Abstract
BACKGROUND
A prolonged PR interval is common among cardiac resynchronization therapy (CRT) candidates; however, the association between PR interval and outcomes is unclear, and the data are conflicting.
METHODS
We conducted inverse probability weighted analyses of 26 451 CRT-eligible (ejection fraction ≤35, QRS ≥120 ms) patients from the National Cardiovascular Data Registry ICD Registry to assess the association between a prolonged PR interval (≥230 ms), receipt of CRT with defibrillator (CRT-D) versus implantable cardioverter defibrillator (ICD), and outcomes. We first tested the association between a prolonged PR interval and outcomes among patients stratified by device type. Next, we performed a comparative effectiveness analysis of CRT-D versus ICD among patients when stratified by PR interval. Using Medicare claims data, we followed up with patients up to 5 years for incident heart failure hospitalization or death.
RESULTS
Patients with a PR≥230 ms (15%; n=4035) were older and had more comorbidities, including coronary artery disease, atrial arrhythmias, diabetes mellitus, and chronic kidney disease. After risk adjustment, a PR≥230 ms (versus PR<230 ms) was associated with increased risk of heart failure hospitalization or death among CRT-D (hazard ratio, 1.23; 95% confidence interval, 1.14–1.31; P<0.001) but not ICD recipients (hazard ratio, 1.08; 95% confidence interval, 0.97–1.20; P=0.17) (Pinteraction=0.043). CRT-D (versus ICD) was associated with lower rates of heart failure hospitalization or death among patients with PR<230 ms (hazard ratio, 0.79; 95% confidence interval, 0.73–0.85; P<0.001) but not PR≥230 ms (hazard ratio, 1.01; 95% confidence interval, 0.87–1.17; P=0.90) (Pinteraction=0.0025).
CONCLUSIONS
A PR≥230 ms is associated with increased rates of heart failure hospitalization or death among CRT-D patients. The real-world comparative effectiveness of CRT-D (versus ICD) is significantly less among patients with a PR≥230 ms in comparison with patients with a PR<230 ms.
Keywords: atrioventricular block, cardiac resynchronization therapy, cardiomyopathies, defibrillators, pacemaker, artificial
QRS morphology and duration are well-established predictors of outcomes among patients receiving cardiac resynchronization therapy (CRT) devices. The presence of left bundle-branch block and prolonged QRS duration (≥150 ms) have been associated with superior outcomes in many cohorts, including those enrolled in randomized controlled trials.1–4 In contrast, many studies have suggested that CRT may not be efficacious in those with shorter QRS duration, non–left bundle-branch block (LBBB) morphology, or both. Current guidelines on the use of CRT reflect these observations and either weakly recommend or do not recommend the use of CRT in patients with unfavorable QRS profiles, depending on New York Heart Association symptom class.5
The favorable effect of CRT is attributed to achieving both biventricular resynchronization and atrioventricular resynchronization, although the relative contribution of each to the effect of CRT is controversial. It has been hypothesized that the treatment effect of atrioventricular resynchronization may vary on the basis of the PR interval because of the association of a prolonged PR interval with disordered diastolic filling. The existing literature on the association between PR interval and CRT outcomes is conflicting, with some studies suggesting that a prolonged PR interval is associated with increased benefit from CRT,6–8 with others suggesting that the same measure is associated with decreased benefit from CRT.9–12 As such, we performed a comparative effectiveness analysis to determine the association between prolonged PR interval (≥230 ms), CRT with defibrillator (CRT-D) use (versus implantable cardioverter defibrillator [ICD]), and outcomes, in a nationally representative cohort of older CRT-eligible patients with heart failure. We used a 230-ms PR interval threshold (instead of 200 ms) to ensure that our analysis focused on those with unequivocally abnormal atrioventricular conduction. In addition, physiological studies maintain that PR intervals >230 ms are associated with the development of diastolic mitral regurgitation,13,14 providing physiological support for using this threshold in the current analysis.
METHODS
Data sources
National Cardiovascular Data Registry ICD Registry
Patients for this study were identified from the National Cardiovascular Data Registry (NCDR) ICD Registry versions 1.0 and 2.0. According to a mandate from the Centers for Medicare and Medicaid Services, all Medicare beneficiaries who receive a primary prevention ICD are enrolled in the ICD Registry; many sites report implantation of all ICD implants, allowing the registry to capture an estimated 90% of all ICD implantations in the United States.15 The ICD Registry includes extensive information on baseline characteristics, procedure details, and in-hospital outcomes. Rigorous data abstraction processes and standards are used, including standardized variable definitions, electronic quality checks, electronic data submission over a secure website, and annual on-site audits of a few enrolling sites.16 This rigorous approach has led to >90% accuracy for data elements.17 The Human Investigation Committee of the Yale University School of Medicine approved the use of data from the ICD Registry for research purposes.
Medicare Database
Longitudinal outcomes were obtained by linking fee-for-service Medicare claims to the ICD Registry by using indirect identifiers: patient sex, birth date, hospital, admission date, and discharge date.18 Inpatient claims, outpatient claims, and the denominator files were used to assess morbidity and mortality. We used the Chronic Conditions Warehouse database (years 2005–2011), which includes both Part A and Part B Medicare claims, to assess specific covariates and outcomes.
Study Population
We restricted the study population to all fee-for-service Medicare patients ≥65 years old who underwent ICD implantation (with or without CRT) between January 1, 2006, and December 31, 2010; were eligible for CRT (on the basis of an ejection fraction ≤35% and QRS≥120 ms); had reported data on PR interval and QRS morphology; and could be linked to Medicare claims data. We excluded patients who were admitted during a nonelective hospitalization, were enrolled in the ICD Registry at the time of generator change, required an epicardial lead, had a prior pacemaker or defibrillator, and had a second- or third-degree heart block or a paced rhythm before device implantation.
Patient Characteristics
All baseline characteristics, with the exception of the frailty and dementia variables, were obtained directly from the ICD Registry case report form. Dementia and frailty were defined through the use of Hierarchical Condition Categories to allow for the adjustment for 2 important variables that are known to have an effect on prognosis and the decision to implant a CRT-D versus ICD.19
Treatment
The treatment of interest was CRT-D versus ICD alone.
Outcomes
The primary outcome was the composite end point of heart failure (HF) hospitalization or death. Secondary outcomes included HF hospitalization and death, individually. Medicare claims data were available through the end of 2011. Follow-up was censored 5 years after device implant or on the date at which the patient’s data were no longer available (because of death or transition to a managed care plan). We used 2 falsification end points20 (gastrointestinal bleed and bone disorder/fracture) to test the adequacy of the statistical models used for the comparative effectiveness analyses, because these outcomes should not vary on the basis of the receipt of CRT-D versus ICD.
