Abstract
The synaptopodin family of proteins consists of at least 3 members: synaptopodin, the synaptopodin 2 proteins, and the synaptopodin 2-like proteins. Each family member has at least 3 isoforms that are produced by alternative splicing. Synaptopodin family members are basic proteins that are rich in proline and have little regular 2° or 3° structure at physiological temperature, pH and ionic strength. Like other natively unfolded proteins, synaptopodin family members have multiple binding partners including actin and other actin-binding proteins. Several members of the synaptopodin family have been shown to stimulate actin polymerization and to bundle actin filaments either on their own or in collaboration with other proteins. Synaptopodin 2 has been shown to accelerate nucleation of actin filament formation and to induce actin bundling. The actin polymerization activity is inhibited by Ca2+-calmodulin. Synaptopodin 2 proteins are localized in Z-bands of striated and heart muscle and dense bodies of smooth muscle cells. Depending on the developmental status and stress, at least one member of the synaptopodin family can occupy nuclei of some cells. Members of the synaptopodin 2 subfamily have been implicated in cancers.
Keywords: Synaptopodin, Actin polymerization, Cancer, Actin bundling, Nuclear transport, Natively unfolded
Introduction
Actin is a track for myosin motors, it is a major component of the cytoskeleton and is responsible for amoeboid movement and membrane projections of various types. Although actin serves many functions it would appear that sufficient actin binding proteins have been identified to take care of those activities. So why do new actin binding proteins keep turning up? Of what use is another protein that nucleates actin polymerization and that bundles actin? Why is it necessary to have another natively unfolded actin binding protein that interacts with several other actin binding proteins? Why does this relative newcomer have several splice forms and why does it shuttle between the nucleus and cytoplasm? We examine here a cross-section of studies that describe this fascinating and poorly understood family of proteins, the synaptopodin family. Because some aspects of other members of the synaptopodin family have previously been reviewed (Deller et al. 2007), we place emphasis here on synaptopodin 2.
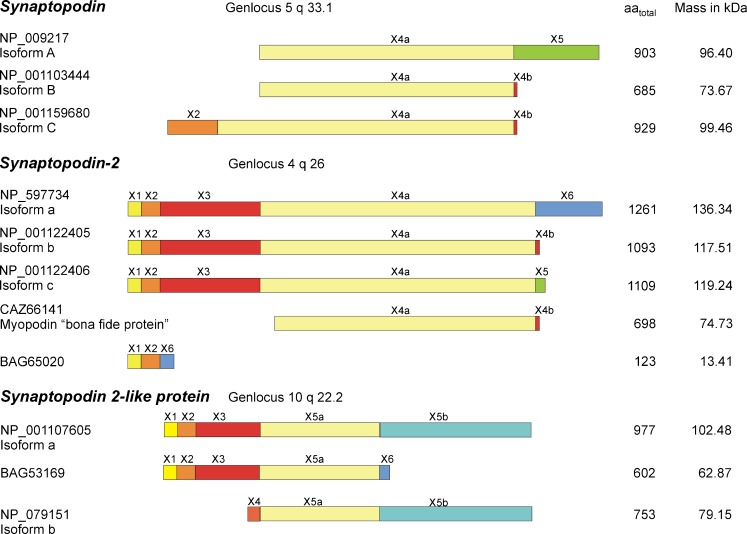
Synaptopodin was first discovered as an actin-associated protein in kidney podocytes and postsynaptic densities of telencephalic synapses (Mundel et al. 1997). Other members of the synaptopodin family were discovered in avian smooth muscle (fesselin or synaptopodin 2) (Leinweber et al. 1999) and in heart and skeletal muscle (myopodin, genethonin-2 or synaptopodin 2) (Weins et al. 2001). The last member of the synaptopodin family of proteins, the synaptopodin 2-like protein is found in heart and skeletal muscle tissue and is better known under the names tritopodin (Claeys et al. 2009) or CHAP (Beqqali et al. 2010). These family members are compared in Fig. 1. In this manuscript, we will use the term synaptopodin to mean the founding member of this family. Synaptopodin 2 will be used to signify either the mammalian or avian muscle form. Synaptopodin 2-like refers to tritopodin and CHAP.
Fig. 1.
Primary protein structure of synaptopodin family members and their splice forms. Each of the three members of the synaptopodin family can exist in at least three different isoforms produced by alternative splicing of the N- and C-terminal exons. The figure shows only the composition of the proteins, therefore non-coding exons 1 and 3 in synaptopodin are not shown. Except for the unnamed protein that is listed with the NCBI accession number BAG65020 the isoforms of each subfamily share a common core region
Physical properties and isolation
Critical to the understanding of the function of synaptopodin’s is the appreciation of the fact that they have little secondary or tertiary structure under native conditions. Fesselin or avian Synaptopodin 2 has CD spectra characteristic of a random coil, an excessively large hydrated volume, and a resistance to denaturation by guanidine-HCl or heat (Khaymina et al. 2007). Avian synaptopodin 2 actually adopts a more regular structure as the temperature is increased to 85°C.
The synaptopodins are basic proteins with isoelectric points of around 9.3 for avian (Leinweber et al. 1999), human and murine synaptopodin family members (Weins et al. 2001; Mundel et al. 1997). Synaptopodin family members also have high proline contents, ranging from 10.6% in human synaptopodin 2, isoform c, to 17.2% in synaptopodin, isoform A.
Analyses of the primary structure of the synaptopodin’s also predict large unfolded regions. Except for the unnamed protein BAG65020 that contains a PDZ domain and a co-repressor motif for the retinoid X receptor RXR, the rest of the synaptopodin family members are predicted to be 70–83% unfolded by the program PONDR.
