Summary
Sensitization of allergic patients normally takes place over several years and is the result of repeated exposure to low levels of allergen. Most mouse asthma models use a high dose of allergen administered over a short period. We have investigated the role of dose in the immune response to an inhaled respiratory allergen (Blomia tropicalis). We observed the effect of priming dose on the allergic response in mice intranasally immunized with low (0·5 μg) and high (50 μg) doses of B. tropicalis extract and killed 1 day after the last challenge. For both doses of allergen, T helper type 2 (Th2) cells and Th2 cytokines were evident as well as eosinophilic inflammation accompanied by mucus hyper‐secretion. By contrast, IgE and IgG1 antibody responses were normally only detected at high‐dose priming. To investigate the mechanism for these effects, we found group 2 innate lymphoid cells (ILC2s) were increased 48 hr after challenge in the low‐dose‐treated but not the high‐dose‐treated mice. Furthermore, we determined whether repeated low‐dose exposure with different priming protocols could induce an antibody response. Repeated low‐dose exposure to 0·5 μg three times weekly for 4 weeks (cumulative 6 μg) had the same effect as a shorter high‐dose exposure (cumulative 80 μg) and increasing cumulative dose induced antibody responses. These data indicate that low doses of allergen are sufficient to prime Th2 cells and ILC2s, but insufficient to induce antibody responses. Cumulative exposure to small amounts of allergen induces both Th2 and antibody responses and may better reflect natural sensitization.
Keywords: allergy, antibodies, innate lymphoid cells, lung, T cells, T helper type 2 cells
Abbreviations
- 4get
IL‐4‐IRES‐eGFP reporter
- APC
allophycocyanin
- BALF
bronchoalveolar lavage fluid
- Blo t
Blomia tropicalis
- BTE
Blomia tropicalis extract
- Foxp3‐eGFP
C.Cg‐Foxp3tm2 (eGFP) Tch/J
- IFN‐γ
interferon‐γ
- IL‐4
interleukin‐4
- ILC2s
type 2 innate lymphoid cells
- MLN
mediastinal lymph node
- OVA
ovalbumin
- PAS
periodic acid‐Schiff's
- PE‐Cy7
phycoerythrin‐Cy7
- PE
phycoerythrin
- Th2
T helper type 2 cells
- TSLP
thymic stromal lymphopoietin
Introduction
Conventional mouse models of asthma have used ovalbumin (OVA), a food antigen that rarely causes asthma in humans. These models usually use intraperitoneal sensitization by OVA accompanied by the T helper type 2 (Th2) cells skewing adjuvant aluminium hydroxide/aluminium sulphate (alum). Two reasons for the popularity of this antigen are its low cost and the availability of transgenic mice whose CD4+ T‐cell receptors recognize OVA. However, OVA is not a natural respiratory allergen and the conventional method does not represent the normal route of sensitization in humans. More recently investigators have turned to asthma allergens, such as house dust mite,1, 2 cockroach3 and fungus extracts4 with or without adjuvant.5 Sensitization to these inhalant allergens through intranasal administration without adjuvant is more representative of real‐life aeroallergen exposure and induces a stronger and more enduring allergic response.
Asthma is a heterogeneous disease with different phenotypes involving a variety of immune cells. The major clinical classification divides asthma into atopic, dependent on the presence of IgE antibodies, and non‐atopic, without IgE. Recently the concept of non‐atopic asthma has been reported to be associated with pollutants, viruses and other microbes that induce/activate type 2 innate lymphoid cells (ILC2s) without producing an IgE response.6 On the other hand, respiratory allergen‐specific Th2 cells can be induced by lung dendritic cells and leading to atopic asthma.7 Protocols for murine asthma models have used sufficient allergen dose to induce antibodies. However, natural exposure involves much lower antigen doses.
For people living in the tropical regions of the world, approximately one‐third of the world's population, the major asthma allergen is Blomia tropicalis (Blo t). This mite has similar sensitizing properties in mice to Dermatophagoides pteronyssinus. In patients living in Singapore, Malaysia, Taiwan and South America who have asthma and other allergic diseases, high frequencies of sensitization to Blo t antigens are described.8, 9, 10, 11, 12 Blo t 5, the major allergen, is the major cause of asthma in these patients.13, 14, 15 We have successfully generated novel transgenic mice which have CD4+ T cells that express T‐cell receptor chains specific for the 55–70 epitope of Blo t 5, which has a triple helical coiled‐coil structure as determined by nuclear magnetic resonance.16 We have shown that the capacity of granulocyte–macrophage colony‐stimulating factor licensed CD11b+ lung DCs to prime and polarize CD4+ Th2 cells was critically dependent on lung epithelium‐derived granulocyte–macrophage colony‐stimulating factor in a Blo t‐immunized mouse model.17 Here we have examined the Th2 cell and antibody responses at different sensitizing doses. We found that low‐dose priming induced a comparable Th2 response to high‐dose priming, but failed to elicit specific IgE and IgG1. Low‐dose sensitization closely resembles the level of allergen exposure at which asthma patients are normally sensitized to a mite allergen. Repeated low‐dose exposure produced the same features of Th2 cells and IgE/IgG1 antibodies to high‐dose priming and so is closer to the way that allergic patients are sensitized.
