Abstract
Monoclonal antibodies now form a key part of the biochemist’s toolbox, and are important reagents for therapeutic applications. This has resulted in a need for high-throughput production to satisfy the demand from the global community. Manual production involves overwhelming amounts of tissue culture and associated liquid handling steps to achieve high-throughput operation. By contrast, automated systems can readily cope with the numbers required. In this review, we address the development of automated systems, and discuss the pros and cons of their operation.
Keywords: Monoclonal antibodies, Automation, Robotics, High through-put
Introduction
Monoclonal antibodies (mAbs) have become an indispensible part of the biological scientist’s toolkit since the pioneering work of Köhler and Milstein (1975), a discovery that revolutionised science and medicine, and for which they were awarded the Nobel Prize in Physiology and Medicine in 1984. This is due to the potential to generate antibodies to a wide range of compounds and the high specificity and sensitivity they display towards their target antigen. This allows them to be used in many antibody-based applications including immunohistochemistry, immunocytochemistry, Western blot analysis, immunoprecipitation, affinity-based purification, epitope-specific tagging (e.g. crystallography), fluorescence-activated cell sorting (FACS) and enzyme-linked immunosorbent assay (ELISA) (Chiarella et al. 2011; Bordeaux et al. 2010). Additionally, mAbs have established themselves as important therapeutic reagents with more than 25 FDA-approved antibody-based therapeutics currently in use clinically in the United States (Nelson et al. 2010), predominantly for the treatment (by inhibiting key signalling pathways or targeted drug delivery) or diagnosis (usually via targeting and imaging) of a variety of solid tumours and haematological malignancies (Scott et al. 2012).
Since antibodies can be raised against virtually any target that is foreign to the host animal, they afford a virtually universal source of reagents for the detection of any protein present in a specific proteome. This has been recognised by the Human Proteome Organisation (HUPO) who, following on from the successful completion of the human genome (Venter et al. 2001), have undertaken to map the entire human protein set of the estimated 20,300 protein-coding genes (Legrain et al. 2011a), where antibody capture forms one of the three HUPO working pillars (along with mass spectrometry and bioinformatics) (Legrain et al. 2011b) . Alongside this, the Swedish Human Protein Atlas project (Berglund et al. 2008) is developing and validating antibodies on a multitude of normal tissues, cancer tissues, cell samples and cell lines with a long-term goal of having one validated antibody toward all non-redundant human proteins and providing a first draft of the human proteome by 2014. However, unlike the human genome, which is constant, the proteome is dynamic and variable. Thus, at the gene level, both alternative splicing and single nucleotide polymorphisms can give rise to new isoforms, while at the protein level, expressed proteins can be subjected to a wide range of post-translational modifications (e.g. phosphorylation, acetylation, glycosylation, acetylation, ubiquitination, methylation, oxidation). Such protein modifications can determine activity, localisation, turnover, and interactions with other proteins (Mann and Jensen 2003), and deregulation of these events is frequently associated with disease (Vidal 2011). Ideally, antibodies are also required which recognise these multitudinous different protein isoforms and post-translational states to facilitate their characterisation. This has stimulated systematic, genome-wide efforts to generate and validate renewable protein binders (Colwill and Graslund 2011) and the development of automated platforms compatible with the large number of reagents required. In this review, we will explain the monoclonal antibody production process, which involves a number of labour-intensive steps, and show how it is ideally suited for transfer to robotic platforms for high-throughput monoclonal antibody production.