Synaptopodin 2 has properties of a hub protein. Hubs bind to many partners and have many connections in signaling cascades. The flexibility of loosely structured proteins may enable them to adapt to different binding sites. This flexibility generally results in readily reversible binding (Shoemaker et al. 2000; Tompa and Fuxreiter 2008). Hub proteins include BRCA1 (Breast cancer 1, early-onset), p53, caldesmon, α-synuclein and calmodulin (Tompa 2009).
Roughly 10% of eukaryotic proteins are largely unfolded under physiological conditions while many more have significant unfolded regions (Le Gall et al. 2007; Sickmeier et al. 2007). Several actin binding proteins contain large unstructured regions. Those proteins include caldesmon (Permyakov et al. 2003), MARCKS (myristoylated alanine-rich C kinase substrate) (Tapp et al. 2005), ARG (Abl-related gene) (Buffa et al. 2007), and dematin (Chen et al. 2009). In fact, proteins involved with the cytoskeleton have a greater than normal tendency to have large unfolded regions. Disorder is associated with other biological functions including regulation of transcription, signal transduction/regulation of the cell cycle, function of ribosomes and chromatin, and mRNA processing (Tompa 2009). These patterns may serve as guides for future investigations of synaptopodin family members.
Another clue to the biological role of the synaptopodin family is that proteins involved in cancer contain a larger than normal fraction of members with large unfolded regions (Iakoucheva et al. 2002). Members of the synaptopodin 2 subfamily have been implicated in some cancers (see below). Examples of other known cancer associated disordered proteins are p53, Cip/Kip (cyclin-dependent kinase inhibitors), BRCA1, and securin (Tompa 2009).
Synaptopodins are difficult to extract from tissues. Avian synaptopodin 2 or fesselin, the most readily purified member of the synaptopodin family, is extracted by heat treatment of a suspension of myofibrils (Leinweber et al. 1999). Two protocols for preparing synaptopodin 2 from gizzard tissue have been described (Leinweber et al. 1999; Kolakowski et al. 2004). Isolated synaptopodin 2 generally contains two polypeptide chains with apparent molecular masses on SDS gels of 79 and 102 kDa (Leinweber et al. 1999). Synaptopodin 2 has also been prepared from rabbit stomach tissue with lower yields than from chicken or turkey gizzards (Schroeter et al. 2008).
Ligand binding
Actin was the first binding partner of synaptopodin to be identified (Mundel et al. 1997) and is probably the most important partner. Actin binding was identified by decoration of actin with synaptopodin antibodies in cultured mouse podocytes and the postsynaptic region of dendrites; depolymerization of actin with cytochalasin B eliminated the synaptopodin pattern. Actin binding was also demonstrated by using an actin co-sedimentation assay with 35 S labeled synaptopodin that was synthesized in a reticulocyte lysate system (Kremerskothen et al. 2005). Transiently expressed GFP-fusion constructs of synaptopodin were used to demonstrate that residues 391–483 and 752–903 of synaptopodin were critical for actin binding (Kremerskothen et al. 2005). Myopodin has a novel actin binding site (Weins et al. 2001) that was identified by producing truncated fragments from myopodin. The smallest fragment that bound to F-actin contained residues 410–563 of mouse myopodin (Acc. No. CAC67798).
Quantitative binding studies were done with synaptopodin 2 isolated from avian gizzards (Leinweber et al. 1999) and rabbit stomach. Measurements of avian gizzard synaptopodin 2 binding to actin were made with a mixture containing 66% of the 79-kDa splice form and 34% of the 103-kDa form. Binding to pyrene-labeled actin (pH 7, 116 mM ionic strength, 20°C) was described by the McGhee and von Hippel equation (McGhee and von Hippel 1974):
This form of the equation corrects an error in the original publication. This equation describes binding of a ligand to multiple protomers of a filament. Theta is the ratio of bound ligand to total actin and Lf is the free ligand concentration. Each molecule of synaptopodin 2 binds to approximately 4 actin protomers in F-actin (n = 4). The affinity to a single isolated site of 4 actin protomers is given by K = 2 × 106 M−1. Binding has slight positive cooperativity with a cooperativity parameter, ω = 1.7. The affinity of synaptopodin 2 to a binding site adjacent to an existing bound synaptopodin 2 is given by ωK or 3.4 × 106 M−1. Binding to a doubly contiguous site on actin is given by ω2 K or 5.8 × 106 M−1.
Synaptopodin 2 also binds to the monomeric form of actin, G-actin, but with an interesting difference. Binding to G-actin is regulated by Ca2+-calmodulin (Schroeter and Chalovich 2004).
Many proteins bind to a hydrophobic pocket that is available in subdomain 1 of both G- and F-actin (Dominguez 2004). This pocket is between subdomains 1 and 3 of actin and is near the reactive sulfhydryl residue in actin, cys 374. Synaptopodin 2 appears to be no exception as it displaces both myosin S1 and caldesmon from actin (Schroeter and Chalovich 2004, 2005). Synaptopodin 2 also perturbs the fluorescence of pyrene attached to cys 374 of actin (Leinweber et al. 1999). If this pattern holds for synaptopodin 2 then the actin binding region may be expected to form an α helix that contains exposed hydrophobic side chains.
Synaptopodin 2 binds to smooth muscle myosin with an affinity of about 2 × 106 M−1 (50 mM ionic strength, pH 7, 10°C) (Schroeter and Chalovich 2005). Each myosin molecule bound to 2 molecules of synaptopodin 2; the myosin head groups appeared to be involved in the binding.
