sanofi Aventis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
ORCID
Ricardo P. Garay http://orcid.org/0000-0001-6209-926X
Pierre-Michel Llorca http://orcid.org/0000-0001-7438-8990
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.McIntyre RS, Filteau M-J, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 2.Holtzmann J, Richieri R, Saba G, et al. How to define treatment-resistant depression? Presse Méd. 2016;45:323–328. doi: 10.1016/j.lpm.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26:222–232. [PubMed] [Google Scholar]
- 4.Gaynes B. Assessing the risk factors for difficult-to-treat depression and treatment-resistant depression. J Clin Psychiatry. 2016;77(Suppl 1):4–8. doi: 10.4088/JCP.14077su1c.01. [DOI] [PubMed] [Google Scholar]
- 5.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72:e18. doi: 10.4088/JCP.8133tx4c. [DOI] [PubMed] [Google Scholar]
- 7.Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17:111–126. doi: 10.31887/DCNS.2015.17.2/dionescu. • Treatment strategies in TRD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–481. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- 9.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coplan JD, Gopinath S, Abdallah CG, et al. A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014;8:189. doi: 10.3389/fnbeh.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knochel C, Alves G, Friedrichs B, et al. Treatment-resistant late-life depression: challenges and perspectives. Curr Neuropharmacol. 2015;13:577–591. doi: 10.2174/1570159X1305151013200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang UE, Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 13.Jaso BA, Niciu MJ, Iadarola ND, et al. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr Neuropharmacol. 2016;15:57–70. doi: 10.2174/1570159X14666160321123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, Na KS. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:117–126. doi: 10.1016/j.pnpbp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 15.FDA Food and Drug Administration. Drugs@FDA. Symbyax. Label information 2014 [cited 2016 Jun 8]. Available from: http://www. accessdata.fda.gov/drugsatfda_docs/label/2014/021520s034lbl.pdf]
- 16.Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122:39–48. doi: 10.3810/pgm.2010.07.2174. [DOI] [PubMed] [Google Scholar]
- 17.Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69:978–997. doi: 10.1111/ijcp.12714. [DOI] [PubMed] [Google Scholar]
- 18.Cha DS, McIntyre RS. Treatment-emergent adverse events associated with atypical antipsychotics. Expert Opin Pharmacother. 2012;13:1587–1598. doi: 10.1517/14656566.2012.656590. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Baumann P, Delucchi K, et al. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord. 2014;168:269–275. doi: 10.1016/j.jad.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 20.Cooper-Kazaz R, Lerer B. Efficacy and safety of triiodothyronine supplementation in patients with major depressive disorder treated with specific serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2008;11:685–699. doi: 10.1017/S1461145707008206. [DOI] [PubMed] [Google Scholar]
- 21.Appelberg BG, Syvalahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001;62:448–452. doi: 10.4088/jcp.v62n0608. [DOI] [PubMed] [Google Scholar]
- 22.Tallon D, Wiles N, Campbell J, et al. Mirtazapine added to selective serotonin reuptake inhibitors for treatment-resistant depression in primary care (MIR trial): study protocol for a randomised controlled trial. Trials. 2016;17:66. doi: 10.1186/s13063-016-1199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodkin JA, Lasser RA, Wines JD, Jr., et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antide-pressant monotherapy. J Clin Psychiatry. 1997;58:137–145. doi: 10.4088/jcp.v58n0401. [DOI] [PubMed] [Google Scholar]
- 24.Howland RH. Sequenced treatment alternatives to relieve depression (STAR*D). Part 2: study outcomes. J Psychosoc Nurs Ment Health Serv. 2008;46:21–24. doi: 10.3928/02793695-20081001-05. [DOI] [PubMed] [Google Scholar]
- 25.Lomas J, Llewellyn A, Soares M, et al. The clinical and cost effectiveness of vortioxetine for the treatment of a major depressive episode in patients with failed prior antidepressant therapy: a critique of the evidence. Pharmacoeconomics. 2016;34:901–912. doi: 10.1007/s40273-016-0417-9. [DOI] [PubMed] [Google Scholar]