Materials and methods
Mice
Age‐ and sex‐matched C57BL/6J mice were bred at the National University of Singapore (NUS) animal centre. IL‐4‐IRES‐eGFP reporter (4get) mice and C.Cg‐Foxp3tm2 (eGFP) Tch/J (Foxp3‐eGFP) mice were purchased from Jackson Laboratory (JAX Mice and Services, Bar Harbor, ME). All experiments were conducted in accordance with institutional guidelines of the NUS Advisory Committee for Laboratory Animal Research (NACLAR) Singapore. The protocol (protocol number 015/12) was approved by the Institutional Animal Care and Use Committee (IACUC) of NUS.
Allergen preparation
Blo t allergen was purchased from The Siriraj Dust Mite Centre for Services and Research (Mahidol University, Bangkok, Thailand) and prepared as described elsewhere.17 Endotoxin levels measured using the EndoLISA (Hyglos GmbH, Bernried am Starnberger See, Germany) were less than 20 EU/mg protein. Ten grams of frozen B. tropicalis (The Siriraj Dust Mite Centre for Services and Research) were extracted overnight with slow stirring at 4° in PBS (pH 7·4). The extract was centrifuged at 13 000 g for 30 min at 4°. The supernatant was filtered through 0·22‐μm filter and stored at −80°. The extract was assayed for endotoxin levels using the EndoLISA (Hyglos GmbH, Starnberg, Germany) and was 19·4 EU/mg protein.
Sensitization of mice
C57BL/6J mice were sensitized intranasally with low‐dose Blomia tropicalis extract (BTE) (0·5 μg) or high‐dose BTE (50 μg) in 25 μl PBS on day 0, and subsequently challenged with 10 μg of BTE on day 21–23. Mice were killed at 24 hr after the last challenge. In some experiments, mice were killed at 5, 24 or 48 hr after the last exposure, and on 1–3 days, 7 days after the single BTE priming. In the additional experiment, C57BL/6J mice were sensitized intranasally with 0·5 μg of BTE in 25 μl PBS on day 0 or day 0–2, and subsequently challenged with 0·5 μg of BTE on day 21–23. Another group of C57BL/6J mice were repeatedly exposed with 0·5 μg of BTE continuously for 3 days per week. Mice were killed at 3 days after the last challenge.
Flow cytometry
The following antibodies were used for staining cells: anti‐CD45‐Alexa Fluor 488 (30‐F11, BD Pharmingen, BD Biosciences, San Jose, CA) or BUV395 (30‐F11, BD Biosciences), anti‐CD3ε‐phycoerythrin‐Cy7 (PE‐Cy7) (145‐2C11, eBioscience, San Diego, CA), Peridinin chlorophyll protein‐Cy5.5 (145‐2C11, eBioscience), Alexa Flour 488 (145‐2C11, BD Pharmingen), or allophycocyanin (APC) (145‐2C11, eBioscience), anti‐CD3‐eFlour450 (17A2, eBioscience), anti‐CD11c‐eFluor450 (N418, eBioscience) or Alexa Fluor 647 (N418, BioLegend, San Diego, CA), anti‐Siglec‐F‐PE (E50‐2440, BD Pharmingen), anti‐Ly‐6G‐Alexa Fluor 647 (1A8, BioLegend), anti‐CD4‐Alexa Fluor 647 (RM4‐5, BD Pharmingen) or Pacific Blue (RM4‐5, BD Pharmingen), anti‐GATA‐3‐eFlour660 (TWAJ, eBioscience), anti‐Foxp3‐PE (FJK‐16s, eBioscience), anti‐CD19‐ FITC (6D5, BioLegend) or Brilliant Violet 650 (1D3, BD Horizon, BD Biosciences), anti‐CD45R (B220)‐Alexa Flour 488 (RA3‐6B2, eBioscience), anti‐Gr‐1 (Ly6G/6C)‐FITC (RB6‐8C5, BioLegend), anti‐FcεRIα‐FITC (MAR‐1, eBioscience) or PE (MAR‐1, eBioscience), anti‐CD11b‐FITC (M1/70, BioLegend), anti‐NK1.1‐Alexa Flour 488 (PK136, BioLegend), anti‐TER‐119‐FITC (TER‐119, BioLegend), anti‐IL‐33Rα (ST2)‐PE (DIH9, BioLegend), anti‐CD25 (IL‐2Rα)‐BV650 (PC61, BioLegend), anti‐CD90.2 (Thy1.2)‐APC (53‐2.1, BD Biosciences), anti‐CD278 (ICOS)‐BV421 (C398.4A BioLegend), anti‐Sca‐1 (Ly6A/E)‐PE‐Cy7 (D7, BD Biosciences), anti‐CD49b‐PE‐Cy7 (Dx5, eBioscience), anti‐CD117 (c‐kit)‐APC (2B8, eBioscience) or BV786 (2B8, BD Horizon). Purified anti‐mouse CD16/32 (93, BioLegend) was used during all staining. Live/dead cells were differentiated by aqua or near IR live/dead fixable dye staining (Molecular Probes, Invitrogen, Singapore), according to the manufacturer's instructions. Flow cytometric analysis were performed using CyAn ADP (Beckman Coulter, Brea, CA), BD LSRFortessa, or BD LSRFortessa X‐20 (BD Biosciences, San Jose, CA). The data were analysed using flowjo software (Tree Star, Ashland, OR).