Vital status was determined by the Medicare denominator file. Longitudinal outcomes were determined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or diagnosis-related group codes as appropriate: HF hospitalization (diagnosis-related group 127 before October 1, 2007, and diagnosis-related groups 291–293 on or after October 1, 2007); gastrointestinal bleed (ICD-9-CM code 578); and bone disorder/fracture (ICD-9-CM codes 730–736, 802–824).19
Statistical Analysis
Baseline characteristics of the study population dichotomized by PR interval (≥ or <230 ms) were described by using proportions for categorical variables and means with standard deviations for continuous variables. Differences between groups were tested using the χ2 test for categorical variables and t tests for continuous variables.
We reported observed event rates by baseline PR interval and device type (CRT-D or ICD). For the primary end point of HF hospitalization or death and the secondary end point of death, the Kaplan-Meier method was used to calculate event rates, and the log-rank test was used to assess differences between groups. For HF hospitalization, the cumulative incidence function was used to calculate event rates and Gray tests to assess differences between groups while accounting for the risk of death.
To estimate the risk-adjusted association between PR interval, device type (CRT-D versus ICD), and each outcome, we used inverse probability weighted Cox proportional hazard or Fine-Gray models. Cox proportional hazard models were used for the end points of HF hospitalization or death and death. Fine-Gray models were used for the end points of HF hospitalization, gastrointestinal bleed, and bone disorder/fracture, to account for the competing risk of death, which is high in this population. Logistic regression models were used to examine factors associated with CRT-D receipt or a prolonged PR interval (depending on the analysis) and derive an inverse probability weight (IPW) on the basis of all covariates detailed in Table 1. Interactions between device type (CRT-D versus ICD), PR interval, and outcome were calculated. The comparative effectiveness of CRT-D versus ICD was also tested in analyses stratified by both PR interval and QRS morphology. We plotted the unadjusted incidence rate of the primary outcome across subgroups defined by binning CRT patients by PR interval using 10-ms bins. Based on the observation that the association between the incidence of HF hospitalization or death and PR interval decreased linearly until a threshold of 170 ms, and increased linearly thereafter, we fitted a linear spline function of PR interval with a knot at 170 ms. Based on this, we created a multivariable Cox model with 2 PR interval variables, each examining PR interval across the continuous range, with one variable for PR intervals <170 ms, and the other for PR intervals ≥170 ms. We constructed restricted cubic spline functions (5 knots at the 5th, 25th, 50th, 75th, and 90th percentiles) to explore the relation between PR interval and outcomes among CRT-D patients; functions were generated on the basis of the β-coefficients estimated from unadjusted and multivariable models and plotted as log hazard on the y axis versus PR interval on the x axis. The comparative effectiveness of CRT-D versus ICD was assessed across the continuous range of PR intervals using restricted cubic spline with 5 knots at the 5th, 25th, 50th, 75th, and 90th quantiles of PR interval using a fully adjusted multivariable model including an interaction term for the device (CRT-D versus ICD) and PR interval. Hazard ratios (HRs) or subdistribution hazard ratios and their 95% confidence intervals (CIs) were reported on the basis of robust sandwich variance estimates to account for clustering of patients within hospitals, as appropriate. Missing variables were addressed with the multiple imputation technique; the coefficients of 5 rounds of imputation were combined to obtain the final estimates for the models. A P value of <0.05 was considered statistically significant for all tests. Analyses were performed by using SAS (version 9.3, SAS institute).
Table 1.
Baseline Patient, hospital, Operator, and Procedure Characteristics
| PR<230 ms (n=22 416) |
PR≥230 ms (n=4035) |
P Value | |
|---|---|---|---|
| Age, y | 75.1 (6.0) | 76.9 (6.2) | <0.0001 |
| Sex, n (%) | |||
| Male | 14 620 (65.2) | 3329 (82.5) | <0.0001 |
| Female | 7796 (34.8) | 706 (17.5) | |
| Race, n (%) | |||
| White non-Hispanic | 19 503 (87.0) | 3610 (89.5) | <0.0001 |
| Black non-Hispanic | 1572 (7.0) | 244 (6.0) | |
| Hispanic | 892 (4.0) | 106 (2.6) | |
| Other | 449 (2.0) | 75 (1.9) | |
| QRS duration, ms | |||
| 120–129 | 3134 (14.0) | 504 (12.5) | <0.0001 |
| 130–139 | 3360 (15.0) | 587 (14.5) | |
| 140–149 | 4046 (18.0) | 625 (15.5) | |
| 150–159 | 4050 (18.1) | 603 (14.9) | |
| 160–169 | 3725 (16.6) | 686 (17.0) | |
| ≥170 | 4101 (18.3) | 1030 (25.5) | |
| Intraventricular conduction, n (%) | |||
| Non–left bundle-branch block | 7031 (31.4) | 1513 (37.5) | <0.0001 |
| Left bundle-branch block | 15 385 (68.6) | 2522 (62.5) | |
| Nonischemic cardiomyopathy | |||
| Missing | 18 (0.1) | 8 (0.2) | <0.0001 |
| No | 14 396 (64.2) | 2980 (73.9) | |
| Yes, within the past 9 mo | 2539 (11.3) | 291 (7.2) | |
| Yes, >9 mo | 5463 (24.4) | 756 (18.7) | |
| Ischemic heart disease, n (%) | 14 909 (66.5) | 3034 (75.2) | <0.0001 |
| Atrial fibrillation/atrial flutter, n (%) | 3871 (17.3) | 1106 (27.4) | <0.0001 |
| Ventricular tachycardia, n (%) | 3849 (17.2) | 745 (18.5) | 0.1006 |
| Primary prevention | 21 302 (95.0) | 3839 (95.1) | 0.7624 |
| New York Heart Association class | |||
| Missing | 14 (0.1) | 4 (0.1) | 0.077 |
| I | 1116 (5.0) | 169 (4.2) | |
| II | 5523 (24.6) | 983 (24.4) | |