- 26.Abel A, Hayes AM, Henley W, et al. Sudden gains in cognitive-behavior therapy for treatment-resistant depression: processes of change. J Consult Clin Psychol. 2016;84:726–737. doi: 10.1037/ccp0000101. [DOI] [PubMed] [Google Scholar]
- 27.Block SG, Nemeroff CB. Emerging antidepressants to treat major depressive disorder. Asian J Psychiatr. 2014;12:7–16. doi: 10.1016/j.ajp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Wijesinghe R. Emerging therapies for treatment resistant depression. Mental Health Clinician. 2014;4:226–230. [Google Scholar]
- 29.Health Quality O Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2016;16:1–66. [PMC free article] [PubMed] [Google Scholar]
- 30.Berry SM, Broglio K, Bunker M, et al. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl) 2013;6:17–35. doi: 10.2147/MDER.S41017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garay RP, Citrome L, Samalin L, et al. Therapeutic improvements expected in the near future for schizophrenia and schizoaffective disorder: an appraisal of phase III clinical trials of schizophrenia-targeted therapies as found in US and EU clinical trial registries. Expert Opin Pharmacother. 2016;17:921–936. doi: 10.1517/14656566.2016.1149164. [DOI] [PubMed] [Google Scholar]
- 32.Garay RP, Citrome L, Grossberg GT, et al. Investigational drugs for treating agitation in persons with dementia. Expert Opin Investig Drugs. 2016;25:973–983. doi: 10.1080/13543784.2016.1193155. [DOI] [PubMed] [Google Scholar]
- 33.Garay RP, Bourin M, De Paillette E, et al. Potential serotonergic agents for the treatment of schizophrenia. Expert Opin Investig Drugs. 2016;25:159–170. doi: 10.1517/13543784.2016.1121995. [DOI] [PubMed] [Google Scholar]
- 34.EMA. European Medicines Agency Guideline on clinical investigation of medicinal products in the treatment of depression 2013 [cited 2016 Jun 8] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143770.pdf]. • Regulatory guidelines for clinical trials in depression, including TRD.
- 35.Naughton M, Clarke G, O′Leary OF, et al. A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord. 2014;156:24–35. doi: 10.1016/j.jad.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 36.DeWilde KE, Levitch CF, Murrough JW, et al. The promise of ketamine for treatment-resistant depression: current evidence and future directions. Ann N Y Acad Sci. 2015;1345:47–58. doi: 10.1111/nyas.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 39.Zarate CA, Jr., Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. •• Demonstration of the rapid and robust antidepressant action of sub-anesthetic doses of intravenous ketamine in TRD. [DOI] [PubMed] [Google Scholar]
- 40.Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8:283–290. doi: 10.4103/0259-1162.143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loo CD. Is ketamine ready to be used clinically for the treatment of depression? Med J Aust. 2015;203:425–426. doi: 10.5694/mja15.00966. [DOI] [PubMed] [Google Scholar]
- 42.McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 43.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- 44.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 45.Brunner E, Tohen M, Osuntokun O, et al. Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder. Neuropsychopharmacology. 2014;39:2549–2559. doi: 10.1038/npp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bobo WV, Voort JL, Croarkin PE, et al. Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety. 2016;33:698–710. doi: 10.1002/da.22505. [DOI] [PubMed] [Google Scholar]
- 47.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2) receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- 48.Pochwat B, Palucha-Poniewiera A, Szewczyk B, et al. NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin Investig Drugs. 2014;23:1181–1192. doi: 10.1517/13543784.2014.918951. [DOI] [PubMed] [Google Scholar]
- 49.Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 50.Li CT, Chen MH, Lin WC, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: a randomized controlled study. Hum Brain Mapp. 2016;37:1080–1090. doi: 10.1002/hbm.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ionescu DF, Swee MB, Pavone KJ, et al. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry. 2016;77:e719–25. doi: 10.4088/JCP.15m10056. [DOI] [PubMed] [Google Scholar]