Bronchoalveolar lavage analysis
Bronchoalveolar lavage fluid (BALF) was collected by three 0·8‐ml instillations of PBS into the trachea of cannulated mice, followed by blood collection through cardiac puncture. The BAL cells were stained and differentiated by flow cytometry with antibodies to CD45, CD3ε, CD11c, CD19, Siglec‐F and Ly‐6G. BAL cytokine levels of interleukin‐4 (IL‐4), IL‐5, IL‐9, IL‐10, IL‐13, IL‐17A, interferon‐ γ (IFN‐γ), IL‐33, IL‐25, and thymic stromal lymphopoietin (TSLP) were measured by ELISA (R&D Systems, Minneapolis, MN).
Lung cell isolation
Lungs were excised and digested in Liberase Blendzyme (Roche Diagnosis, Singapore) for 45 min at 37° before physical disruption into single‐cell suspensions by passing through a 70‐μm cell strainer (Fischer Scientific, Singapore). Single‐cell suspensions were layered on Ficoll‐Paque (GE Healthcare Life Sciences, Singapore) and centrifuged at 600 g for 20 min at room temperature. Cells accumulating at the interface were collected, washed and cultured at a density of 1·0 × 106 cells/well in RPMI medium (Invitrogen) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μm 2‐mercaptoethanol (Sigma‐Aldrich, Singapore), and 10% fetal calf serum (HyClone, GE Healthcare Life Sciences) with or without 10 μg/ml of BTE in U‐bottomed 96‐well plates for 5 days.
Intracellular transcription factor staining of lung and mediastinal lymph node cells
To assess transcription factors, lung cells after the isolation and mediastinal lymph node (MLN) cells after the 5 days ex vivo culture with 10 μg/ml of BTE were used. The cells were washed, fixed using fixation/permeabilization buffer (eBioscience) for 1 hr, washed with permeabilization buffer (eBioscience), and stained for transcription factors.
Lung histological analysis
Lung tissues were obtained after perfusion with PBS and inflated with 1 ml of 4% paraformaldehyde (Sigma‐Aldrich) instilled through a tracheostomy tube. Tissues were further fixed in 4% paraformaldehyde for 3–5 days, dehydrated and embedded in paraffin. Sections (5 μm thick) were cut and stained with haematoxylin & eosin reagent (Sigma‐Aldrich) for the detection of inflammatory infiltration and periodic acid‐Schiff (PAS) reagent (Sigma‐Aldrich) for mucus‐secreting cells. To determine the severity of inflammatory cell infiltration, the airways and blood vessels in lung sections were scored blind by using a five‐point scoring system as follows. The score system was 0, normal; 1, few cells; 2, a ring of inflammatory cells of one cell‐layer deep; 3, a ring of inflammatory cells two to four cell‐layers deep; 4, a ring of inflammatory cells more than four cell‐layers deep.18 Fifty airways and blood vessels were scored per lung and the mean was calculated in each mouse. To assess the mucus‐secreting cells, the lung sections were scored blind at five points over 200 μm on the bronchi per sample. In the central airway, five locations were randomly selected, measured over 200 μm on bronchi and PAS‐positive cells per spot were counted. The score was determined by the sum of the number of those cells at the five points and expressed as the number of PAS‐positive cells per unit length (mm) of basement membrane on bronchus.19
Detection of Foxp3‐eGFP+ CD4+ T cells
Foxp3‐eGFP+ CD4+ T cells were collected from BALF, lung and MLN of BTE‐immunized Foxp3‐eGFP mice that were killed on day 1 after the last challenge. The cells were stained and analysed by flow cytometry with antibodies to CD3ε and CD4. Foxp3‐eGFP+ CD4+ T cells were identified as eGFP+ CD4+ CD3ε+ cells.