| III | 15 164 (67.6) | 2751 (68.2) | |
| IV | 599 (2.7) | 128 (3.2) | |
| Previous percutaneous coronary intervention | 7380 (32.9) | 1388 (34.4) | 0.1546 |
| Previous coronary artery bypass grafting | 8612 (38.4) | 1999 (49.5) | <0.0001 |
| Diabetes mellitus | 8323 (37.1) | 1726 (42.8) | <0.0001 |
| Previous myocardial infarction | 11 746 (52.4) | 2395 (59.4) | <0.0001 |
| Chronic lung disease | 5201 (23.2) | 809 (20.0) | <0.0001 |
| Cerebrovascular disease | 3302 (14.7) | 706 (17.5) | <0.0001 |
| Cardiac arrest | 452 (2.0) | 73 (1.8) | 0.4454 |
| Hypertension | 17 567 (78.4) | 3213 (79.6) | 0.1129 |
| Syncope | 2315 (10.3) | 461 (11.4) | 0.0324 |
| Congestive heart failure duration | |||
| Missing | 25 (0.1) | 6 (0.1) | 0.0001 |
| No | 2166 (9.7) | 362 (9.0) | |
| <9 mo | 5682 (25.3) | 904 (22.4) | |
| >9 mo | 14 543 (64.9) | 2763 (68.5) | |
| Ejection fraction, % | 24.8 (6.3) | 25.2 (6.1) | <0.0001 |
| Glomerular filtration rate | |||
| Missing | 94 (0.4) | 14 (0.3) | <0.0001 |
| >60 mL·min−1·1.73m2 | 9473 (42.3) | 1426 (35.3) | |
| 30–59 mL·min−1·1.73m2 | 10 782 (48.1) | 2127 (52.7) | |
| 15–29 mL·min−1·1.73m2 | 1386 (6.2) | 328 (8.1) | |
| <15 mL·min−1·1.73 m2 including those on dialysis | 681 (3.0) | 140 (3.5) | |
| Dialysis | 581 (2.6) | 129 (3.2) | 0.0901 |
| Blood urea nitrogen level, mg/dL | |||
| Missing | 126 (0.6) | 22 (0.5) | <0.0001 |
| ≤ 20 | 9642 (43.0) | 1380 (34.2) | |
| 20–40 | 10 498 (46.8) | 2060 (51.1) | |
| >40 | 2150 (9.6) | 573 (14.2) | |
| Sodium level, mEq/L | |||
| Missing | 141 (0.6) | 23 (0.6) | 0.0641 |
| ≤135 | 2689 (12.0) | 521 (12.9) | |
| 135–145 | 19 327 (86.2) | 3429 (85.0) | |
| >145 | 259 (1.2) | 62 (1.5) | |
| Systolic blood pressure, mm Hg | |||
| Missing | 88 (0.4) | 17 (0.4) | 0.284 |
| ≤ 100 | 1178 (5.3) | 188 (4.7) | |
| 100–130 | 9235 (41.2) | 1635 (40.5) | |
| >130 | 11 915 (53.2) | 2195 (54.4) | |
| Cardiac resynchronization therapy | 15 994 (71.4) | 2906 (72.0) | 0.3864 |
| Angiotensin-converting enzyme inhibitor | 13 765 (61.4) | 2362 (58.5) | 0.0006 |
| Amiodarone | 1751 (7.8) | 574 (14.2) | <0.0001 |
| Hydralazine | 820 (3.7) | 194 (4.8) | 0.0005 |
| Angiotensin receptor blocker | 4597 (20.5) | 879 (21.8) | 0.0654 |
| Aspirin | 16 373 (73.0) | 2900 (71.9) | 0.1238 |
| β-Blocker | 19 583 (87.4) | 3471 (86.0) | 0.0192 |
| Warfarin | 3636 (16.2) | 978 (24.2) | <0.0001 |
| Digoxin | 4729 (21.1) | 1017 (25.2) | <0.0001 |
| Diuretic | 15 122 (67.5) | 2900 (71.9) | <0.0001 |
| Long-acting nitrate | 2659 (11.9) | 539 (13.4) | 0.0073 |
| Clopidogrel | 5391 (24.0) | 980 (24.3) | 0.7451 |
| Statin | 15 348 (68.5) | 2864 (71.0) | 0.0015 |
| Dementia | 605 (2.7) | 120 (3.0) | 0.3246 |
| Disability/frailty | 1378 (6.1) | 277 (6.9) | 0.0832 |
| Operator Training | |||
| Unknown | 4593 (20.5) | 817 (20.2) | 0.2905 |
| Board-certified EP/EP fellowship | 14 143 (63.1) | 2596 (64.3) | |
| Surgeon | 194 (0.9) | 38 (0.9) | |
| Other | 3486 (15.6) | 584 (14.5) | |
| Operator cardiac resynchronization therapy volume, implants/y | |||
| ≤ 20 | 12 439 (55.5) | 2208 (54.7) | 0.3648 |
| >20 | 9977 (44.5) | 1827 (45.3) | |
| Region | |||
| Other | 598 (2.7) | 128 (3.2) | 0.2015 |
| New England | 982 (4.4) | 180 (4.5) | |
| Atlantic | 7528 (33.6) | 1287 (31.9) | |
| Central | 10 599 (47.3) | 1936 (48.0) | |
| Mountain | 932 (4.2) | 167 (4.1) | |
| Pacific | 1777 (7.9) | 337 (8.4) | |
| Teaching status | |||
| Unknown | 594 (2.6) | 128 (3.2) | 0.1194 |
| Council of teaching hospitals | 6475 (28.9) | 1112 (27.6) | |
| Teaching hospital | 6276 (28.0) | 1141 (28.3) | |
| Not teaching hospital | 9071 (40.5) | 1654 (41.0) | |
| Center cardiac resynchronization therapy volume, implants/y | |||
| ≤20 | 2179 (9.7) | 343 (8.5) | 0.0151 |
| >20 | 20 237 (90.3) | 3692 (91.5) | |
| Beds set up and staffed | |||
| Unknown | 594 (2.6) | 128 (3.2) | 0.0443 |
| ≤100 | 1185 (5.3) | 221 (5.5) | |
| 101–500 | 13 093 (58.4) | 2404 (59.6) | |
| >500 | 7544 (33.7) | 1282 (31.8) | |
| Implant year | |||
| 2006 | 3926 (17.5) | 741 (18.4) | 0.0015 |
| 2007 | 4299 (19.2) | 871 (21.6) | |
| 2008 | 4933 (22.0) | 853 (21.1) | |
| 2009 | 4863 (21.7) | 832 (20.6) | |
| 2010 | 4395 (19.6) | 738 (18.3) | |
The values shown are n (%), unless stated otherwise. EP indicates electrophysiology.
The glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula.21
RESULTS
Between January 1, 2006, and December 31, 2010, 122 643 individuals with an ejection fraction ≤35% and QRS≥120 ms underwent ICD implantation and were enrolled in the NCDR ICD Registry. We excluded individuals with a prior pacemaker or defibrillator (n=54 897), implantation during a nonelective hospital admission (n=27 236), an epicardial left ventricular lead (n=1058), second or third degree heart block (n=1084), paced rhythm (n=843), missing QRS morphology (n=4264), or an unobtainable PR interval (because of atrial fibrillation or other abnormal atrial rhythm, atrioventricular (AV) block, or paced rhythm) (n=6810). No patients were excluded on the basis of a missing PR interval. Fifteen percent of patents (n=4035) had a PR interval of ≥230 ms, and the median follow-up was 34 months.
Baseline patient, hospital, operator, and procedure characteristics stratified by PR interval are detailed in Table 1. Patients with a prolonged PR interval were more often male, older, and more frequently comorbid with ischemic heart disease, atrial arrhythmias, cerebrovascular disease, diabetes mellitus, and chronic kidney disease. Forty-five percent of all implants were performed by operators who implanted >20 CRT devices per year, and this did not vary by PR interval. Device implants in patients with a PR<230 ms were slightly more common in hospitals with >500 beds. Although LBBB was less common among those with a PR≥230 ms, CRT rates did not differ by PR interval.