- 52.Sos P, Klirova M, Novak T, et al. Relationship of ketamine’s anti-depressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34:287–293. [PubMed] [Google Scholar]
- 53.Zhang MW, Harris KM, Ho RC. Is Off-label repeat prescription of ketamine as a rapid antidepressant safe? Controversies, ethical concerns, and legal implications. BMC Med Ethics. 2016;17:4. doi: 10.1186/s12910-016-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin SA, Iglewicz A, Nelesen RA, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med. 2013;16:958–965. doi: 10.1089/jpm.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Domani Y, Bleich-Cohen M, Stoppelman N, et al. Oral ketamine for treatment resistant major depression – a double blind randomized controlled trial. European Psychiatry (24th European Congress of Psychiatry) 2016;33S:S523. [Google Scholar]
- 56.Zarate C, Duman RS, Liu G, et al. New paradigms for treatment-resistant depression. Ann N Y Acad Sci. 2013;1292:21–31. doi: 10.1111/nyas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. • In mice, the antidepressant action of ketamine is independent of NMDAR inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deakin JF, Lees J, McKie S, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychological Med. 2016;46:2449–2451. doi: 10.1017/S0033291716000969. [DOI] [PubMed] [Google Scholar]
- 60.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 61.Daly EJ, Singh JB, Fedgchin M, et al. Intranasal esketamine in treatment-resistant depression, a dose response study – double-blind and open-label extension data; 54th Annual Meeting of the American College of Neuropsychopharmacology 2015;(ACNP); Hollywood, Florida, USA. Dec 6th-10th, 2015. p. 74. •• Antidepressant action of intranasal esketamine in TRD. [Google Scholar]
- 62.Zhang J, Li S, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Canuso CM, Singh J, Fedgchin M, et al. Presented at American Society of Clinical Psychopharmacology 2016;(ASCP), May 30-June 3, 2016. Scottsdale, AZ, USA: PeRSEVERe: a study of esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in patients assessed to be at imminent risk for suicide. [Google Scholar]
- 64.Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2015;173:816–826. doi: 10.1016/j.biopsych.2015.10.018. • Antidepressant action of intravenous esketamine in TRD. [DOI] [PubMed] [Google Scholar]
- 65.Jarventausta K, Chrapek W, Kampman O, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J Ect. 2013;29:158–161. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- 66.FDA. Food and Drug Administration Drugs@FDA. Buprenorphine. Drug approval package. 2002 [cited 2016 Jun 8]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/20-732_20-733_Subutex.cfm]
- 67.Karp JF, Butters MA, Begley AE, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75:e785–93. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sher L. Buprenorphine and the treatment of depression, anxiety, non-suicidal self-injury, and suicidality. Acta Psychiatr Scand. 2016;134:84–85. doi: 10.1111/acps.12577. [DOI] [PubMed] [Google Scholar]
- 69.Bodkin JA, Zornberg GL, Lukas SE, et al. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Norelli LJ, Smith HS, Sher L, et al. Buprenorphine in the treatment of non-suicidal self-injury: a case series and discussion of the literature. Int J Adolesc Med Health. 2013;25:323–330. doi: 10.1515/ijamh-2013-0069. [DOI] [PubMed] [Google Scholar]
- 71.Ehrich E, Turncliff R, Du Y, et al. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacol. 2015;40:1448–1455. doi: 10.1038/npp.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fava M, Memisoglu A, Thase ME, et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2016;173:499–508. doi: 10.1176/appi.ajp.2015.15070921. [DOI] [PubMed] [Google Scholar]
- 73.FDA. Food and Drug Administration Drugs@FDA (FDA approved drug products) Brexpiprazole. 2015 [cited 2016 Jun 8]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm]
- 74.Citrome L, Stensbol TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. 2015;15:1219–1229. doi: 10.1586/14737175.2015.1086269. [DOI] [PubMed] [Google Scholar]
- 75.Gyertyan I, Kiss B, Saghy K, et al. Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int. 2011;59:925–935. doi: 10.1016/j.neuint.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Durgam S, Earley W, Guo H, et al. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry. 2016;77:371–378. doi: 10.4088/JCP.15m10070. [DOI] [PubMed] [Google Scholar]