Measurement of serum immunoglobulins
Blood was directly collected by the cardiac puncture and serum was collected after centrifugation and stored at −80° until analysed. Total serum IgE was measured by ELISA according to the manufacturer's instructions (BD Biosciences). BTE‐specific IgE and IgG1 were measured using plates coated with 5 μg/ml of BTE in carbonate buffer (0·1 m sodium carbonate, pH 9·5). Samples were diluted 1 : 5 or 1 : 10 in assay diluent (PBS with 10% fetal bovine serum, pH 7·0) and incubated overnight at 4°. Detection was performed with biotinylated rat anti‐mouse IgE (LO‐ME‐3) or IgG1 (LO‐MG1‐2) antibodies (5 μg/ml) (AbD Serotec, Oxford, UK), avidin alkaline phosphatase (Sigma‐Aldrich, Singapore), and p‐nitrophenyl phosphate (Sigma‐Aldrich).
Identification of type 2 ILC2s
The ILC2s were collected from lung and MLN of BTE‐sensitized C57BL/6J mice that were killed at 5, 24 and 48 hr after the last exposure. The cells were stained by flow cytometry with antibodies. ILC2s were identified as CD45+ Lineage− (CD3ε, CD19, B220, Gr‐1, FcεRIα, CD11b, NK1.1, Ter‐119) IL‐33Rα+ CD25+ CD90.2+ ICOS+ Sca‐1+ cells.
Kinetics of IL‐4‐eGFP+ CD4+ T cells
IL‐4‐eGFP+ CD4+ T cells were collected from lung and MLN of BTE‐sensitized 4get mice on days 1, 2, 3 and 7 after single‐dose BTE priming, stained and analysed by flow cytometry. IL‐4‐eGFP+ CD4+ T cells were identified as eGFP+ CD45+ CD3ε+ CD4+ CD19− CD49b− CD117− FcεRIα− cells.
Statistical analysis
Statistical analysis was performed using graphpad prism (GraphPad Software, San Diego, CA). The unpaired Student's t‐test was used for comparison between two groups, and one‐way analysis of variance with Dunnett's or Tukey's test was used for multiple group comparisons.
Results
Low‐dose sensitization augments the Th2 response in respiratory tissue and is comparable to high‐dose priming
To investigate the effect of priming dose on the allergic response mice were intranasally immunized with two key priming doses (low dose 0·5 μg and high dose 50 μg) of BTE and killed 1 day after the last challenge (Fig. 1a). In BALF, cell numbers in the low‐dose‐primed group were comparable to those in the high‐dose group (Fig. 1b) whereas the levels of IL‐5 and IL‐13 in the low‐dose, but not the high‐dose, group were higher than controls (Fig. 1c). The quantity of IL‐10 in BALF was low but was significantly higher in the low‐dose compared with the high‐dose group. In addition, we evaluated the levels of Th2 cytokines produced by lung and MLN cells cultured with BTE. Higher levels of IL‐5, IL‐10 and IL‐13 were detected from cultured lung cells at both doses (Fig. 1d) compared with controls. Th2 cytokine levels secreted by MLN cells from high‐dose‐primed mice were comparable to those primed with the low dose (Fig. 1e). Also, we measured epithelium‐derived cytokines in BALF. Interleukin‐33 was significantly higher than control at both doses (Fig. 1f), but IL‐25 and TSLP were not different (data not shown). We also noted that numbers of B cells in BALF were greater with high‐dose priming (Fig. 1g). Accordingly, Th2 cytokine production induced by low‐dose sensitization was equivalent or greater compared with high‐dose priming but B cell numbers were greater with high‐dose priming.
Figure 1.