Manual production of monoclonal antibody-producing hybridomas
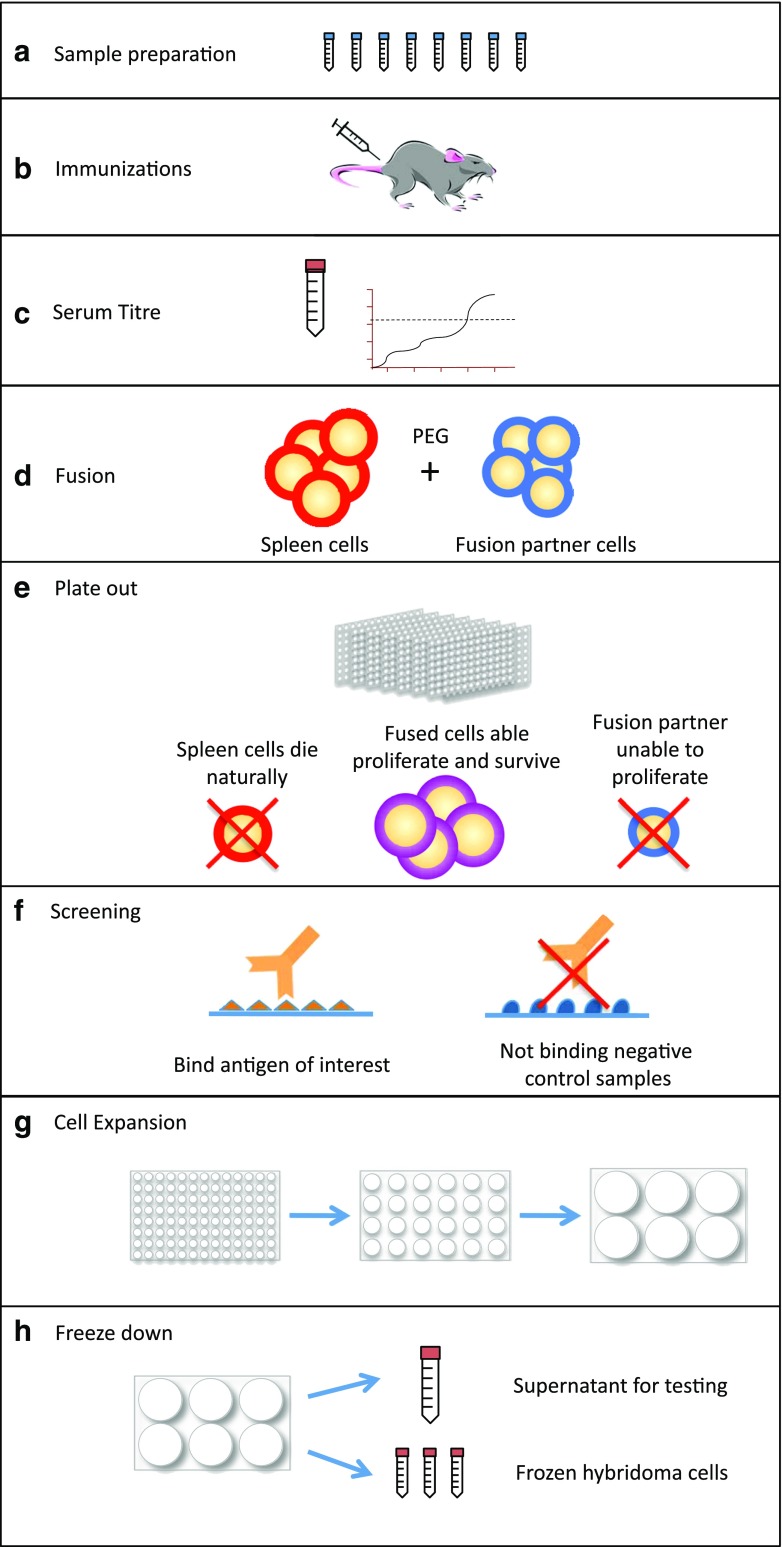
Although there have been minor improvements with regards to the production of traditional hybridomas, the basic technique remains largely the same as that reported in the original publication of Köhler and Milstein (1975). The process (Fig. 1) starts with preparation of the antigen that will be used for antibody production (Fig. 1a). The most frequently used antigens are proteins (native or recombinant) or synthetic peptides, although many other types of molecule can be used (e.g. carbohydrates, lipids, cell extracts, nucleic acids, small molecules). Small polypeptides (<10 kD) and non-protein antigens generally need to be conjugated or crosslinked to larger, immunogenic, carrier proteins to increase immunogenicity [e.g. keyhole limpet hemocyanin (KLH), ovalbumin, diphtheria toxin]. The next step is immunisation of the host species, most commonly rodents, with both the target of interest as well as an immune adjuvant (Fig. 1b). This immunisation, much like vaccination, causes the proliferation of B cells producing antibodies that bind the antigen and ultimately generate a high serum titre of target reactive antibodies. Once this serum response reaches a suitable level, measured as fold increase over pre-immunisation serum level (Fig. 1c), B cells from the immunised mouse are extracted, typically from spleen or lymph nodes, and fused with a myeloma cell line (Fig. 1d). The fusing of B cells to a myeloma cell line allows the B cells to proliferate indefinitely. The cell fusion is typically performed with the use of polyethylene glycol (PEG). A number of suggestions have been made as to the mechanism by which PEG is able to catalyse cell fusion. These include membrane crosslinking, detergent effects and membrane disruption (reviewed in Lentz 1994). However, it has now been shown that PEG can drive close contact between membranes via a thermodynamic force and membrane aggregates are formed through water exclusion (Arnold et al. 1990). It is these dehydrated membrane aggregates that are thought to allow cell fusion. Once this fusion has taken place, the cells are aliquoted into 96- or 384-well tissue culture plates and allowed to grow for 10–14 days (Fig. 1e). The cell numbers used are typically titrated to give approximately 1 growing hybridoma (B cell and myeloma fusion) colony per well. Obviously, the more wells available for screening, the higher the chance of obtaining a useful mAb. However, with manual production, this has to be weighed against the amount of antibody screening that needs to take place in the relatively short timeframe before the cells need to be passaged, or they will overgrow and die (∼48–72 h). A critical step in hybridoma production is the ability to distinguish between fused hybridoma cells and the unfused spleen and myeloma cells. After the ∼14 days of growth, most of the unfused spleen cells will die naturally as they are unable to survive under normal tissue culture conditions. Myeloma cells are, however, able to survive in standard tissue culture media, and the two key discoveries of Köhler and Milstein (1975) were a myeloma cell line that was devoid of the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT) coupled with the development of a growth medium that blocked survival of cells unable to produce this enzyme. This growth medium contains hypoxanthine, aminopterin and thymidine (HAT). Typically, nucleic acid synthesis occurs via what is known as the de novo pathway; however, when grown in HAT medium, the aminopterin blocks dihydrofolate reductase, required for de novo synthesis, and forces the cells to use the salvage pathway. The salvage pathway is reliant on HGPRT and thymidine kinase to metabolise hypoxanthine and thymidine from the HAT media for nucleic acid synthesis to occur. Therefore, the cells lacking HGPRT die and only cells fused with HGPRT containing spleen cells can divide and survive. The cells are often ‘fed’ with HT (hypoxantine/thymidine) media after 10 days to encourage surviving cells to divide more rapidly.