- 77.Allergan Allergan and Richter Provide Update on Cariprazine Program. 2016 [cited 2016 Dec 21]. Available from: http://www. allergan.com/investors/news/thomson-reuters/allergan-and-rich ter-provide-update-on-cariprazine]
- 78.FDA. Food and Drug Administration Drugs@FDA. Nuedexta. Drug approval package. 2010 [cited 2016 June 8]. Available from: http://www.accessd ata.fda.gov/dru gsat f d a _docs/nd a/2010/021879Orig1s000TOC.cfm]
- 79.EMA. European Medicines Agency Nuedexta. Product Information. 2013 [cited 2016 June 8]. Available from: http://www.ema.europa. eu/ema/index.jsp?curl=pages/medicines/human/medicines/002560/human_med_1652.jsp&mid=WC0b01ac58001d124]
- 80.Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with alzheimer disease dementia: a randomized clinical trial. Jama. 2015;314:1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 81.Avanir Avanir pharmaceuticals announces initiation of phase III trial of AVP-786 for agitation in patients with Alzheimer’s Disease. 2015 Available from: http://www.avanir.com/press/avanir-pharma ceuticals-announces-initiation-phase-iii-trial-avp-786-agitation-patients]
- 82.Garner R, Gopalakrishnan S, McCauley JA, et al. Preclinical pharmacology and pharmacokinetics of CERC-301, a GluN2B-selective N-methyl-D-aspartate receptor antagonist. Pharmacol Res Perspect. 2015;3:e00198. doi: 10.1002/prp2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim L, Diaz Granados N, Jolkovsky L, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paterson B, Fraser H, Wang C, et al. A Randomized, double-blind, placebo-controlled, sequential parallel study of CERC-301 in the adjunctive treatment of subjects with severe depression and recent active suicidal ideation despite antidepressant treatment; Presented at the 2015 National Network of Depression Centers Annual Conference, November-2015; Ann Arbor, MI, USA. 2015. [Google Scholar]
- 85.Salituro FG, Tomlinson RC, Baron BM, et al. Enzyme-activated antagonists of the strychnine-insensitive glycine/NMDA receptor. J Med Chem. 1994;37:334–336. doi: 10.1021/jm00029a003. [DOI] [PubMed] [Google Scholar]
- 86.Zanos P, Piantadosi SC, Wu HQ, et al. The prodrug 4-Chlorokynurenine causes ketamine-like antidepressant Effects, but not side effects, by NMDA/GlycineB-Site inhibition. J Pharmacol Exp Ther. 2015;355:76–85. doi: 10.1124/jpet.115.225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mealing GA, Lanthorn TH, Small DL, et al. Structural modifications to an N-methyl-D-aspartate receptor antagonist result in large differences in trapping block. J Pharmacol Exp Ther. 2001;297:906–914. [PubMed] [Google Scholar]
- 88.Sanacora G, Smith MA, Pathak S, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zarate CA, Jr., Mathews D, Ibrahim L, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moskal JR, Burgdorf JS, Stanton PK, et al. The Development of Rapastinel (Formerly GLYX-13); a rapid acting and long lasting antidepressant. Curr Neuropharmacol. 2016;15:47–56. doi: 10.2174/1570159X14666160321122703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Preskorn S, Macaluso M, Mehra DO, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 92.Javelot H. [Psychopharmacology of anxiety and depression: historical aspects, current treatments and perspectives] Ann Pharm Fr. 2016;74:93–118. doi: 10.1016/j.pharma.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Roche Pipeline summary. 2015:79. Available from: http://www.roche.com/irp150128-annex.pdf]
- 94.Lawrence J. The secret life of ketamine. Pharm J. 2015 Available from http://www.pharmaceutical-journal.com/news-and-analysis/features/the-secret-life-of-ketamine/20068151.article]
- 95.Chocano-Bedoya PO, Mirzaei F, O’Reilly EJ, et al. C-reactive protein, interleukin-6, soluble tumor necrosis factor alpha receptor 2 and incident clinical depression. J Affect Disord. 2014;163:25–32. doi: 10.1016/j.jad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papakostas GI, Fava M, Baer L, et al. Ziprasidone augmentation of escitalopram for major depressive disorder: efficacy results from a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2015;172:1251–1258. doi: 10.1176/appi.ajp.2015.14101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt AW, Lebel LA, Howard HR, Jr., et al. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;425:197–201. doi: 10.1016/s0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- 98.Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry. 2004;65:217–221. doi: 10.4088/jcp.v65n0212. [DOI] [PubMed] [Google Scholar]