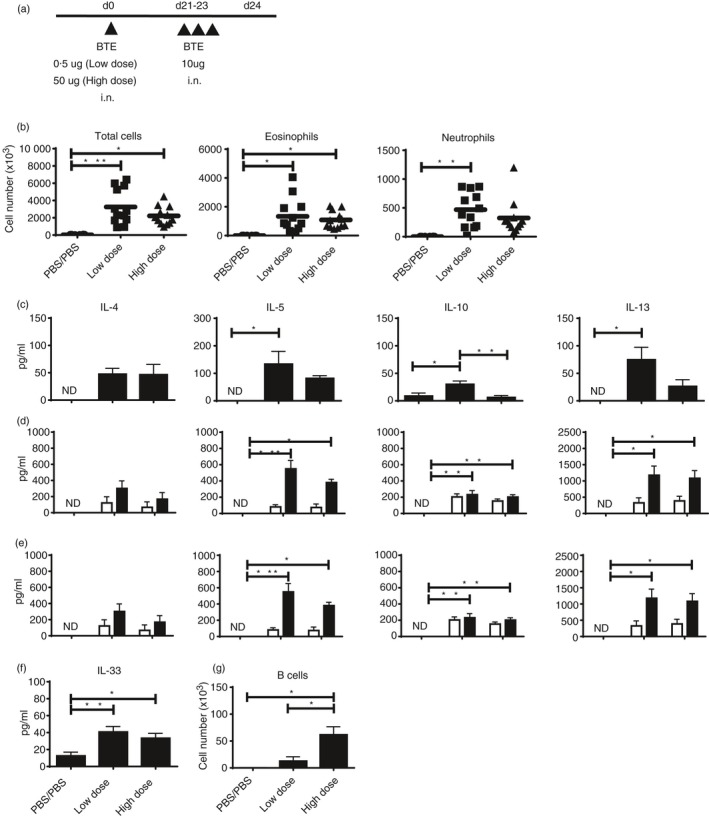
Low‐dose sensitization produces a strong T helper type 2 (Th2) response in respiratory tissues. (a) C57BL/6J mice were sensitized intranasally with low (0·5 μg) or high (50 μg) dose Blomia tropicalis extract (BTE) in 25 μl PBS on day 0, and subsequently challenged with 10 μg of BTE on days 21–23 and killed 24 hr after the last challenge. (b) Differential cell counts in bronchoalveolar lavage fluid (BALF). Each dot represents an individual mouse. Bars indicate mean. (c) Levels of Th2 cytokines in BALF. (d) Cytokines from lung cells after the re‐stimulation with (black bars) or without (white bars) 10 μg/ml of BTE for 5 days. (e) Cytokines from mediastinal lymph node (MLN) cells after the re‐stimulation with (black bars) or without (white bars) 10 μg/ml of BTE for 5 days. (f) Levels of epithelium‐derived cytokines in BALF. Data presented are pooled from four independent experiments with three or four mice/group/experiment (n = 12 mice). (g) The number of B cells in BALF from five mice. Data represent means ± SEM and significance determined by one‐way analysis of variance with Tukey's test or unpaired Student's t‐test, *P < 0·05, **P < 0·01, ***P < 0·001; ND, not detected; NA, not available.
High‐ and low‐dose priming elicit comparable inflammatory cell infiltration and airway mucus secretion and similar GATA‐3 and Foxp3 expressions, but low‐dose priming does not produce a significant IgE or IgG1 response
Intranasal immunization of mice with BTE (Fig. 1a) resulted in inflammatory cell infiltration around airways and vessels (Fig. 2a) and airway mucus production (Fig. 2b) demonstrated using haematoxylin & eosin and PAS staining, respectively. The severity of lung inflammation and PAS staining was quantified using a previously described scoring system.18, 19 The inflammation score for both immunized groups was significantly increased compared with control mice (Fig. 2c) as was the score for mucus secretion (Fig. 2d).
Figure 2.
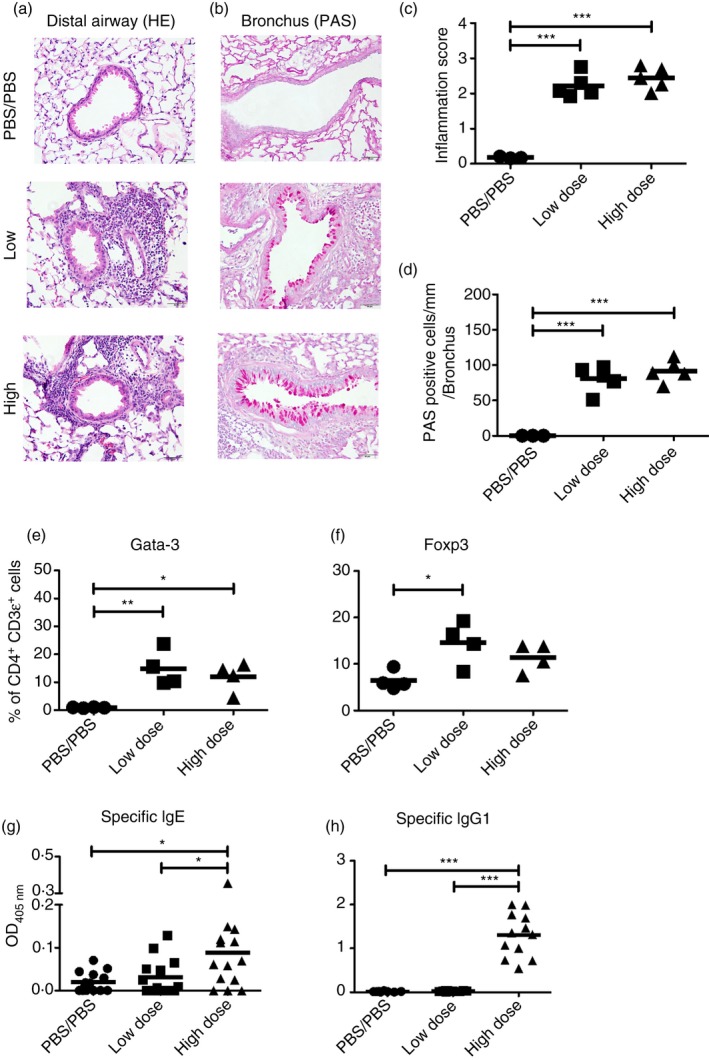
Low‐dose and high‐dose priming elicit comparable inflammatory cell infiltration around airways and vessels and airway mucus secretion and similar GATA‐3 and Foxp3 expression, but low‐dose priming does not produce a significant IgE or IgG1 response. Representative images for (a) haematoxylin and eosin (H&E) and (b) periodic acid‐Schiff's (PAS) stained lung tissue sections are shown. Magnification × 400. Scale black bar; 50 μm. (c) Scoring of H&E‐stained lung sections for severity of inflammation. The sections scored blind by using a five‐point scoring system as follows, 0, normal; 1, few cells; 2, a ring of inflammatory cells one‐cell‐layer deep; 3, a ring of inflammatory cells two to four cell‐layers deep; 4, a ring of inflammatory cells more than four cell‐layers deep. Fifty airways and blood vessels were scored per lung. (d) Scoring of PAS‐stained lung section for mucus‐secreting cells on airways. The lung sections were scored blind, using five points along 200 μm of bronchi per sample. In central airway, five spots per lung were randomly selected, 200 μm was measured along the bronchi, and PAS‐positive cells were counted. The score was determined as the sum of cells expressed as the number of PAS‐positive