Fig. 1.
Manual monoclonal antibody production. The schematic shows the protocol for the production of hybridoma cells lines, beginning with the preparation of samples for immunisation and screening (Fig. 1a). A laboratory animal, most commonly rodents, is immunised with the antigen of interest as well as an immune activating adjuvant (Fig. 1b). To assess the immune response a sample of serum is taken and the quantity of antigen-specific antibody is determined by ELISA. The spleen or lymph nodes are taken from an immunised animal and fused with a myeloma cells line (Fig. 1d). The resulting hybridoma cells are aliquoted into many tissue culture plates at 1 clone per well (Fig. 1e). From each well of the hybridoma containing tissue culture plates, a sample of culture supernatant is screened for reactivity to the antigen of interest (Fig. 1f). Hybridoma cell lines that are reactive for the antigen of interest are expanded (Fig. 1g) and frozen for later use (Fig. 1h)
Once the cells have had an opportunity to divide and secrete antibody into the media, an aliquot from each well is taken for screening (Fig. 1f). In theory, any assay for which the mAbs will be useful can be used for screening. Common assays include ELISA, flow cytometry and Western and dot blot analysis. The caveat is that the timeframe before the cells need to be passaged is quite short as they will most likely overgrow and die within ∼48–72 h. Once positive clones have been identified they are expanded (Fig. 1g) and frozen (Fig. 1h). The cells then need to be cloned by limiting dilution (regrown from a single cell) to isolate a monoclonal cell line.
The problems with large-scale manual hybridoma production
When attempting to meet the mAb needs of the global community and eventually cover the proteome with high quality binders, a significant scale up from the existing production capacity is required. The problem that arises is the overwhelming amount of tissue culture and associated liquid handling steps needed to achieve a high-throughput operation. When evaluating capacity for the production of monoclonal antibodies, the throughput can be defined as the maximum number of hybridoma fusion projects to be undertaken in a given week. Consider the situation in which a laboratory wishes to undertake 20 independent mAb projects per week (∼1,000 per year). Based on existing protocols for manual production, for a throughput of 20 projects per week this equates to 400 × 96-well microtitre plates per week (Fig. 1) for hybridoma plating. This results in 38,400 independent wells that require screening for binding to the antigen of interest. Furthermore, on average, each mAb project will require screening against one additional control protein (tag, fusion protein, post-translational modification, etc.) making the number of samples required for screening 76,800 samples. When working at this scale, several issues arise beside the large-scale pipetting, such as sample tracking/labelling, maintenance of sterility and data evaluation. It has been estimated that approximately 50 full-time equivalent (FTE) staff (Chiarella et al. 2011) would be required to generate 1,000 monoclonal antibodies in a year using standard manual methods, a number which is often not financially or logistically possible. One possible solution to this problem is the use of laboratory automation. We have found that this staff number can be reduced ten-fold to 5 FTEs generating 1,000 monoclonal antibodies per year by addressing the two major bottle necks in monoclonal antibody production: the screening of thousands of potential clones and the ability to handle very large numbers of liquid handling steps, and applying automation and high throughput technologies to them.