In unadjusted analyses, a prolonged PR interval was associated with an increased risk of HF hospitalization or death, death, and HF hospitalization alone, among ICD and CRT-D patients at 1, 3, and 5 years of followup (Table 2). However, after IPW risk adjustment, a PR≥230 ms (versus PR<230 ms) remained associated with increased risk of HF hospitalization or death among CRT-D (HR, 1.23; 95% CI, 1.14–1.31; P<0.001) but not ICD recipients (HR, 1.08; 95% CI, 0.97–1.20; P=0.17) (Pinteraction=0.043) (Table 3); results were consistent using the individual end points of HF hospitalization and death. Online-only Data Supplement Table I contains the baseline characteristics of the IPW cohort for this analysis. Among CRT-D recipients, a plot of the unadjusted incidence of HF hospitalization or death by PR interval (continuous) was created and fitted with a linear spline function with a single knot at 170 ms (online-only Data Supplement Figure I). Among CRT-D recipients, there was no association between PR interval (across the continuous range) and HF hospitalization or death among those with a PR<170 ms (HR, 0.9997; 95% CI, 0.9979–1.0014; P=0.70, per 1-ms increase in PR interval); among those with a PR interval ≥170 ms, there was a linear relationship between PR interval (across the continuous range) and HF hospitalization or death (HR, 1.0014; 95% CI, 1.0008–1.0021; P<0.001, per 1-ms increase in PR interval). When the association between PR interval and outcomes among CRT-D patients was additionally assessed using restricted cubic splines, we again identified ≈170 ms as a clinically relevant cut point at which risk for HF hospitalization and death increased (online-only Data Supplement Figure II).
Table 2.
Unadjusted Event Rates by PR Interval After Stratification by Device type
| Outcome | ICD | CRT-D | ||||
|---|---|---|---|---|---|---|
| PR<230 ms | PR≥230 ms | P Value | PR<230 ms | PR≥230 ms | P Value | |
| Heart failure hospitalization or death, n (%) | ||||||
| 1 y | 1015 (15.8) | 212 (18.8) | 0.0108 | 2709 (16.9) | 645 (22.2) | <0.0001 |
| 3 y | 2083 (32.4) | 441 (39.1) | <0.0001 | 4900 (30.6) | 1192 (41.0) | <0.0001 |
| 5 y | 2540 (39.6) | 530 (46.9) | <0.0001 | 5727 (35.8) | 1365 (47.0) | <0.0001 |
| Death, n (%) | ||||||
| 1 y | 459 (7.1) | 93 (8.2) | 0.2034 | 1262 (7.9) | 329 (11.3) | <0.0001 |
| 3 y | 1322 (20.6) | 29 1(25.8) | 0.0003 | 3137 (19.6) | 761 (26.2) | <0.0001 |
| 5 y | 1787 (27.8) | 398 (35.3) | <0.0001 | 4009 (25.1) | 957 (32.9) | <0.0001 |
| Heart failure hospitalization, n (%) | ||||||
| 1 y | 702 (10.9) | 151 (13.4) | 0.0105 | 1903 (11.9) | 432 (14.9) | <0.0001 |
| 3 y | 1281 (19.9) | 282 (25.0) | 0.0002 | 3080 (19.3) | 779 (26.8) | <0.0001 |
| 5 y | 1513 (23.6) | 329 (29.1) | 0.0001 | 3466 (21.7) | 866 (29.8) | <0.0001 |
The log rank test was used for heart failure hospitalization or death and death end points; the Gray test was used for the heart failure hospitalization end point.
CRT-D indicates cardiac resynchronization therapy with defibrillator; and ICD, implantable cardioverter defibrillator.
Table 3.
Unadjusted and Adjusted Analyses Comparing the Association Between PR Interval ≥230 ms Versus PR Interval <230 ms and Outcomes in Analyses Stratified by Device Type
| ICD | CRT-D | Pinteraction* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted by IPW | Unadjusted | Adjusted by IPW | ||||||||||
| HR or sHR | 95% CI | P Value | HR or sHR | 95% CI | P Value | HR or sHR | 95% CI | P Value | HR or sHR | 95% CI | P Value | ||
| Heart failure hospitalization or death† | 1.242 (1.134–1.360) | <0.0001 | 1.079 (0.968–1.201) | 0.1692 | 1.423 (1.344–1.506) | <0.0001 | 1.225 (1.141–1.314) | <0.0001 | 0.043 | ||||
| Death† | 1.290 (1.164–1.429) | <0.0001 | 1.059 (0.938–1.195) | 0.3547 | 1.377 (1.279–1.481) | <0.0001 | 1.120 (1.025–1.224) | 0.012 | 0.4437 | ||||
| Heart failure hospitalization‡ | 1.264 (1.116–1.431) | 0.0002 | 1.127 (0.978–1.299) | 0.0987 | 1.413 (1.316–1.517) | 0.0019 | 1.279 (1.180–1.387) | <0.0001 | 0.118 | ||||
CI indicates confidence interval; CRT-D, cardiac resynchronization therapy with defibrillator; HR, hazard ratio; ICD, implantable cardioverter defibrillator; IPW, inverse probability weighted; and sHR, subdistribution hazard ratio.
For device type (CRT-D versus ICD), PR interval (≥230 ms versus <230ms), and outcome.
Hazard ratio.
Subdistribution hazard ratio.
We subsequently tested the comparative effectiveness of CRT-D versus ICD in IPW analyses stratified by PR interval. CRT-D (versus ICD) was associated with lower rates of HF hospitalization or death among patients with PR<230 ms (HR, 0.79; 95% CI, 0.73–0.85; P<0.001) but not PR≥230 ms (HR, 1.01; 95% CI, 0.87–1.17; P=0.90) (Pinteraction=0.0025) (Table 4, Figure 1); this was consistent in sensitivity analyses using multivariable adjustment including an interaction term for device type and PR interval (online-only Data Supplement Table II), when examining the components of the composite end point individually, and among the LBBB patients. CRT-D was not associated with a reduction in HF hospitalization or death in non-LBBB patients in either PR-interval group. Online-only Data Supplement Tables III and IV contain the baseline characteristics of the IPW-adjusted LBBB and non-LBBB populations, respectively. In a series of IPW analyses using the falsification end points of gastrointestinal bleed and bone disorder/fracture, we found that neither outcome varied by receipt of CRT-D versus ICD, suggesting adequate statistical adjustment among the cohorts used in the comparative effectiveness analyses (online-only Data Supplement Table V). We performed additional sensitivity analyses for the 2 IPW models where covariate assessment demonstrated standardized differences of ≥10%; in these models, we additionally adjusted for any variable with a standardized difference of >10%, and the results were unchanged.