cells per unit length (mm) of basement membrane on bronchus (n = 5). (e) The expression of GATA‐3 and (f) Foxp3 in lung cells 1 day after the last challenge. Numbers indicate the percentage of positive CD4+ CD3ε+ (n = 4). (g) Blomia tropicalis extract (BTE) ‐specific IgE and (h) IgG1 levels in serum. Data are pooled from four independent experiments with three to four mice/group/experiment (n = 8−14). Statistical analysis was performed using analysis of variance with Tukey's test, *P < 0·05, **P < 0·01, ***P < 0·001.
We examined GATA‐3, a Th2 transcription factor, and Foxp3, a regulatory T‐cell transcription factor, in lung cells by intracellular staining 1 day after the last exposure (Fig. 1a). Low‐ and high‐dose priming increased the expression of GATA‐3 by lung CD4+ T cells compared with control mice (Fig. 2e). Foxp3 expression, however, was significantly increased in mice given the low dose of BTE but not at the high dose (Fig. 2f). Therefore, we examined Foxp3‐eGFP mice immunized using the low‐ or high‐dose protocol and analysed cell number and the percentage of Foxp3‐eGFP+ cells in respiratory tissues on day 1 following re‐exposure to BTE. The number and percentage in Foxp3‐eGFP+ cells were not significantly different between the two doses (see Supplementary material, Fig. S1). Consequently, the priming dose did not relate to expression of Foxp3. We next measured serum‐specific immunoglobulins by ELISA. High‐dose priming produced stronger specific IgE (Fig. 2g) and IgG1 (Fig. 2h) responses against BTE than low‐dose sensitization. In addition, we examined measurement of other immunoglobulin subtypes by multiplex immunoassay. Consistent with the results of specific IgE by ELISA in the low‐dose and high‐dose model, we found that total (non‐specific) IgE and IgG2a in high‐dose priming were higher than those in control and low‐dose priming (see Supplementary material, Fig. S2). Regarding the difference in IgG2a, we investigated the levels of IFN‐γ, which is a key cytokine for secretion of IgG2a in both priming models. The level of IFN‐γ from MLN cells cultured with BTE following high‐dose priming was significantly higher than following low‐dose priming (see Supplementary material, Fig. S3).
Low‐dose priming elevated ILC2s in lung after the re‐exposure, while high‐dose sensitization strongly polarized IL‐4‐producing CD4+ T (Th2) cells in respiratory tissues
We hypothesized that the Th2 response after allergen re‐exposure in the low‐dose priming model might stimulate innate immune cells such as ILC2s. To verify this, we determined the number of ILC2 cells (IL‐33Rα+, CD25+, CD90.2+, ICOS+, Sca1+) in the lung and MLN by flow cytometry (Fig. 3a). ILC2s in the low‐dose priming model increased 24 hr after exposure in the lung, while ILC2s in the high‐dose model plateaued (Fig. 3b). The percentages of ILC2s of lung in the low‐dose‐primed model increased significantly more than controls from 24 hr and there was a significant difference between low dose and control at 48 hr. However, there was no significant difference of ILC2s between the low‐dose and high‐dose priming models at 48 hr, as between high dose and control. ILC2s did not accumulate in MLN, and were not different in either priming model.
Figure 3.