- 99.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 101.Kircik LH. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9:1407–1411. [PubMed] [Google Scholar]
- 102.Husain MI, Chaudhry IB, Rahman RR, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: study protocol for a pilot randomised controlled trial. Trials. 2015;16:410. doi: 10.1186/s13063-015-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyaoka T, Wake R, Furuya M, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–226. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Dean OM, Maes M, Ashton M, et al. Protocol and rationale-the efficacy of minocycline as an adjunctive treatment for major depressive disorder: a double blind, randomised, placebo controlled trial. Clin Psychopharmacol Neurosci. 2014;12:180–188. doi: 10.9758/cpn.2014.12.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkiteshwaran A. Tocilizumab. MAbs. 2009;1:432–438. doi: 10.4161/mabs.1.5.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smolen JS, Weinblatt ME, Sheng S, et al. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;73:1616–1625. doi: 10.1136/annrheumdis-2013-205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Johnson&Johnson Esketamine receives Breakthrough Therapy Designation from U.S. Food and Drug Administration for major depressive disorder with imminent risk for suicide. 2016 [cited 2016 June 8]. Available from: http://www.jnj.com/news/all/Esketamine-Receives-Breakthrough-Therapy-Designation-from-US-Food-and-Drug-Administration-for-Major-Depressive-Disorder-with-Imminent-Risk-for-Suicide]. •• The FDA grants breakthrough therapy designation for intranasal esketamine in MDD with imminent risk for suicide.
- 108.Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7:51–54. doi: 10.1016/0740-5472(90)90035-o. [DOI] [PubMed] [Google Scholar]
- 109.Alkermes Alkermes announces topline Results of FORWARD-3 and FORWARD-4, two phase 3 studies of ALKS 5461 in major depressive disorder 2016 [cited 2016 June 8th] Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=92211&p=irol-corporateNewsArticle&ID=2131031.
- 110.Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76:1232–1240. doi: 10.4088/JCP.14m09689. [DOI] [PubMed] [Google Scholar]
- 111.Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76:1224–1231. doi: 10.4088/JCP.14m09688. [DOI] [PubMed] [Google Scholar]
- 112.FDA. Food and Drug Administration Drugs@FDA. Cariprazine. Label. 2015 [cited 2016 December 21st]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204370lbl.pdf]
- 113.Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75–82. doi: 10.2147/SAR.S36761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen L, Thomas KL, Lucke-Wold BP, et al. Dextromethorphan: an update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther. 2016;159:1–22. doi: 10.1016/j.pharmthera.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Kulkarni SK, Dhir A. sigma-1 receptors in major depression and anxiety. Expert Rev Neurother. 2009;9:1021–1034. doi: 10.1586/ern.09.40. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen L, Robson MJ, Healy JR, et al. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. Plos One. 2014;9:e89985. doi: 10.1371/journal.pone.0089985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cerecor CERC-301 2016. [cited 2016 June 9]. Available from: http://www.cerecor.com/pipeline/cerc-301.php.
- 118.Bolshakov KV, Gmiro VE, Tikhonov DB, et al. Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem. 2003;87:56–65. doi: 10.1046/j.1471-4159.2003.01956.x. [DOI] [PubMed] [Google Scholar]
- 119.AdisInsight AZD 6765. 2015 [cited 2016 May 27th]. Available from: http://adisinsight.springer.com/drugs/800026805]
- 120.Allergan Allergan’s rapastinel receives FDA breakthrough therapy designation for adjunctive treatment of major depressive disorder (MDD) 2016 Avaialble from: http://www.allergan.com/news/news/thomson-reuters/allergan-s-rapastinel-receives-fda-breakthroughth]. • The FDA grants breakthrough therapy designation for rapastinel for adjunctive treatment of MDD.