Table 4.
Association Between CRT-D Versus ICD and Outcomes Among All Patients and Among Subgroups When Stratified by PR Interval and QRS Characteristics
| Group | Outcome | PR<230 ms | PR≥230 ms | Pinteraction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted by IPW | Unadjusted | Adjusted by IPW | |||||||
| HR/sHR 95% CI | P Value | HR/sHR 95% CI | P Value | HR/sHR 95% CI | P Value | HR/sHR 95% CI | P Value | |||
| All patients | HF hospitalization or death | 0.965 (0.918–1.014) | 0.16 | 0.789 (0.734–0.849) | <0.0001 | 1.110 (1.008–1.222) | 0.0347 | 1.010 (0.870–1.173) | 0.90 | 0.0025 |
| Death | 0.979 (0.923–1.039) | 0.49 | 0.803 (0.738–0.875) | <0.0001 | 1.047 (0.934–1.173) | 0.43 | 0.922 (0.782–1.087) | 0.33 | 0.13 | |
| HF hospitalization | 0.969 (0.908–1.033) | 0.33 | 0.794 (0.723–0.871) | <0.0001 | 1.093 (0.962–1.242) | 0.17 | 1.033 (0.854–1.248) | 0.74 | 0.01 | |
| Non LBBB | HF hospitalization or death | 1.305 (1.219–1.396) | <0.0001 | 0.924 (0.826–1.034) | 0.17 | 1.374 (1.195–1.579) | <0.0001 | 1.061 (0.832–1.352) | 0.63 | 0.30 |
| Death | 1.225 (1.129–1.330) | <0.0001 | 0.851 (0.750–0.966) | 0.0128 | 1.237 (1.051–1.456) | 0.0106 | 0.896 (0.686–1.171) | 0.42 | 0.73 | |
| HF hospitalization | 1.352 (1.240–1.475) | <0.0001 | 1.035 (0.905–1.184) | 0.62 | 1.344 (1.116–1.618) | 0.0018 | 1.135 (0.854–1.510) | 0.38 | 0.57 | |
| LBBB | HF hospitalization or death | 0.907 (0.847–0.970) | 0.0048 | 0.717 (0.652–0.788) | <0.0001 | 1.034 (0.898–1.192) | 0.64 | 1.047 (0.835–1.313) | 0.69 | 0.0015 |
| Death | 0.965 (0.888–1.050) | 0.41 | 0.764 (0.684–0.853) | <0.0001 | 1.019 (0.862–1.203) | 0.83 | 0.968 (0.754–1.244) | 0.80 | 0.08 | |
| HF hospitalization | 0.877 (0.805–0.956) | 0.0028 | 0.685 (0.605–0.776) | <0.0001 | 1.007 (0.841–1.207) | 0.94 | 1.022 (0.785–1.331) | 0.87 | 0.0048 | |
CI indicates confidence interval; CRT-D, cardiac resynchronization therapy with defibrillator; HF, heart failure; HR, hazard ratio; ICD, implantable cardioverter defibrillator; IPW, inverse probability weighted; LBBB, left bundle-branch block; and sHR, subdistribution hazard ratio.
Figure 1. Heart failure hospitalization or death among patients stratified by device type and PR interval among the overall, lBBB, and non-lBBB populations.
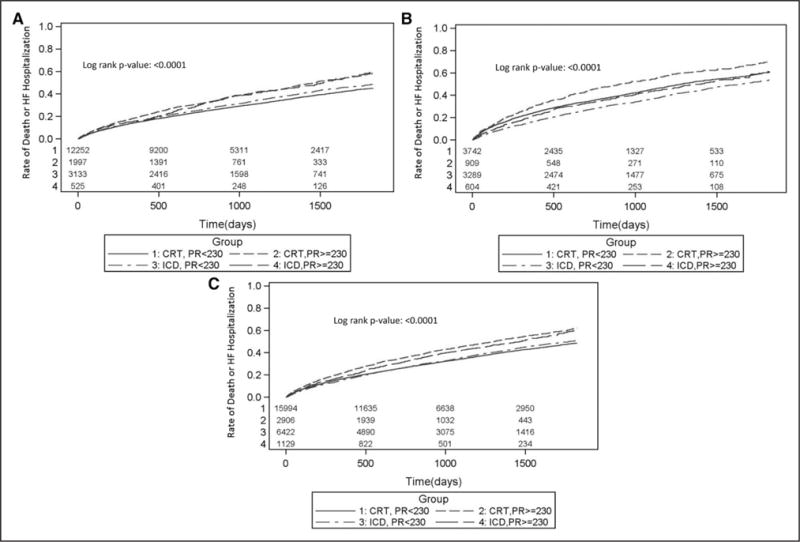
Cumulative incidence plots depicting rates of HF hospitalization or death among the entire population (A), non-LBBB patients (B), and LBBB patients (C), when stratified based on device type (CRT-D versus ICD) and PR interval (≥230 ms versus <230 ms). CRT indicates cardiac resynchronization therapy; CRT-D, CRT with defibrillator; HF, heart failure; ICD, implantable cardioverter defibrillator; and LBBB, left bundle-branch block.
To further explore the relationship between PR interval and outcomes in CRT, we performed a sensitivity analysis looking at the comparative effectiveness of CRT-D (versus ICD) within 3 subpopulations, defined by PR interval: PR<180 ms, PR 180 ms to 229 ms, and PR≥230 ms. CRT-D use was associated with a significantly lower risk for HF hospitalization or death among those with a PR<180 ms (HR, 0.79; 95% CI, 0.72–0.88; P<0.0001) and a PR of 180 to 229 ms (HR, 0.78; 95% CI, 0.71–0.86; P<0.0001), but not among those with a PR≥230 ms (HR, 1.01; 95% CI, 0.87–1.17; P=0.90) (Pinteraction=0.0086) (Figure 2). Online-only Data Supplement Table VI contains the baseline characteristics for the IPW-adjusted groups constructed for this sensitivity analysis. When assessed using a restricted cubic spline, it is evident that the comparative effectiveness of CRT versus ICD decreases in a linear manner across the continuous range of PR intervals (Figure 3).
Figure 2. Comparative effectiveness of CRT-D versus ICD alone among patient subgroups defined by PR interval.