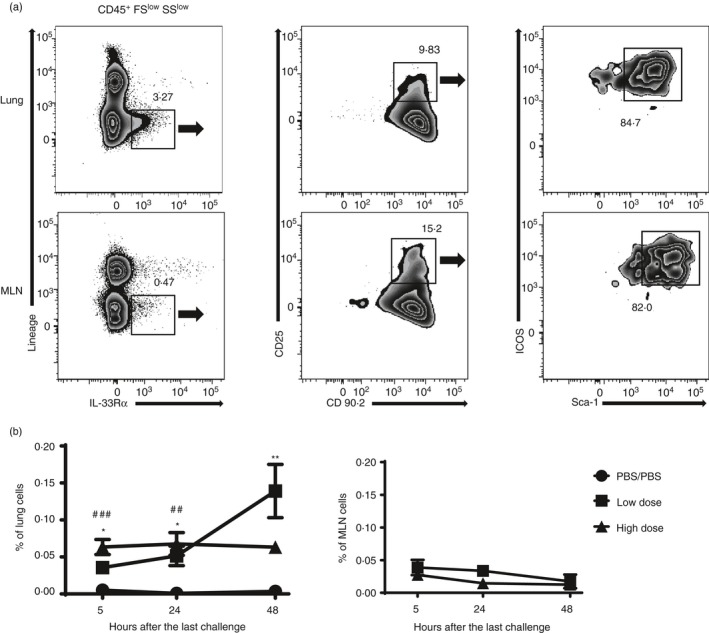
Low‐dose priming increases type 2 innate lymphoid cells (ILC2s) in lung after the re‐exposure. (a) The ILC2s were identified in lung mediastinal lymph node (MLN) of Blomia tropicalis extract (BTE) immunized mice at 5, 24 and 48 hr after the last challenge. The cells were stained and analysed by flow cytometry using antibodies as described in the Materials and methods. Representative ILC2s gating of lung cells at 48 hr and MLN cells at 24 hr after the last challenge. Numbers indicate the percentage of gated cells in the panel. The percentage (b) of ILC2s in lung and MLN after the last challenge. Circles indicate PBS/PBS control group, squares the low‐priming‐dose group and triangles the high‐priming‐dose group. Data are shown as the mean ± SEM with four mice/group/experiment (n = 4). Statistical analysis was performed using analysis of variance with Tukey's test or unpaired Student's t‐test at each time‐point. *P < 0·05, **P < 0·01, compared with the low‐dose priming group and PBS/PBS control group. # P < 0·05, ## P < 0·01, ### P < 0·001, compared the high‐dose priming group and PBS/PBS control group.
Next, we examined the activation of IL‐4 in CD4+ T cells using 4get mice in which eGFP is linked to the IL‐4 promoter. We analysed the percentage of lung and MLN cells that were positive for eGFP by flow cytometry at various time‐points after priming. High‐dose single priming resulted in the robust induction of Th2 cells from day 2 in lung and MLN as previously reported17 (Fig. 4a–d). On the other hand, low‐dose single priming did not strongly induce IL‐4‐producing CD4+ T cells.
Figure 4.
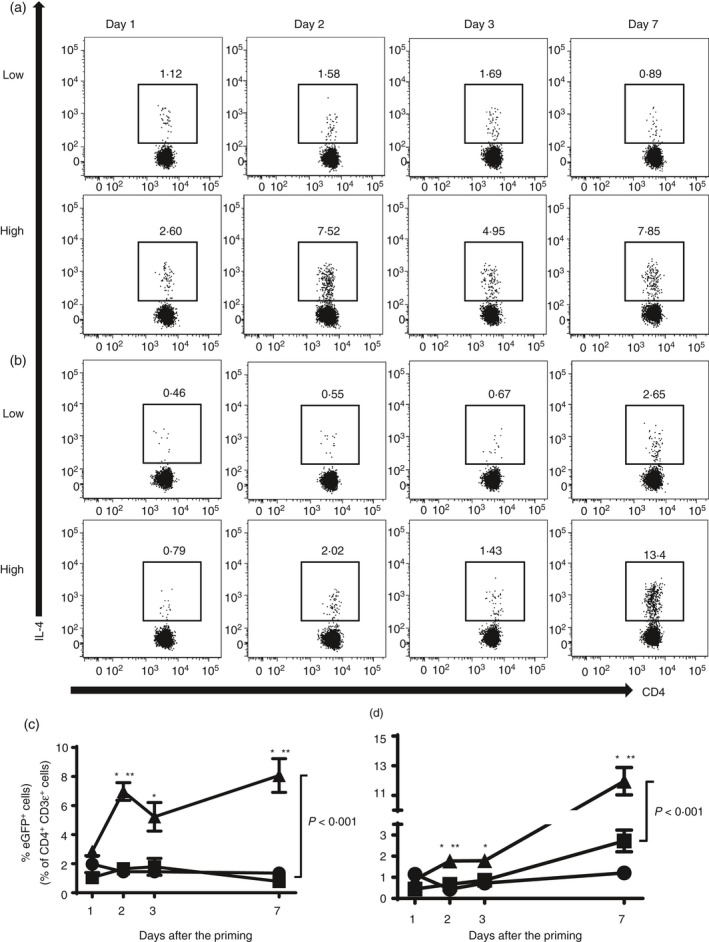
Low‐dose single priming does not strongly polarize interleukin‐4 (IL‐4) ‐producing T helper type 2 (Th2) cells. IL‐4‐eGFP+ CD4+ T cells were collected from lung and mediastinal lymph nodes (MLN) of Blomia tropicalis extract (BTE) sensitized IL‐4/GFP‐enhanced transcript (4get) mice on days 1, 2, 3 and 7 after a single priming dose of BTE. IL‐4 producing Th2 cells were stained and analysed by flow cytometry using antibodies as described in the Materials and methods. A representative result is shown for IL‐4‐eGFP‐positive cells in lung (a) and MLN (b). The upper row is low‐dose priming and the lower row is high‐dose priming. Numbers indicate the percentage of IL‐4‐eGFP‐positive CD4+ CD3ε+ cells. Kinetics of IL‐4‐producing Th2 cells expressed as a percentage of IL‐4eGFP‐positive cells in the lung (c) and MLN (d) at different cells after priming. Circles indicate the PBS‐treated group; squares indicate low‐dose single priming group; triangles indicate high‐dose single priming group. Data represent mean ± SEM with four mice/group/experiment (n = 4). Statistical analysis was performed using analysis of variance with Tukey's test at each time‐point. *P < 0·05, ***P < 0·001, compared with the high‐dose priming group and PBS/PBS control group.