Robotics and automation
The origins of automated liquid handling and robotics can be traced back to the invention of the syringe by Martin Overlach in 1889 (U.S. patent 404105) which was significantly developed by Clark Hamilton in 1947 to deliver microlitre quantities of fluids into the gas chromatographs being developed at the National Institute of Medical Research at Mill Hill in London. By the 1970s, significant advances in both micro-scale dc motor and valve technology led to the introduction of the Digital Dilutor by the Hamilton Company, a semi-automated pipetting device based on Hamilton’s own syringes. The first true automated liquid handling workstations were developed by the Hamilton Company in collaboration with Tecan AG, and resulted in the production of the AMICA system (Bartels and Walser 1983). Subsequently, automation has been applied to a vast array of different scientific applications. Some of these applications are on a significantly large scale, such as the fully automated liquid handling ‘barns’ that pharmaceutical companies use to manage the millions of compounds they test and synthesise every year (Cui et al. 2011; Schmitt et al. 2010), or the automated liquid handling with integrated testing and detection instrumentation for ultra high throughput screening of those compounds (Michael et al. 2008). On the other end of the scale is the relatively low throughput but fully automated in vivo sample collection system used in pharmacokinetic and other pre-clinical studies (Clark et al. 2011; Aryal et al. 2012). Many laboratories use some type of automation in the form of liquid handling systems (Joelsson et al. 2008) (automated sample analysis for Mass Spectrometry and HPLC and automated plate readers, for example) to facilitate their daily scientific research (Uyeda et al. 2011). When comparing automated processes to the manual equivalent, automation has many advantages including increased throughput, unattended operation, decreased variability and sample size. However, the key to successfully automating a given laboratory procedure is in knowing which steps to automate and, most importantly, when not to automate.
Design of an automated monoclonal antibody production facility
Throughput and protocol
In the initial stages of developing an automated platform, it is essential to fully define what is required in that system and the expectation of how that system will work when installed and up and running in the laboratory. In the example of monoclonal antibody production, the expectations are quite clear: to develop an automated system to reproduce the manual tasks involved in mAb production (Fig. 1), to the equivalent, or superior, quality in a high throughput manner. For each manual production step detailed in Fig. 1, there is a well-established and defined protocol or standard operating procedure (SOP) that forms the basis for automating each task. However, when automating a manual process, it is sometimes useful to challenge the status quo, as it is not always possible to re-create the manual procedure in exactly the same way on a robot. This is perfectly acceptable, providing the outcome is equal to the manual operation, and often results in a far more effective and productive process. In mAb production, the only task that is not readily automated is the immunisation of the animals (Fig. 1b), as this is a highly complex and intricate procedure that is far more suited to human operation; a good example of when automation is not appropriate or beneficial. The next consideration is the desired maximum throughput of the automated system. Maximum throughput and assay protocol allow for the calculation of the maximum number of samples that the system can process in a given batch (i.e. per day or week). This information is critical as the system hardware is designed around it, and essentially defines what instrumentation and accessories are required.
Robotic equipment
Once the desired maximum throughput and operational protocols have been defined, the system hardware and instrumentation required can be planned. There are a number of global vendors that offer solutions for automated liquid handling of the type required for automated antibody production, with the expertise and capabilities to provide both off-the-shelf and customised systems for an extensive array of applications. Table 1 lists some of the vendors that offer customised integrated solutions for automated liquid handling in the global life science market.
Table 1.
A list of vendors and their websites offering customised solutions for automation of scientific processes in the life sciences market
| Vendor | Website address |
|---|---|
| Agilent | www.chem.agilent.com |
| BeckmanCoulter | www.beckmancoulter.com |
| Caliper | www.caliperls.com |
| Hamilton | www.hamiltonrobotics.com |
| PerkinElmer | www.perkinelmer.com |
| ttplabtech | www.ttplabtech.com |
| Tecan | www.tecan.com |
| TAP Biosystems | www.tapbiosystems.com |
The Monash Antibody Technologies Facility (MATF) was among the first of such high throughput systems established worldwide for the production of monoclonal antibodies. The facility is based on an original concept developed by Alan Sawyer at EMBL Montorotondo, the final configuration comprising eight separate workstations each with a specific step to run. Table 2 details the steps involved in mAb production along with a comparison of the FTEs required for manual and automated operation, as well as the specific instrumentation layout for each robotic workstation. It can be seen that the use of automation results in a significant reduction in the number of FTEs required to operate the facility. At MATF, each workstation was fully customised to perform a specific task (or set of related tasks) for at least 20 fusion projects per week. The instrumentation common to the workstations is: a robotic deck, a liquid handling arm and a plate moving arm. The remainder of the individual workstation configurations are highly customised around the task they are designed to perform. For example, the sample reception and management robot, which is designed to generate all the various antigen samples required throughout the entire operation (immunisation and screening), has a integrated barcode reader to allow for comprehensive sample tracking, the ELISA platforms have integrated plate washers and readers to enable unattended operation, and the fusion plate-out and screen sampling robot has an integrated CO2 incubator and a 96-pipette head to accommodate the massive number of plates that result from those tasks. Figure 2 shows a schematic of the robotic workstation customised for the fusion of B cells from the immunised mouse with a myeloma cell line (Fig. 1d). The workstation has heated tube holders to maintain the fusion mixture at 37 °C and 16 pipettes: 8 for general reagent transfer (e.g. PEG addition to the spleen and SP2 cells) and 8 for the sole use with SP2 cells. It has an in-line cell counter and fully integrated centrifuge and CO2 incubator. Some interesting customised solutions for this workstation include a tool for transferring tubes into and out of the centrifuge baskets and a vacuum-controlled plate de-lidding station, where the microtitre plates are automatically de-lidded as they move from the CO2 incubator onto the robotic deck, minimising human intervention and therefore the potential for contamination.