Synaptopodin binds to the actin bundling protein α-actinin in podocytes cells (Asanuma et al. 2005). Binding of synaptopodin to α-actinin 2 and 4 was also shown in the yeast 2 hybrid system (Kremerskothen et al. 2005), and binding to α-actinin 2 was confirmed with GST pull-down assays. The 4th spectrin repeat contains the main binding determinant on α-actinin (Kremerskothen et al. 2005). The COOH terminal region of synaptopodin, residues 752–903 of isoform A, contains the major interacting site on synaptopodin.
Synaptopodin 2 also binds to α-actinin. That interaction has been shown to involve the central spectrin domain repeat region of α-actinin (Pham and Chalovich 2006). In solution the affinity for α-actinin is about 4 × 107 M−1 with a stoichiometry of 1:1 (116 mM ionic strength, pH 7, 25°C).
Synaptopodin 2 and α-actinin are co-localized in dense bodies and Z-lines (Renegar et al. 2009), while synaptopodin and α-actinin colocalize in kidney podocytes and the hippocampus (Asanuma et al. 2005). Synaptopodin family members might be involved in the organization and anchoring of actin in the cell and might be necessary for the correct localization of α-actinin. That is supported by recent findings that synaptopodin 2 expression precedes α-actinin expression (Linnemann et al. 2010). On the other hand, overexpression of CHAP resulted in disorganization of α-actinin (Beqqali et al. 2010).
Myopodin was shown to bind to the adaptor protein zyxin by yeast 2 hybrid analyses and co-immunoprecipitation assays. Deletion mutants of myopodin (Yu and Luo 2006) were used to show that 19 residues located in the C-terminus of human myopodin form a binding site for zyxin. Zyxin and myopodin were found to be localized at the same sites within PC3 cells; those sites included both nuclear and cytoplasmic locations.
A potentially important interaction of synaptopodin 2 is that with calmodulin. Both avian synaptopodin 2 (Schroeter and Chalovich 2004; Kolakowski et al. 2004) and human synaptopodin 2 bind to calmodulin (Shen et al. 2005). Binding to synaptopodin 2 requires Ca2+ (Schroeter and Chalovich 2004). This was demonstrated by calmodulin affinity chromatography and by fluorescence spectroscopy with MIANS-labeled calmodulin. The association constant in Ca2+ was ≈8 × 108 M–1 with 2 molecules of calmodulin bound per molecule of avian synaptopodin 2 (105 mM ionic strength, 25°C, pH 7). This affinity is typical for proteins regulated by Ca2+-calmodulin (O'Neil and DeGrado 1990). The stoichiometry of binding could not be determined with certainty by the fluorescence assay used and should be verified directly.
The binding of F-actin to synaptopodin 2 reduces the affinity to Ca2+-calmodulin to a value estimated to be 1.4 × 108 M−1.
The kinetics of binding of synaptopodin 2 to MIANS-labeled calmodulin are biexponential suggesting that the initial binding reaction is followed by a conformational change. That conformational change is probably linked to the change in avian synaptopodin 2 that weakens its interaction with F-actin. The initial association is a very rapid reaction; the second-order rate constant for the reaction is about 108 M−1 s−1. The subsequent isomerization had an apparent rate constant of 22/sec at 15°C in a buffer containing 200 mM KCl. The complex of synaptopodin 2 and Ca2+-calmodulin was readily reversed by chasing with an excess of unlabeled calmodulin or by sequestering Ca2+. The half-life for the dissociation in both cases was 30−35 ms indicating that the interaction of avian synaptopodin 2 with calmodulin can respond rapidly to environmental changes.
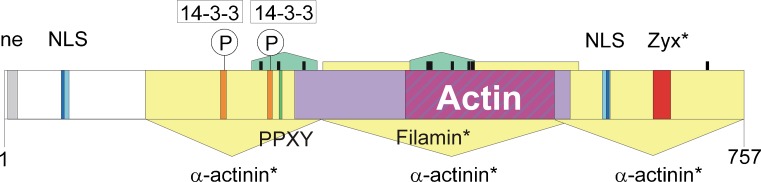
Beside 2 lysine-rich NLS sites (consensus motif K-K/R-X-K/R) myopodin provides 2 binding sites for 14-3-3 (consensus motif a: RSXpS/TXP; b: RXXXpS/TXP; see Fig. 2). These sites seem to be indispensable for effective shuttling of myopodin from the Z-line, where myopodin is bound to α-actinin, into the nucleus (Faul et al. 2005). Phosphorylation of myopodin within the 14-3-3 binding sites (S225 and T272) by protein kinase A and Ca2+/calmodulin-dependent protein kinase II abrogates the binding with α -actinin and is a sine qua non condition for 14-3-3 binding to myopodin, which is a prerequisite for binding of importin α (Faul et al. 2007). Using an importin α binding site that was not restricted to the classical NLS-consensus motif, De Ganck et al. (2005) showed nuclear localization by a singular importin α binding-motif.
Fig. 2.
Binding motifs in mouse myopodin CAC67798. This region forms the core region of synaptopodin 2 encoded by exon 4a. Motifs that have only been described in human myopodin are marked with a star. The green domains represent proline-rich stretches. The heavy black bars are PXXP motifs that are putative SH3 interacting domains. The yellow arrows show three independent α-actinin binding regions (Linnemann et al. 2010). The filamin binding domain (Linnemann et al. 2010), shown by the light violet bar contains the main actin binding region (Weins et al. 2001) (dark purple diagonals). The region marked ne has weak nuclear export activity without a classical NES-motif (Van Impe et al. 2003). NLS is the nuclear localization site; the consensus motifs are in dark blue while additional motifs (De Ganck et al. 2005) are in light blue. Binding motifs for 14-3-3 are shown along with the phosphorylation sites S225 and T272, that are targets of protein kinase A and calcium-calmodulin kinase II (Faul et al. 2005, 2007). The PPXY motif may interact with WW-domain containing proteins. Zyx is the Zyxin binding motif discovered in human myopodin (Yu and Luo 2006)
Synaptopodin 2 and synaptopodin 2-like protein contain PDZ domains of unknown function (De Ganck et al. 2008). Synaptopodin family members also contain multiple PXXP motifs that may be involved in binding to SH3-domain-containing proteins. The C-terminus of synaptopodin isoform A contains 3 PXXP motifs that bind to the SH3 domain of IRSp53 (Yanagida-Asanuma et al. 2007).