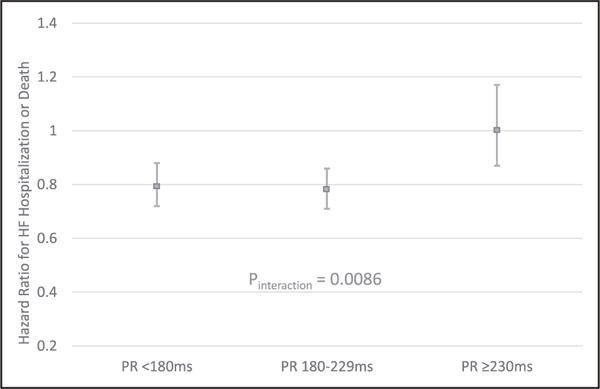
The interaction P value was generated by combining the 3 PR-interval subgroups (with IPW) and running a model that included device type, PR interval (3-level categorical variable), and an interaction term for device type and PR interval. CRT-D indicates cardiac resynchronization therapy with defibrillator; HF, heart failure; ICD, implantable cardioverter defibrillator; and IPW, inverse probability weighted.
Figure 3. Comparative effectiveness of CRT-D versus ICD across the continuous range of PR intervals.
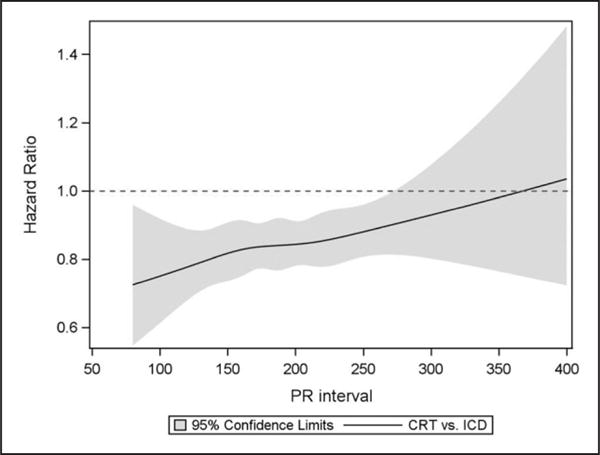
Restricted cubic spline function depicting the comparative effectiveness of CRT-D versus ICD across the continuous range of PR intervals for the primary end point of HF hospitalization or death. CRT indicates cardiac resynchronization therapy; CRT-D, CRT with defibrillator; HF, heart failure; and ICD, implantable cardioverter defibrillator.
DISCUSSION
In this nationally representative study, we report a series of key observations underscoring the importance of the PR interval in older CRT-eligible patients. First, we demonstrated that 15% of CRT-eligible patients have a PR interval ≥230 ms, and these patients have a higher comorbidity burden including diabetes mellitus, atrial arrhythmias, chronic kidney disease, and ischemic heart disease, in comparison with patients with a PR<230 ms. Second, we demonstrated that after adjustment for increased comorbidity burden, a PR≥230 ms was associated with an increased risk of HF hospitalization and death among patients implanted with a CRT-D; it is interesting to note that a prolonged PR interval was not associated with an increased risk of HF hospitalization and death among those implanted with an ICD. In a comparative effectiveness analysis, we demonstrated that CRT-D use (versus ICD) was only associated with a reduction in HF hospitalization and death among those with a PR<230 ms, a finding that was consistent among LBBB patients. CRT-D (versus ICD only) use was not associated with the primary end point among non-LBBB patients, regardless of the PR interval.
The lack of an independent association between a prolonged PR interval and outcomes in the ICD-only group suggests that, in isolation, a prolonged PR interval is not itself harmful in this population (at least in the presence of an ICD), but rather is a marker of accumulated comorbidities. However, a prolonged PR interval either directly or indirectly appears to be associated with reduced real-world comparative effectiveness of CRT-D (versus ICD), even in LBBB patients, the subgroup of patients who have historically been most likely to benefit.
Prior studies on the association between PR interval and outcomes in CRT have reported conflicting findings. A retrospective analysis of CRT recipients in the MIRACLE trial (Multicenter InSync Randomized Clinical Evaluation) and MIRACLE ICD studies demonstrated that the absence of first-degree heart block was associated with an increased likelihood of New York Heart Association class improvement among those in MIRACLE, but not in MIRACLE ICD, despite nearly identical enrollment criteria.11 CARE-HF investigators (Cardiac Resynchronization – Heart Failure) found that a prolonged PR interval was an independent predictor of death or unplanned cardiovascular hospitalization among the entire study cohort (medical and CRT arms).9 In an analysis of the COMPANION trial (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure), a PR≥200 ms was a predictor of increased risk of HF hospitalization or death among control but not CRT patients.7 It is interesting to note that the COMPANION investigators also found that CRT effectiveness was greater in the PR≥200 ms group (0.54, P<0.01) than in the PR<200 ms group (0.71, P=0.02), although there was not a significant interaction between treatment, PR interval, and outcomes (P=0.17). In a retrospective analysis of the non-LBBB patients from the MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy), CRT use in patients with a PR≥230 ms was associated with a 73% reduction in HF hospitalization or death, whereas there was a trend toward increased risk among those with a shorter PR interval (Pinteraction<0.001).6 In a secondary analysis of the RethinQ study (Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS), CRT was associated with improvements in Vo2max, ejection fraction, and New York Heart Association class, exclusively among those with a PR≥180 ms.8 A retrospective analysis from the Mayo Clinic reported that patients with a PR>200 ms had lower rates of reverse remodeling and a trend toward increased mortality.12 Last, a recent single-center study reported that a PR>200 ms was associated with an increased risk of HF hospitalization and reduced reverse remodeling, particularly among those with a non-LBBB QRS morphology.10
The varied findings among studies assessing PR interval in CRT recipients may be, at least in part, related to physiologically plausible mechanisms relating a prolonged PR interval to outcomes in CRT. A prolonged PR interval is associated with a number of factors that have been associated with reduced CRT response (including male sex, ischemic heart disease, atrial fibrillation, and diabetes mellitus) and may be a marker for increased ventricular fibrosis and scar. Thus, it is possible that a prolonged PR interval is a marker of a ventricular substrate that is less amenable to resynchronization. Second, a prolonged PR interval reflects a combination of intrinsic intra-atrial and atrioventricular conduction, both of which impact diastolic filling. A prolonged PR interval is often considered detrimental to diastolic filling because it leads to a decrease in diastolic filling time. However, patients with a prolonged PR interval often have delayed interatrial conduction times22 and thus may benefit from a longer PR interval because it may allow for complete atrial emptying during atrial systole. Thus, the interaction between a prolonged PR, CRT, diastolic filling, and outcomes, is likely complex, depending, at least in part, on atrial conduction times and the programmed AV delay.
Echocardiogram guided AV optimization was used in MIRACLE, CARE-HF, and a single-center report,10,23,24 and ECG-based optimization (on the basis of QRS and AV intervals) was used in COMPANION and MADIT-CRT.25,26 It is interesting to note that studies using the ECG-guided approach to AV optimization in standard CRT patients found that CRT was associated with improved outcomes in long-PR patients. The RethinQ analysis included a very different population (ie, narrow-QRS patients) than in the current study (ie, wide-QRS patients) making comparisons difficult.8 CRT devices in the Mayo Clinic report were programmed with a fixed AV delay (100 ms sensed and 130 ms paced AV delays).12 Our study did not demonstrate that a prolonged PR interval was a marker of favorable outcomes in non-LBBB as was demonstrated in a retrospective analysis of the MADIT-CRT trial6; this discrepancy may reflect a difference between AV delay programming strategies used in MADIT-CRT versus the real world.