Table 2.
Instrumentation details for the robotic workstations required for each task in the automated production of mAbs and the comparison of Full Time Equivalent (FTE) staff required to run those tasks in traditional manual or fully automated mode
| Manual Task – challenges and bottlenecks | Manual mode - FTE to complete 20 fusions per week | Customised workstation instrumentation | Automated mode - FTE to complete 20 fusions per week | Features of workstation to address challenges and bottlenecks |
|---|---|---|---|---|
| Sample management | 3 | 1.5 m work deck | 0.5 | Barcode reader enables comprehensive sample tracking |
| High volume of samples to track | 8 pipette liquid handling arm | |||
| Plate moving arm | ||||
| Barcode reader | −20 °C storage allows for individual sample storage and retrieval, maximising sample quality | |||
| −20 °C barcoded, automated sample storage | ||||
| Immunisation | 2 | Task performed manually | 2 | |
| ELISA – serum titre | 4 | 1.5 m work deck | 0.3 | Integrated plate washer and reader allow for unattended and out-of-hours operation |
| Repetitive liquid handling | 8 pipette liquid handling arm | |||
| Plate moving arm | ||||
| High volume of plates to handle | Integrated plate washer | |||
| Integrated absorbance plate reader | ||||
| Fusion (see Fig. 2) | 6 | 2.0 m work deck | 0.2 | Integrated incubator, cell counter and centrifuge allow for unattended and out-of-hours operation |
| Repetitive liquid handling | 8 pipette liquid handling arm × 2 | |||
| Plate moving arm | ||||
| Integrated CO2 incubator | ||||
| Many manually intensive cell counting steps | Integrated cell counter | |||
| Integrated centrifuge | ||||
| Biosafety cabinet Class II containment | ||||
| Plate out | 4 | 1.5 m work deck | 0.5 | 96 pipette head offers very fast reagent dispensing and plate-to-plate transfer |
| Repetitive liquid handling | 4 pipette liquid handling arm | |||
| 96 pipette liquid handling arm | ||||
| High volume of plates to handle | Plate moving arm | Integrated incubator allows for unattended and out-of-hours operation | ||
| Integrated CO2 incubator | ||||
| Biosafety cabinet Class II containment | ||||
| Screening | 8 | 1.5 m work deck | 0.4 | 96 pipette head offers very fast reagent dispensing and plate-to-plate transfer |
| Repetitive liquid handling | 4 pipette liquid handling arm | |||
| 96 pipette liquid handling arm | Integrated incubator allows for total unattended and out-of-hours operation | |||
| High volume of plates and samples to handle | Plate moving arm | |||
| Integrated CO2 incubator | ||||
| Narrow window of time to perform screening to avoid cell degradation or death | Biosafety cabinet Class II containment | AMA enables rapid HTS of thousands of cell supernatant samples with high degree of flexibility | ||
| Antigen MicroArray (AMA) | ||||
| Cell Expansion | 6 | 1.5 m work deck | 0.5 | Integrated incubator and cell imager allow for unattended and out-of-hours operation |
| Repetitive liquid handling | 4 pipette liquid handling arm | |||
| Plate moving arm | ||||
| High volume of plates to handle | Integrated CO2 incubator | |||
| Integrated cell imager | ||||
| Biosafety cabinet Class II containment | ||||
| Cell Freeze Down | 4 | 1.5 m work deck | 0.2 | Integrated incubator allows for unattended and out-of-hours operation |
| Repetitive liquid handling | 8 pipette liquid handling arm | |||
| High volume of plates to handle | Plate moving arm | |||
| Integrated CO2 incubator | ||||
| Biosafety cabinet Class II containment |
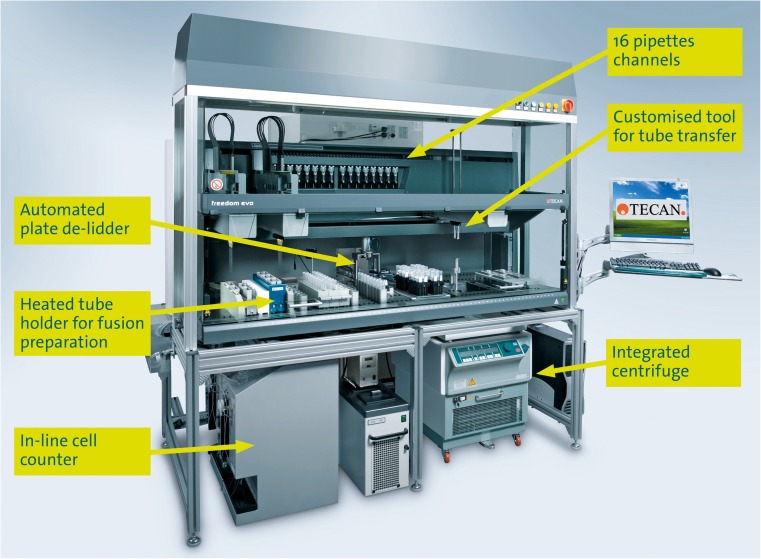
Fig. 2.