The core region of synaptopodin 2, equivalent to the protein myopodin, contains several key binding sites. Figure 2 shows the ligand binding sites in mouse myopodin CAC67798. Binding sites that were discovered in human myopodin are explicitly stated.
Actin polymerization
Synaptopodin 2 from avian gizzards stimulates the rate of polymerization of both rabbit skeletal and turkey gizzard actin (Beall and Chalovich 2001). The polymerization rate increased with increasing avian synaptopodin 2 concentrations; at low ionic strength conditions 75 nM avian synaptopodin 2 increased the rate of actin polymerization by 33-fold. Avian synaptopodin 2 also increased the elongation rate by 3-fold. Synaptopodin 2 increased the critical concentration from 0.3 to 0.49 μM suggesting growth at the pointed ends of actin filaments (Schroeter et al. 2010).
Evidence for the ability of synaptopodin to polymerize actin also comes from studies in cellular systems. Synaptopodin -/- mice podocytes had an impaired ability to regenerate actin filaments following washout of cytochalasin D (Asanuma et al. 2005).
Although synaptopodin 2 stimulates actin polymerization in solution, it is thought that synaptopodin family members work together with specific isoforms of α-actinin in cells to promote actin polymerization (Asanuma et al. 2005; Beqqali et al. 2010). HEK cells do not contain appreciable α-actinin 4 or synaptopodin and have no stress fibers. Transfection of a construct of synaptopodin resulted in colocalization with actin aggregates. Transfection with α-actinin 4 produced short-branched actin filament bundles. Transfections with both α-actinin 4 and synaptopodin produced long parallel unbranched actin bundles (Asanuma et al. 2005). The suggestion was made that synaptopodin eliminates the branching activity of α-actinin and that synaptopodin elongates actin filaments induced with α-actinin. Furthermore, different synaptopodin constructs had selectivities for specific α-actinin isoforms.
The potent stimulation of actin polymerization by synaptopodin 2 is likely to be regulated. Ca2+-calmodulin inhibits the ability of synaptopodin 2 to stimulate actin polymerization apparently by inhibiting nucleation (Schroeter and Chalovich 2004). However, Ca2+-calmodulin accelerates the elongation of actin filaments to a small extent. That is, Ca2+-calmodulin-synaptopodin 2 binds to actin and these complexes can add to growing actin chains. Calmodulin stimulates the effects of synaptopodin 2 in the absence of Ca2+. The net effect is that Ca2+ has a large effect on the rate of actin polymerization in the presence of synaptopodin 2 and calmodulin.
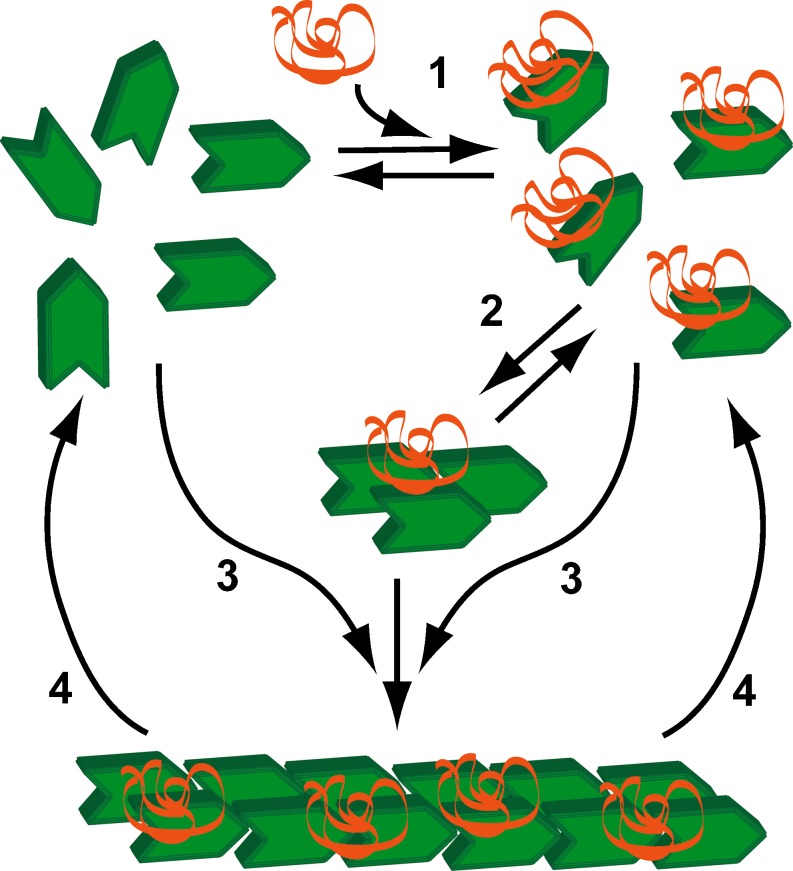
The inhibition of polymerization by Ca2+-calmodulin is in part due to a reduction in the affinity of avian synaptopodin 2 for G-actin (Schroeter and Chalovich 2004). In contrast, the affinity of Ca2+-calmodulin-synaptopodin 2 to F-actin remained high (∼108 M−1) (Schroeter and Chalovich 2004) and Ca2+-calmodulin did not reverse inhibition of actin-S1 ATPase activity by avian synaptopodin 2 (Schroeter and Chalovich 2005). A model of the synaptopodin 2 stimulated polymerization of actin is shown in Fig. 3.