- 121.Park M, Niciu MJ, Zarate CA., Jr Novel glutamatergic treatments for severe mood disorders. Curr Behav Neurosci Rep. 2015;2:198–208. doi: 10.1007/s40473-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.FDA. Food and Drug Administration Drugs@FDA. Ziprasidone. Approval Package. 2009 [cited 2016 June 8]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/020825Orig1s034.pdf]
- 123.Shelton RC, Papakostas GI. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand. 2008;117:253–259. doi: 10.1111/j.1600-0447.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- 124.Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ) CNS Neurosci Ther. 2010;16:322–325. doi: 10.1111/j.1755-5949.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 126.Khandaker GM, Pearson RM, Zammit S, et al. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression - The Maastricht Study. Brain Behav Immun. 2016;56:390–396. doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 128.Kohler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 129.Penn E, Tracy DK. The drugs don’t work? antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol. 2012;2:179–188. doi: 10.1177/2045125312445469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am Psychiatr Assoc. 2010 APA Guidelines:1–151. [cited 2016 Dec 21]. Available from: https://psychiatryonline.org/pb/assets/raw/site wide/practice_guidelines/guidelines/mdd.pdf • Practice guideline for the treatment of MDD, including TRD.
- 131.Saba G, Nieto I, Bation R, et al. [Other therapeutic strategies] Presse Med. 2016;45:323–332. doi: 10.1016/j.lpm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 132.Guzman F. Differences between tricyclic antidepressants and SNRIs mechanism of action. Pharmacol Corner. 2016 [cited 2016 June 17]. Available from: http://pharmacologycorner. com/differences-between-tricyclic-antidepressants-and-selec tive-serotonin-norepinephrine-reuptake-inhibitors-mechanism-of-action/
- 133.Trindade E, Menon D, Topfer LA, et al. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antide-pressants: a meta-analysis. Cmaj. 1998;159:1245–1252. [PMC free article] [PubMed] [Google Scholar]
- 134.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 135.Borges S, Chen YF, Laughren TP, et al. Review of maintenance trials for major depressive disorder: a 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry. 2014;75:205–214. doi: 10.4088/JCP.13r08722. [DOI] [PubMed] [Google Scholar]
- 136.Machado-Vieira R, Baumann J, Wheeler-Castillo C, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010;3:19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El-Hage W. [Roadmap for therapeutic strategies of treatment resistant depression] Presse Med. 2016;45:320–322. doi: 10.1016/j.lpm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 138.Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;53(Suppl. 13):23–29. [PubMed] [Google Scholar]
- 139.Hassan AK, Farmer KC, Brahm NC, et al. Evaluating antidepressant treatment prior to adding second-line therapies among patients with treatment-resistant depression. Int J Clin Pharm. 2016;38:429–437. doi: 10.1007/s11096-016-0272-y. [DOI] [PubMed] [Google Scholar]
- 140.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- 142.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duman RS. Pathophysiology of depression and innovative treat-ments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Freedman R. Further Investigation of Ketamine. Am J Psychiatry. 2016;173:761–762. doi: 10.1176/appi.ajp.2016.16050581. [DOI] [PubMed] [Google Scholar]
- 146.Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415–2423. doi: 10.1001/jama.2016.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 148.Citrome L. Drug safety evaluation of ziprasidone. Expert Opin Drug Saf. 2011;10:437–448. doi: 10.1517/14740338.2011.560114. [DOI] [PubMed] [Google Scholar]
- 149.Tian G, Mi J, Wei X, et al. Circulating interleukin-6 and cancer: a meta-analysis using Mendelian randomization. Sci Rep. 2015;5:11394. doi: 10.1038/srep11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 151.Lam RW, Milev R, Rotzinger S, et al. Discovering biomarkers for antidepressant response: protocol from the Canadian biomarker integration network in depression (CAN-BIND) and clinical characteristics of the first patient cohort. BMC Psychiatry. 2016;16:105. doi: 10.1186/s12888-016-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 153.Lam RW, Parikh SV, Michalak EE, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27:142–149. [PubMed] [Google Scholar]