A fully automated workstation for hybridoma cell fusion. Schematic of the fully automated fusion workstation showing the 16 pipette channels, the customised tool for tube transfer between the robotic deck and the integrated centrifuge, the automated plate de-lidder, the heated manifold to house the tubes where the fusion is performed and the in-line cell counter (photograph courtesy of Tecan Group Ltd)
Monoclonal antibody production is heavily dependent on good cell culture techniques. Hybridoma fusion, fusion plate-out, supernatant sampling for screening, clone hitpicking, clone expansion and clone freeze down must all be performed in a sterile environment, so the workstations designed for these steps are contained in hepafiltered biosafety cabinets (Fig. 2). The advantage of the robotic systems for cell culture is that human intervention is minimal once the work decks are setup, which greatly reduces the potential for contamination.
The final design of an automated system is critically dependent on where the system is going to be housed and how much laboratory space is available. If an automated system comprises more than one workstation, then a decision needs to be made on whether, if appropriate, the workstations should be integrated and physically connected to each other (fully integrated) or set up as standalone units that depend on human intervention to move plates and samples between them (modular). Table 3 compares the advantages and disadvantages of these two different models for large automated systems. To maximise flexibility when producing mAbs in a high throughput mode, the approach taken at MATF is to have each individual workstation physically separate from another. However, the instrumentation for each workstation (e.g. liquid handler, CO2 incubator, plate washer and plate reader) is fully integrated with automated robotic plate movements, resulting in the scenario whereby each task or assay can be performed in a fully automated, unattended manner but simple manual intervention is required to move plates and samples from one station to the next. It is worth pointing out that automation is not always faster than performing the task manually, but is generally considered more reproducible and can be performed unattended. In turn, this liberates laboratory staff for other tasks, or allows the tasks to be performed outside of normal operating hours, leading to higher throughput. Using robotics, the typical turnaround time for mAb production is 12–16 weeks, of which the immunisation schedule accounts for ∼7–8 weeks.
Table 3.
A comparison between fully integrated and modular automated systems typically found in a laboratory
| Fully integrated | Modular | |
|---|---|---|
| Explanation | Typically, all components of the workstation(s) are connected together physically by the use of a shuttle, robotic arm and deck integration etc. | Typically, each workstation is separate from another physically but may have equipment such as a plate reader integrated or in a separate location. |
| Operation | Has the potential to be run in a fully automated fashion with unattended, out-of-hours operation. | May require manual intervention for the process to proceed (move plates from one workstation to the next). This has greater potential for operator error. |
| The entire system is in-use at any one time, which reduces flexibility of how resources can be used. | Reduced potential for unattended, out-of-hours operation. | |
| Process is run in a sequential manner. | Different workstations can be used in parallel. | |
| Cost considerations | Costs generally higher due to a more complex system and additional development and installation time. | Costs generally lower if the modules themselves are not overly complex. |
| Space | Fully integrated systems have a large space footprint as all components need to be close to each other to allow for physical connection via robotic arm or plate shuttle etc. | Modular systems tend to have a smaller space footprint as the various workstations can be placed in smaller spaces that don’t need to be close to each other. |
| Software | May require another level of software complexity above standard liquid handling robotic software. | Will usually only require the standard liquid handling robotic software to run each module. |
Antigen Microarray is a key assay for high throughput mAb development
As mentioned above, one of the most critical steps in high throughput mAb production is the screening, both in terms of timing and quality, of the mAb that is produced. In order to increase the number of mAbs that a given laboratory can realistically generate, the screening process must also be amenable to scale up. Typically, ELISAs are used for the preliminary screening of mAbs. There are, however, several drawbacks to ELISA, the most important of which is the amount of screening material needed (both mAb and antigen). A typical ELISA will use 10–50 μL of antibody supernatant and ∼0.2 μg of antigen per well of mAb. It is also quite common to need to screen multiple controls such as irrelevant proteins with the same affinity tag, non-phospho counterparts, etc. During production, the amount of mAb available for these screens is limited to that present in the tissue culture well (typically 96- or 384-well format) and therefore obtaining more than 50 μL from each mAb for