Fig. 3.
Proposed effect of synaptopodin 2 on actin polymerization. 1 G-actin (green) binds to synaptopodin 2 (orange ribbon). 2 Actin-synaptopodin 2 complexes rapidly form nuclei consisting of 1 synaptopodin 2 and 3 actin monomers. 3 Elongation of synaptopodin 2 nuclei occurs rapidly; G-actin-synaptopodin 2 complexes add more rapidly than plain G-actin. 4 Actin can detach from both the barbed (fast growing) and pointed ends of actin filaments. The primary effect of Ca2+-calmodulin appears to be to reduce the rate of nucleation (step 2)
Several other actin binding proteins that stimulate actin polymerization are inhibited by Ca2+-calmodulin. These include caldesmon (Galazkiewicz et al. 1985) and myelin basic protein (Dobrowolski et al. 1986; Boggs and Rangaraj 2000). These proteins differ from synaptopodin 2 in that their binding to both F-actin and G-actin are weakened by Ca2+-calmodulin.
Synaptopodin also promotes actin stress fiber formation by an indirect mechanism (Asanuma et al. 2006). Synaptopodin blocks Smurf1-mediated ubiquinitation of RhoA. The elevated RhoA promotes actin fiber formation.
The in vivo significance of Ca2+-calmodulin binding to synaptopodin 2 is still obscure, but recent reports show that this interaction could be involved in nuclear transport. Ca2+-dependent calmodulin triggered facilitated nuclear transport has been shown for the neural cell adhesion molecule NCAM (Kleene et al. 2010) and members of the high-mobility group (HMG) box family including SRY (Kaur et al. 2010). Since synaptopodin 2 features two NLS motifs, there is even the potential of an NLS-/importin-dependent nuclear import that can be regulated by actin-dependent movement towards the nucleus, as it has been shown for the transcription factor NF-kappa B (Fazal et al. 2007). This hypothesis would bridge the observation that in vitro synaptopodin 2-induced actin polymerization is regulated by Ca2+-calmodulin.
Actin aggregation
Mixtures of synaptopodin 2 and actin become turbid at a rate that is much slower than the rate of initial binding (Leinweber et al. 1999). Aggregation of actin filaments was also noted in cells. For example, overexpression of synaptopodin in CV1 cells disrupted actin filaments and produced large aggregates (Kremerskothen et al. 2005). Also, myopodin forms actin bundles in the cytoplasm of muscle cells. Overexpression of GFP-myopodin forms loops in nuclei that are sensitive to the actin depolymerizing agent latrunculin A (Weins et al. 2001).
The cellular role of the interaction of Ca2+-calmodulin with synaptopodin 2 is unclear. Calcium is normally seen as a signal to disassemble the cytoskeleton (Janmey 1994). This occurs as crosslinking proteins are inhibited and severing proteins are activated. On the other hand, puncta (Vasioukhin et al. 2000) and stress fibers (Schaeffer et al. 2003) increase with elevated Ca2+. The picture is complex and there are not yet data showing the effect of Ca2+-calmodulin on synaptopodin 2 in cells.
Role of synaptopodin family members in cancer
Evidence for a protective role of synaptopodin 2 comes from observations that myopodin expression is decreased in urothelial cancer (Sanchez-Carbayo et al. 2003). Myopodin expression also suppresses tumor growth and invasiveness of prostate cancer cells (Jing et al. 2004). Deletion of the zyxin binding region of myopodin eliminates the anti-invasive effect of myopodin (Yu and Luo 2006).
In contrast, overexpressioin of myopodin in human endothelial kidney cells (HEK) and in mouse C2C12 myoblast cells made the cells more invasive in a collagen matrix (Van Impe et al. 2003). RNA interference was used to reduce myopodin expression in human cancer cells PC3 (prostate) and RT4 (bladder) (De Ganck et al. 2009). Reduced invasiveness was observed in type I collagen matrix and in the Matrigel invasion assay. Myopodin knockdown cells exhibited slower wound healing in a confluent layer of cells. E-cadherin-dependent cell–cell contacts were also increased in the myopodin knockdown cells. Based on these results, myopodin was thought to be a tumor activator (De Ganck et al. 2009). It is important to note that the variant of myopodin used in these studies did not enter the nucleus. Thus, nuclear myopodin may be a tumor suppressor while cytoplasmic myopodin may be a tumor activator. Alternatively, the role of myopodin in cancer may differ with the type of cells and with the stage of the cancer.
There is another level of complexity regarding the role of actin bundling proteins in cancer. Metastasis requires cell motility, a property normally associated with plasticity of actin filaments. However, there is ample evidence that bundling of actin filaments is a prerequisite for formation of invadosomes. Elevated levels of some actin bundling proteins are associated with more aggressive cancer phenotypes. High levels of the crosslinking protein fascin are associated with low survival rates (Donnelly et al. 1993; Li et al. 2010; Iguchi et al. 2009). Blocking the effects of fascin inhibits metastatic tumor cell migration (Chen et al. 2010). Fascin is expressed at the invasive front of primary human colon cancer cells but is not present in metastases (Vignjevic et al. 2007). Apparently, fascin is essential for migration of cancer cells. It appears that actin filament formation in the specialized invadosome (Albiges-Rizo et al. 2009) is critical for the metastatic phenotype.