In contrast to our study, analyses of the COMPANION and CARE-HF studies demonstrated an independent association between a prolonged PR interval and worse outcomes among those without CRT. These differences may be related to (1) the absence of ICD therapy in COMPANION and CARE-HF subgroups and (2) more complete adjustment for baseline comorbidities associated with a prolonged PR interval in our study.
Although AV optimization is a physiologically plausible strategy for improving outcomes among patients with a prolonged PR interval, multiple randomized studies of AV optimization have failed to show efficacy.27–29 It is notable that these studies included patients with both long and short PR intervals and 2 studies27,29 included concomitant ventriculo-ventricular optimization and compared a device-based algorithm with echocardiogram-guided optimization (ie, both groups received AV optimizations). It is possible that AV optimization may provide benefit to at least some patients with a prolonged PR interval in comparison with no optimization; however, this needs to be proven by randomized clinical trials.
Limitations
There are important limitations associated with this study. Treatment (CRT-D versus ICD) was not randomized and it is not known why certain CRT-eligible individuals did not receive CRT; although we used robust statistical methods to account for differences between groups, we cannot rule out the potential for residual confounding. Specifically, it is possible that receipt of an ICD rather than CRT could be related to provider inexperience or other factors associated with poor response to CRT. Adequate adjustment may also be affected by the limited data granularity associated with registry studies. However, the well-balanced characteristics among the treatment groups in the cohorts resulting from the inverse probability weighted estimator analyses and the lack of association between tested treatment groups and the falsification end points suggest that our adjustment techniques were adequate. NCDR ICD Registry data are obtained from individual sites and may be subject to inaccuracies that could affect our results; however, we note that a prior analysis suggested >90% accuracy for data fields.17 The study population included adults ≥65 years of age with fee-for-service Medicare, and therefore the results may not be generalizable to younger individuals or those with different insurance. Although HF hospitalization was defined using claims data and not blinded adjudication, a recent study demonstrated excellent agreement between Medicare claims and adjudicated HF hospitalizations.30 The spline function assessing the comparative effectiveness of CRT-D versus ICD could not be constructed using an IPW model because of the inability to achieve create balance among treatment groups, necessitating the use of a multivariable model that may be more prone to residual confounding; as such, although illustrative, this plot cannot be used to determine the correct threshold at which CRT-D may no longer demonstrate comparative effectiveness. We did not have data on many potentially important device-related parameters, including left ventricular lead position, long-term lead integrity, longitudinal medical therapy, arrhythmia burden, percent biventricular pacing, and a variety of programming parameters (at baseline or during follow-up). It is notable that we did not have information regarding the programmed sensed or paced AV delays or use of AV optimization that may be important modifying factors in the relationship between PR interval, CRT, and outcomes.
Clinical Implications and Future Directions
This study demonstrates that CRT patients with a prolonged PR interval are at increased risk for adverse outcomes. Patients with a prolonged PR interval may require special consideration (ie, individualized AV delay programming, possibly via AV optimization) to optimally correct AV dyssynchrony and reduce diastolic mitral regurgitation without impairing active ventricular filling (ie, A-wave truncation) in efforts to maximize CRT response. Although prior work has suggested that routine echocardiogram and ECG-guided AV optimization may not be a necessary strategy, in general, our current study suggests that further research on AV delay programming and AV optimization in patients with a prolonged PR interval is warranted. It is possible that, with optimal AV delay programming, CRT may have the capacity to significantly improve AV synchrony, diastolic filling, and CRT outcomes, among long-PR patients with a variety of QRS morphologies.
CONCLUSIONS
A PR≥230 ms is present in 15% of CRT-eligible patients and is associated with increased rates of HF hospitalization and death among CRT-D patients. The comparative effectiveness of CRT-D (versus ICD) appears to be significantly reduced among patients with a PR≥230 ms. More research is needed to better understand the most favorable programming parameters and AV optimization strategies in CRT patients with a PR interval of ≥230 ms to improve the outcomes of these patients.
Supplementary Material
Clinical Perspective.
What Is New?
The association between PR prolongation and outcomes among cardiac resynchronization therapy (CRT) patients is controversial because of multiple conflicting reports.
This study represents the largest published study on the association between PR interval, CRT with defibrillator versus implantable cardioverter defibrillator, and outcomes, and reflects real-world (as opposed to clinical trial) outcomes.
In this study, a PR≥230 ms was associated with increased rates of heart failure hospitalization or death among CRT with defibrillator but not implantable cardioverter defibrillator patients.
The real-world comparative effectiveness of CRT with defibrillator (versus implantable cardioverter defibrillator) was significantly less among patients with a PR≥230 ms than among patients with a PR<230 ms.
What Are the Clinical Implications?
These findings may be attributable to the association between a prolonged PR interval and factors associated with lower rates of CRT response (eg, non–left bundle-branch block morphology, ischemic heart disease, atrial arrhythmias) and the association between delayed atrioventricular conduction, disordered diastolic filling, and contemporary CRT programming strategies.
Current real-world CRT strategies do not appear to be sufficient for patients with a prolonged PR interval.
CRT patients with a prolonged PR interval are at high risk for poor outcomes and merit close follow-up and consideration of atrioventricular optimization.
Acknowledgments
The ICD Registry is an initiative of the American College of Cardiology with partnering support from the Heart Rhythm Society.
SOURCE OF FUNDING
This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry. The views expressed in this article represent those of the authors and do not necessarily represent the official views of the National Cardiovascular Data Registry or its associated professional societies identified at CVQuality.ACC.org/NCDR. Dr Friedman is funded by the National Institutes of Health T 32 training grant HL069749.
Dr Curtis owns stock in Medtronic (significant), receives research funding from Boston Scientific (significant), and receives salary support from the American College of Cardiology to provide data analytic services (significant). Dr Daubert reports: research grants from Biosense Webster, Medtronic, Boston Scientific, and Gilead (all significant); honoraria, advisory board, or consultation fees from ARCA Biopharma, Biosense-Webster, Biotronik, Boston Scientific, Cardiofocus, Gilead, Medtronic, Orexigen, St. Jude, and Vytronus (all modest); and fellowship support to Duke University provided by Biosense-Webster, Boston Scientific, Medtronic, and St. Jude (all significant). Dr Friedman has received educational grants from Boston Scientific (modest) and St. Jude (modest), research grants from the National Cardiovascular Data Registry (significant), and is funded by the National Institutes of Health T 32 training grant HL069749-13 (significant).
Footnotes
DISCLOSURES
Drs Al-Khatib, Bao, and Spatz have nothing to disclose.