a second or even third screening sample is not always possible. Furthermore, to increase the chances of obtaining a number of good quality clones, it stands to reason that more clones should be screened: however, screening larger numbers of clones will also increase the amount of antigen needed. At MATF, we routinely screen 1,920 wells for each fusion project, which, if they were screened by ELISA, would require 384 μg of antigen. An alternative approach to ELISA, which has already been used successfully for high throughput antibody screening (De Masi et al. 2005), is Antigen Microarray (AMA). AMA holds many advantages over traditional ELISA screening. The process follows established protein–protein microarray techniques, whereby the antigen of interested or screening control samples are coated onto a microarray slide and antibodies are spotted onto the surface. MATF currently use an ArrayJet Super Marathon inkjet microarrayer (Pentlandfield, Scotland, UK). This instrument can accommodate up to 48 microtitre plates, has a capacity to print 100 slides per run at a speed of 8.3 slides per minute, and the potential to spot up to 53,000 spots per slide (we typically run at 2,000–6,000 spots). AMA requires only 20 μg of antigen per slide for the 1,920 wells, a significant reduction on the 384 μg required for ELISA. Furthermore, only picolitres of mAb supernatant are required for screening and can be spotted onto multiple screening slides simultaneously, which is advantageous for scale-up purposes. Binding to the antigen or control samples can be detected with both an anti-IgG and anti-IgM antibody. By using a dual color fluorescence reader, the IgG-containing wells can be specifically identified, a scenario that is not readily possible with traditional ELISA. Identification of IgG clones is a critical step for the selection of high quality antibodies as the IgMs tend to have a lower specificity and are harder to purify. Importantly, the AMA also has the added advantages of higher sensitivity than traditional ELISA (De Masi et al. 2005).
Installation and operation of a fully automated monoclonal antibody production facility
Once an automated system has been designed and purchased, the installation and testing phase is next. This can be challenging and often unpredictable in terms of timescale. Although any large customised system would have a factory acceptance test (FAT) and site acceptance test (SAT), there can still be a considerable amount of work to be performed in the laboratory post-installation. This includes fully testing the system with ‘real’ reagents such as live cells and to fully validate the system under the full range of conditions or variables of the given application. This highlights any weaknesses of the system that can be addressed prior to using it in production.
Experience suggests that it is good practice to have a staff member that is responsible and fully trained in the software and running of the robotic system in the laboratory. This person can address not only the method development issues and applications challenges but can also provide day-to-day maintenance of the systems. They can also provide training to other laboratory staff. On this point, it is essential that all people running the systems have some training in error handling and basic running of the systems to ensure consistent and safe use of the robots. These days, most automated liquid handling system have very user-friendly software so that, once an assay has been developed and validated, the routine running of that assay can easily be performed by junior staff in the laboratory once they have been trained.
Conclusions
In this review, we have discussed the design and operation of an automated facility for the high-throughput production of mAbs. The advantages and disadvantages of operating robotic workstations to perform the heavily labour-intensive tasks involved in making mAbs can be summarised as follows;
It is possible to run at least 20 mAb projects per week with approximately 5 FTEs compared to 50 FTEs if run in the traditional manual mode. This equates to a highly efficient and cost-effective laboratory with rapid turnaround times allowing screening of many thousands of samples per week.
Productivity and consistency is maximised, whilst variability and operator error is minimised when using robotics. These advantages are realised on a daily basis, but the reliance on automation can pose the challenge of what to do when, or if, the workstations fail. Building in redundancy is not always possible due to financial and space restrictions, so it can be cost-effective to purchase fully comprehensive service cover to ensure minimum response time in the event of a breakdown. It is also wise to have practised manual backup procedures in place in the event of a serious robotic failure.
The capital outlay for eight customised robotic workstations and the AMA, and the space and laboratory modifications to support the instrumentation is considerable compared to the set-up costs for the manual equivalent. However, the labour costs for running a facility to produce 1,000 mAbs in manual mode is also very high and those costs scale with increasing throughput. By contrast, in an automated facility, where many of the operating costs are fixed, production costs decrease significantly with increasing throughput.