An indicator of poor survival from primary bladder cancer is methylation of the enriched CpG island at the transcription start site of myopodin (Cebrian et al. 2008). The extent of methylation was associated with increasing tumor stage and grade. Hypermethylation was associated with low nuclear myopodin expression but was unrelated to cytoplasmic levels.
Synaptopodin family members in the nucleus
Myopodin and CHAP, but not synaptopodin, can translocate into the nucleus and induce actin bundling (Weins et al. 2001; Beqqali et al. 2010). Myopodin is expressed primarily in nuclei of proliferating myoblast cells. Cytoplasmic myopodin appears within hours of differentiation of myoblasts into myotubes and is found in Z-lines of mature myotubes (Weins et al. 2001). Heat shock (43°C) of C2C12 cells causes the accumulation of nuclear myopodin and formation of actin loops in the nucleus (Weins et al. 2001).
Faul et al. (2005) proposed the following sequence of events to describe nuclear transport of myopodin: serine/threonine kinases phosphorylate two sites on myopodin, S225 and T272. Phosphorylated myopodin then binds to 14-3-3. The 2 nuclear localization signal sites bind to importin α which mediates the nuclear uptake of myopodin.
Clues to the potential roles of nuclear myopodin come from reviews on the function of nuclear actin (Gieni and Hendzel 2009; Castano et al. 2010). Nuclear actin is involved in chromatin remodeling. The transcriptional activator MAL senses changes in cellular G-actin content. All three RNA polymerases bind to actin; this binding seems to be important for transcription. The pre-mRNA’s remain attached to actin. Thus, it is apparent that actin and its associated proteins play key roles in transcription. In addition to synaptopodin 2, nuclei contain various G-actin binding proteins and actin polymerizing proteins such as Arp2/3 (Campellone and Welch 2010).
Roles of synaptopodin family members
So why do new actin binding proteins keep turning up? The obvious answer is that we do not fully appreciate the biological roles of actin. Because a limited amount of cellular actin carries out many functions, one actin structure may form at the expense of another. Thus, a specific cadre of actin binding proteins may be associated with specific structures. Synaptopodin family members are in Z-disks, dense bodies, podocytes and the spine apparatus (Kremerskothen et al. 2005). The biological function of these structures is diverse and ranges from organization of the cytoskeleton to learning (spine apparatus) (Deller et al. 2003). The association of synaptopodin family members with particular tissues and organelles appears to be splice form-specific thus further assuring segregation and control.
The abilities of some members of the synaptopodin family to stimulate actin polymerization and bundle actin and their co-localization with key actin binding proteins such as α-actinin (Papa et al. 1999) are consistent with a role as actin organizing centers. Furthermore, the natively unfolded structure facilitates binding to multiple ligands including regulatory molecules such as Ca2+-calmodulin. Synaptopodin family members may respond to other cellular signals to promote assembly or disassembly of the actin structures to which they are associated.
The distribution of synaptopodin 2 changes with the onset of cancer. Changes in cytoplasmic synaptopodin 2 may be associated with morphological changes seen in transformed cells. The function of changes in nuclear synaptopodin family members is unclear because the role of actin in the nucleus is poorly understood. If isoforms of synaptopodin 2 are involved with actin in transcription, the role of nuclear actin in cancer would be easy to rationalize. It is clear that the synaptopodin family story is incomplete, but the next chapter in this story will be interesting reading.
Acknowledgments
This work was funded by grant AR035216 from the National Institutes of Health (to J.M.C.) and a grant from the Brody Brothers Endowment (J.M.C. and M.M.S.).
References
- Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- Beall B, Chalovich JM. Fesselin, a synaptopodin-like protein, stimulates actin nucleation and polymerization. Biochemistry. 2001;40:14252–14259. doi: 10.1021/bi011806u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beqqali A, Monshouwer-Kloots J, Monteiro R, Welling M, Bakkers J, Ehler E, Verkleij A, Mummery C, Passier R. CHAP is a newly identified Z-disc protein essential for heart and skeletal muscle function. J Cell Sci. 2010;123:1141–1150. doi: 10.1242/jcs.063859. [DOI] [PubMed] [Google Scholar]
- Boggs JM, Rangaraj G. Interaction of lipid-bound myelin basic protein with actin filaments and calmodulin. Biochemistry. 2000;39:7799–7806. doi: 10.1021/bi0002129. [DOI] [PubMed] [Google Scholar]
- Buffa P, Manzella L, Consoli ML, Messina A, Vigneri P. Modelling of the ABL and ARG proteins predicts two functionally critical regions that are natively unfolded. Proteins. 2007;67:1–11. doi: 10.1002/prot.21161. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano E, Philimonenko VV, Kahle M, Fukalova J, Kalendova A, Yildirim S, Dzijak R, Dingova-Krasna H, Hozak P. Actin complexes in the cell nucleus: new stones in an old field. Histochem Cell Biol. 2010;133:607–626. doi: 10.1007/s00418-010-0701-2. [DOI] [PubMed] [Google Scholar]
- Cebrian V, Alvarez M, Aleman A, Palou J, Bellmunt J, Gonzalez-Peramato P, Cordon-Cardo C, Garcia J, Piulats JM, Sanchez-Carbayo M. Discovery of myopodin methylation in bladder cancer. J Pathol. 2008;216:111–119. doi: 10.1002/path.2390. [DOI] [PubMed] [Google Scholar]