Guest Editor for this article was N.A. Mark Estes III, MD.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.022913/-/DC1.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Circulation is available at http://circ.ahajournals.org.
References
- 1.Dupont M, Rickard J, Baranowski B, Varma N, Dresing T, Gabi A, Finucan M, Mullens W, Wilkoff BL, Tang WH. Differential response to cardiac resynchronization therapy and clinical outcomes according to QRS morphology and QRS duration. J Am Coll Cardiol. 2012;60:592–598. doi: 10.1016/j.jacc.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 2.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL, Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 3.Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ, MADIT-CRT Investigators Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 4.Peterson PN, Greiner MA, Qualls LG, Al-Khatib SM, Curtis JP, Fonarow GC, Hammill SC, Heidenreich PA, Hammill BG, Piccini JP, Hernandez AF, Curtis LH, Masoudi FA. QRS duration, bundle-branch block morphology, and outcomes among older patients with heart failure receiving cardiac resynchronization therapy. JAMA. 2013;310:617–626. doi: 10.1001/jama.2013.8641. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 6.Kutyifa V, Stockburger M, Daubert JP, Holmqvist F, Olshansky B, Schuger C, Klein H, Goldenberg I, Brenyo A, McNitt S, Merkely B, Zareba W, Moss AJ. PR interval identifies clinical response in patients with non-left bundle branch block: a Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ Arrhythm Electrophysiol. 2014;7:645–651. doi: 10.1161/CIRCEP.113.001299. [DOI] [PubMed] [Google Scholar]
- 7.Olshansky B, Day JD, Sullivan RM, Yong P, Galle E, Steinberg JS. Does cardiac resynchronization therapy provide unrecognized benefit in patients with prolonged PR intervals? The impact of restoring atrioventricular synchrony: an analysis from the COMPANION Trial. Heart Rhythm. 2012;9:34–39. doi: 10.1016/j.hrthm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Joshi NP, Stopper MM, Li J, Beshai JF, Pavri BB. Impact of baseline PR interval on cardiac resynchronization therapy outcomes in patients with narrow QRS complexes: an analysis of the ReThinQ Trial. J Interv Card Electrophysiol. 2015;43:145–149. doi: 10.1007/s10840-015-9999-y. [DOI] [PubMed] [Google Scholar]
- 9.Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, Freemantle N, Cleland JG, Tavazzi L, Daubert C, CARE-HF investigators Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705. doi: 10.1093/eurjhf/hfp074. [DOI] [PubMed] [Google Scholar]
- 10.Januszkiewicz Ł, Vegh E, Borgquist R, Bose A, Sharma A, Orencole M, Mela T, Singh JP, Parks KA. Prognostic implication of baseline PR interval in cardiac resynchronization therapy recipients. Heart Rhythm. 2015;12:2256–2262. doi: 10.1016/j.hrthm.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Pires LA, Abraham WT, Young JB, Johnson KM, MIRACLE and MIRACLE-ICD Investigators Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) trials. Am Heart J. 2006;151:837–843. doi: 10.1016/j.ahj.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Wu JH, Asirvatham SJ, Del Carpio Munoz F, Webster T, Brooke KL, Hodge DO, Wiste HJ, Friedman PA, Cha YM. Effects of atrioventricular conduction delay on the outcome of cardiac resynchronization therapy. J Electrocardiol. 2014;47:930–935. doi: 10.1016/j.jelectrocard.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Kimura K, Miyazaki N, Tochikubo O, Usui T, Kashiwagi M, Ishii M. Diastolic mitral regurgitation in patients with first-degree atrioventricular block. Pacing Clin Electrophysiol. 1992;15(11 pt 2):1927–1931. doi: 10.1111/j.1540-8159.1992.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 14.Schnittger I, Appleton CP, Hatle LK, Popp RL. Diastolic mitral and tricuspid regurgitation by Doppler echocardiography in patients with atrioventricular block: new insight into the mechanism of atrioventricular valve closure. J Am Coll Cardiol. 1988;11:83–88. doi: 10.1016/0735-1097(88)90170-2. [DOI] [PubMed] [Google Scholar]
- 15.Hammill SC, Kremers MS, Stevenson LW, Heidenreich PA, Lang CM, Curtis JP, Wang Y, Berul CI, Kadish AH, Al-Khatib SM, Pina IL, Walsh MN, Mirro MJ, Lindsay BD, Reynolds MR, Pontzer K, Blum L, Masoudi F, Rumsfeld J, Brindis RG. Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–1345. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers del M, Reynolds MR, Heidenreich PA, Al-Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA, NCDR Science and Quality Oversight Committee Data Quality Workgroup The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DJ, Singh JP, Curtis JP, Tang WH, Bao H, Spatz ES, Hernandez AF, Patel UD, Al-Khatib SM. Comparative Effectiveness of CRT-D Versus Defibrillator Alone in HF Patients With Moderate-to-Severe Chronic Kidney Disease. J Am Coll Cardiol. 2015;66:2618–2629. doi: 10.1016/j.jacc.2015.09.097. [DOI] [PubMed] [Google Scholar]
- 20.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. doi: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Waggoner AD, Kalathiveetil S, Spence KE, Dávila-Román VG, de las Fuentes L. Interatrial conduction time and left atrial function in patients with left ventricular systolic dysfunction: effects of cardiac resynchronization therapy. J Am Soc Echocardiogr. 2009;22:472–477. doi: 10.1016/j.echo.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham WT. Rationale and design of a randomized clinical trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with advanced heart failure: the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) J Card Fail. 2000;6:369–380. doi: 10.1054/jcaf.2000.20841. [DOI] [PubMed] [Google Scholar]
- 24.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Klein W, Tavazzi L, CARE-HF study Steering Committee and Investigators The CARE-HF study (CArdiac REsynchronisation in Heart Failure study): rationale, design and end-points. Eur J Heart Fail. 2001;3:481–489. doi: 10.1016/s1388-9842(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 25.Brenyo A, Kutyifa V, Moss AJ, Mathias A, Barsheshet A, Pouleur AC, Knappe D, McNitt S, Polonsky B, Huang DT, Solomon SD, Zareba W, Goldenberg I. Atrioventricular delay programming and the benefit of cardiac resynchronization therapy in MADIT-CRT. Heart Rhythm. 2013;10:1136–1143. doi: 10.1016/j.hrthm.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 27.Abraham WT, Gras D, Yu CM, Guzzo L, Gupta MS, FREEDOM Steering Committee Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159:944–948.e1. doi: 10.1016/j.ahj.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 29.Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, Gasparini M, Starling RC, Milasinovic G, Rogers T, Sambelashvili A, Gorcsan J, 3rd, Houmsse M, Adaptive CRT Study Investigators Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9:1807–1814. doi: 10.1016/j.hrthm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM, Chambless L. Identification of heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2016;22:48–55. doi: 10.1016/j.cardfail.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