In conclusion, to facilitate high-throughput mAb production, the use of automation is essential to maximise quality and productivity whilst minimising operator error, product variability and overall production cost. There is the potential to implement automated solutions to many other biological processes, and the authors hope that others will find some of the approaches discussed in this review useful when setting up any high-throughput operation.
Acknowledgements
We gratefully acknowledge Alan Sawyer (European Molecular Biology Laboratory Monterotondo, Italy) for helpful discussion during the preparation of this manuscript. We thank the Tecan Group Ltd. for their expert technical support with the design and installation of the facility at MATF and the Victorian State Government (The Department of Innovation, Industry and Regional Development), the Australian Federal Government (Bioplatforms Australia) and Monash University for financial support for MATF.
Conflict of interest
None
Footnotes
Daniel Layton and Caroline Laverty made equal contributions
References
- Arnold K, Zschoernig O, Barthel D, Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-V. [DOI] [PubMed] [Google Scholar]
- Aryal B, Aryal D, Kim EJ, Kim HG. Pharmacokinetics of venlafaxine and its major metabolite o-desmethylvenlafaxine in freely moving mice using automated dosing/sampling system. Indian J Pharmacol. 2012;44(1):20–25. doi: 10.4103/0253-7613.91861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels H, Walser P. Automation of wet chemical analysis with AMICA. Fresenius J Anal Chem. 1983;315(1):6–11. doi: 10.1007/BF00476397. [DOI] [Google Scholar]
- Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7(10):2019–2027. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, et al. Antibody validation. Biotechniques. 2010;48(3):197–209. doi: 10.2144/000113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarella P, Leuener M, Fasci C, de Marco A, Santini MP, Fazio VM, et al. Comparison and critical analysis of robotized technology for monoclonal antibody high-throughput production. Biotechnol Prog. 2011;27(2):571–576. doi: 10.1002/btpr.564. [DOI] [PubMed] [Google Scholar]
- Clark GT, Giddens G, Burrows L, Strand C. Utilization of dried blood spots within drug discovery: modification of a standard DiLab((R)) AccuSampler((R)) to facilitate automatic dried blood spot sampling. Lab Anim. 2011;45(2):124–126. doi: 10.1258/la.2010.010155. [DOI] [PubMed] [Google Scholar]
- Colwill K, Graslund S. A roadmap to generate renewable protein binders to the human proteome. Nat Methods. 2011;8(7):551–558. doi: 10.1038/nmeth.1607. [DOI] [PubMed] [Google Scholar]
- Cui J, Chai SC, Shelat AA, Guy RK, Chen T. An automated approach to efficiently reformat a large collection of compounds. Curr Chem Genomics. 2011;5:42–47. doi: 10.2174/1875397301105010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Masi F, Chiarella P, Wilhelm H, Massimi M, Bullard B, Ansorge W, et al. High throughput production of mouse monoclonal antibodies using antigen microarrays. Proteomics. 2005;5(16):4070–4081. doi: 10.1002/pmic.200401279. [DOI] [PubMed] [Google Scholar]
- Joelsson D, Moravec P, Troutman M, Pigeon J, DePhillips P. Optimizing ELISAs for precision and robustness using laboratory automation and statistical design of experiments. J Immunol Methods. 2008;337(1):35–41. doi: 10.1016/j.jim.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Legrain P, Aebersold R, Archakov A, Bairoch A, Bala K, Beretta L (2011a) The human proteome project: Current state and future direction. Mol Cell Proteomics. Apr 29. [DOI] [PubMed]
- Legrain P, Aebersold R, Archakov A, Bairoch A, Bala K, Beretta L, et al. The human proteome project: current state and future direction. Mol Cell Proteomics. 2011b;10(7):M111. doi: 10.1074/mcp.M111.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz BR. Polymer-induced membrane fusion: potential mechanism and relation to cell fusion events. Chem Phys Lipids. 1994;73(1–2):91–106. doi: 10.1016/0009-3084(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nat Biotechnol. 2003;21(3):255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- Michael S, Auld D, Klumpp C, Jadhav A, Zheng W, Thorne N, et al. A robotic platform for quantitative high-throughput screening. Assay Drug Dev Technol. 2008;6(5):637–657. doi: 10.1089/adt.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Traphagen L, Hajduk P. High throughput cherry-picking of solvated samples. Comb Chem High Throughput Screen. 2010;13(6):482–489. doi: 10.2174/138620710791516076. [DOI] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- Uyeda C, Pham R, Fide S, Henne K, Xu G, Soto M, et al. Application of automated dried blood spot sampling and LC-MS/MS for pharmacokinetic studies of AMG 517 in rats. Bioanalysis. 2011;3(20):2349–2356. doi: 10.4155/bio.11.227. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vidal C. Post-translational modifications in health and disease. Berlin: Springer; 2011. [Google Scholar]