- Chen L, Jiang ZG, Khan AA, Chishti AH, McKnight CJ. Dematin exhibits a natively unfolded core domain and an independently folded headpiece domain. Protein Sci. 2009;18:629–636. doi: 10.1002/pro.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang S, Jakoncic J, Zhang JJ, Huang XY. Migrastatin analogues target fascin to block tumour metastasis. Nature. 2010;464:1062–1066. doi: 10.1038/nature08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys KG, van der Ven PF, Behin A, Stojkovic T, Eymard B, Dubourg O, Laforet P, Faulkner G, Richard P, Vicart P, Romero NB, Stoltenburg G, Udd B, Fardeau M, Voit T, Furst DO. Differential involvement of sarcomeric proteins in myofibrillar myopathies: a morphological and immunohistochemical study. Acta Neuropathol. 2009;117:293–307. doi: 10.1007/s00401-008-0479-7. [DOI] [PubMed] [Google Scholar]
- De Ganck A, Hubert T, Van Impe K, Geelen D, Vandekerckhove J, De Corte V, Gettemans J. A monopartite nuclear localization sequence regulates nuclear targeting of the actin binding protein myopodin. FEBS Lett. 2005;579:6673–6680. doi: 10.1016/j.febslet.2005.10.054. [DOI] [PubMed] [Google Scholar]
- De Ganck A, De Corte V, Staes A, Gevaert K, Vandekerckhove J, Gettemans J. Multiple isoforms of the tumor suppressor myopodin are simultaneously transcribed in cancer cells. Biochem Biophys Res Commun. 2008;370:269–273. doi: 10.1016/j.bbrc.2008.03.086. [DOI] [PubMed] [Google Scholar]
- De Ganck A, De Corte V, Bruyneel E, Bracke M, Vandekerckhove J, Gettemans J. Down-regulation of myopodin expression reduces invasion and motility of PC-3 prostate cancer cells. Int J Oncol. 2009;34:1403–1409. [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci USA. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Orth CB, Del TD, Vlachos A, Burbach GJ, Drakew A, Chabanis S, Korte M, Schwegler H, Haas CA, Frotscher M. A role for synaptopodin and the spine apparatus in hippocampal synaptic plasticity. Ann Anat. 2007;189:5–16. doi: 10.1016/j.aanat.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dobrowolski Z, Osinska H, Mossakowska M, Barylko B. Ca2 + -calmodulin-dependent polymerization of actin by myelin basic protein. Eur J Cell Biol. 1986;42:17–26. [PubMed] [Google Scholar]
- Dominguez R. Actin-binding proteins - a unifying hypothesis. Trends Biochem Sci. 2004;29:572–578. doi: 10.1016/j.tibs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Donnelly SF, Pocklington MJ, Pallotta D, Orr E. A proline-rich protein, verprolin, involved in cytoskeletal organization and cellular growth in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1993;10:585–596. doi: 10.1111/j.1365-2958.1993.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Faul C, Hüttelmaier S, Oh J, Hachet V, Singer RH, Mundel P. Promotion of importin alpha-mediated nuclear import by the phosphorylation-dependent binding of cargo protein to 14-3-3. J Cell Biol. 2005;169:415–424. doi: 10.1083/jcb.200411169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007;27:8215–8227. doi: 10.1128/MCB.00950-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal FM, Minhajuddin M, Bijli KM, McGrath JL, Rahman A. Evidence for actin cytoskeleton-dependent and –independent pathways for RelA/p65 nuclear translocation in endothelial cells. J Biol Chem. 2007;282:3940–3950. doi: 10.1074/jbc.M608074200. [DOI] [PubMed] [Google Scholar]
- Galazkiewicz B, Mossakowska M, Osinska H, Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985;184:144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Gieni RS, Hendzel MJ. Actin dynamics and functions in the interphase nucleus: moving toward an understanding of nuclear polymeric actin. Biochem Cell Biol. 2009;87:283–306. doi: 10.1139/O08-133. [DOI] [PubMed] [Google Scholar]
- Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK. Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol. 2002;323:573–584. doi: 10.1016/S0022-2836(02)00969-5. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Aishima S, Umeda K, Sanefuji K, Fujita N, Sugimachi K, Gion T, Taketomi A, Maehara Y, Tsuneyoshi M. Fascin expression in progression and prognosis of hepatocellular carcinoma. J Surg Oncol. 2009;100:575–579. doi: 10.1002/jso.21377. [DOI] [PubMed] [Google Scholar]
- Janmey PA. Phosphoinosites and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, Cieply K, Wells A, Luo J-H. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004;164:1799–1806. doi: 10.1016/S0002-9440(10)63738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur GA, Delluc-Clavieres A, Poon IK, Forwood JK, Glover DJ, Jans DA. Calmodulin-dependent nuclear import of HMG-box family nclear factors: importance of the role of SRY in sex reversal. Biochem J. 2010;430:39–48. doi: 10.1042/BJ20091758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaymina SS, Kenney JM, Schroeter MM, Chalovich JM. Fesselin is a natively unfolded protein. J Proteome Res. 2007;6:3648–3654. doi: 10.1021/pr070237v. [DOI] [PubMed] [Google Scholar]
- Kleene R, Mzoughi M, Joshi G, Kalus I, Bormann U, Schulze C, Xiao MF, Dityatey A, Schachner M. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J Neurosci. 2010;30:10784–10798. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski J, Wrzosek A, Dabrowska R. Fesselin is a target protein for calmodulin in a calcium-dependent manner. Biochem Biophys Res Commun. 2004;323:1251–1256. doi: 10.1016/j.bbrc.2004.08.224. [DOI] [PubMed] [Google Scholar]
- Kremerskothen J, Plaas C, Kindler S, Frotscher M, Barnekow A. Synaptopodin, a molecule involved in the formation of the dendritic spine apparatus, is a dual actin/α-actinin binding protein. J Neurochem. 2005;92:597–606. doi: 10.1111/j.1471-4159.2004.02888.x. [DOI] [PubMed] [Google Scholar]
- Le Gall T, Romero PR, Cortese MS, Uversky VN, Dunker AK. Intrinsic disorder in the protein data bank. J Biomol Struct Dyn. 2007;24:325–342. doi: 10.1080/07391102.2007.10507123. [DOI] [PubMed] [Google Scholar]
- Leinweber BD, Fredricksen RS, Hoffman DR, Chalovich JM. Fesselin: a novel synaptopodin-like actin binding protein from muscle tissue. J Muscle Res Cell Motil. 1999;20:539–545. doi: 10.1023/A:1005597306671. [DOI] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010;89:681–692. doi: 10.1016/j.ejcb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- McGhee JD, von Hippel PH. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-X. [DOI] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil KT, DeGrado WF. How calmodulin binds its targets: sequence independent recognition of amphiphilic a-helicies. Trends Biochem Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-D. [DOI] [PubMed] [Google Scholar]
- Papa I, Astier C, Kwiatek O, Raynaud F, Bonnal C, Lebart MC, Roustan C, Benyamin Y. Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. J Muscle Res Cell Motil. 1999;20:187–197. doi: 10.1023/A:1005489319058. [DOI] [PubMed] [Google Scholar]
- Permyakov SE, Millett IS, Doniach S, Permyakov EA, Uversky VN. Natively unfolded C-terminal domain of caldesmon remains substantially unstructured after the effective binding to calmodulin. Proteins. 2003;53:855–862. doi: 10.1002/prot.10481. [DOI] [PubMed] [Google Scholar]
- Pham M, Chalovich JM. Smooth muscle alpha-actinin binds tightly to fesselin and attenuates its activity toward actin polymerization. J Muscle Res Cell Motil. 2006;27:45–51. doi: 10.1007/s10974-005-9053-2. [DOI] [PubMed] [Google Scholar]
- Renegar RH, Chalovich JM, Leinweber BD, Zary JT, Schroeter MM. Localization of the actin-binding protein fesselin in chicken smooth muscle. Histochem Cell Biol. 2009;131:191–196. doi: 10.1007/s00418-008-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003;22:5298–5305. doi: 10.1038/sj.onc.1206616. [DOI] [PubMed] [Google Scholar]
- Schaeffer G, Levak-Frank S, Spitaler MM, Fleischhacker E, Esenabhalu VE, Wagner AH, Hecker M, Graier WF. Intercellular signalling within vascular cells under high D-glucose involves free radical-triggered tyrosine kinase activation. Diabetologia. 2003;46:773–783. doi: 10.1007/s00125-003-1091-y. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Chalovich JM. Ca2+-calmodulin regulates fesselin-induced actin polymerization. Biochemistry. 2004;43:13875–13882. doi: 10.1021/bi0487490. [DOI] [PubMed] [Google Scholar]
- Schroeter MM, Chalovich JM. Fesselin binds to actin and myosin and inhibits actin activated ATPase activity. J Muscle Res Cell Motil. 2005;26:183–189. doi: 10.1007/s10974-005-9009-6. [DOI] [PubMed] [Google Scholar]
- Schroeter MM, Beall B, Heid HW, Chalovich JM. The actin binding protein, fesselin, is a member of the synaptopodin family. Biochem Biophys Res Commun. 2008;371:582–586. doi: 10.1016/j.bbrc.2008.04.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter MM, Orlova A, Beall B, Egelman EH, Chalovich JM. Organization of F-actin by avian smooth muscle synaptopodin 2 (fesselin) Biophys J. 2010;98:157a. doi: 10.1016/j.bpj.2009.12.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Valencia CA, Szostak J, Dong B, Liu R. Scanning the human proteome for calmodulin-binding proteins. Proc Natl Acad Sci USA. 2005;102:5969–5974. doi: 10.1073/pnas.0407928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc Natl Acad Sci USA. 2000;97:8868–8873. doi: 10.1073/pnas.160259697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmeier M, Hamilton JA, Le Gall T, Vacic V, Cortese MS, Tantos A, Szabo B, Tompa P, Chen J, Uversky VN, Obradovic Z, Dunker AK. DisProt: the database of disordered proteins. Nucleic Acids Res. 2007;35:D786–D793. doi: 10.1093/nar/gkl893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp H, Al Naggar IM, Yarmola EG, Harrison A, Shaw G, Edison AS, Bubb MR. MARCKS is a natively unfolded protein with an inaccessible actin-binding site: evidence for long-range intramolecular interactions. J Biol Chem. 2005;280:9946–9956. doi: 10.1074/jbc.M414614200. [DOI] [PubMed] [Google Scholar]
- Tompa P. Structure and function of intrinsically disordered proteins. Boca Raton, Fl: Taylor and Francis; 2009. [Google Scholar]
- Tompa P, Fuxreiter M. Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci. 2008;33:2–8. doi: 10.1016/j.tibs.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Van Impe K, De Corte V, Eichinger L, Bruyneel E, Mareel M, Vandekerckhove J, Gettemans J. The Nucleo-cytoplasmic actin-binding protein CapG lacks a nuclear export sequence present in structurally related proteins. J Biol Chem. 2003;278:17945–17952. doi: 10.1074/jbc.M209946200. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Laé M, Louvard D, Ben-Ze'ev A, Robine S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol. 2001;155:393–404. doi: 10.1083/jcb.200012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young CH, Hyung CJ, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YP, Luo JH. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Res. 2006;66:7414–7419. doi: 10.1158/0008-5472.CAN-06-0227. [DOI] [PubMed] [Google Scholar]