Low‐dose continuous exposure to BTE produces a stronger antibody response
Finally, we determined whether repeated low‐dose exposure (continuous) could induce a comparable antibody response compared with high‐dose priming. Mice were immunized using different protocols: single priming (SP) 0·5 µg followed by three challenges of 0·5 μg; multiple priming (MP) 3 × 0·5 μg followed by three challenges of 0·5 μg; and continuous exposure (CE) 12 × 0·5 μg of BTE (Fig. 5a). The number of eosinophils and neutrophils was similarly elevated with all immunization schedules (Fig. 5b). The levels of IL‐4, IL‐5, IL‐10, IL‐33 and TSLP in BALF of all immunized groups were also similarly increased (Fig. 5c). Interleukin‐13 was only increased in the MP group. Importantly, the levels of total IgE and specific IgG1 were strongly induced by continuous low‐dose allergen exposure (Fig. 5d). Specific IgE responses were variable and so were not significantly increased; however, specific IgE was detected in five of six CE mice (83·3%) and in only one of six SP mice (16·7%). Interestingly the cumulative doses administered were SP 2 μg, MP 3 μg, and CE 6 μg. Hence, continuous exposure to low doses of allergen (6 μg) induced a comparable response to high‐dose allergen (80 μg) (Fig. 1a) and may better reflect natural sensitization.
Figure 5.
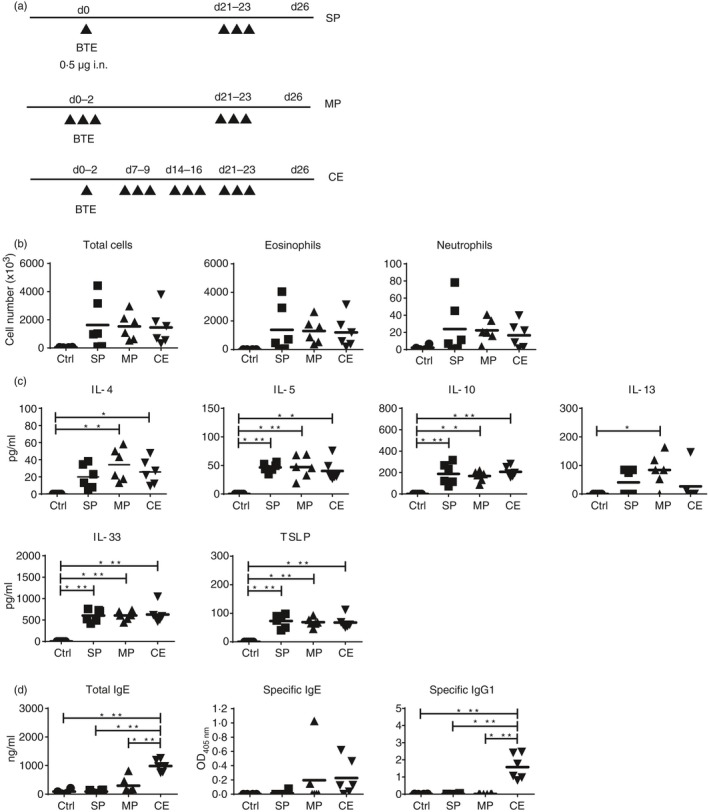
Low‐dose continuous exposure produces a stronger antibody response. (a) Schematic protocol of different low‐dose priming models. The upper protocol is single low‐dose priming (SP) group (0·5 μg followed by three challenges of 0·5 µg), the middle is the multiple priming (MP) group (3 × 0·5 µg followed by three challenges of 0·5 µg), and the lower is the continuous exposure (CE) group (12 × 0·5 µ). (b) Differential cell counts in bronchoalveolar lavage fluid (BALF); (c) levels of T helper type 2 cytokines and epithelium‐derived cytokines in BALF; (d) total IgE, Blomia tropicalis extract (BTE) ‐specific IgE and IgG1 levels in serum. Each dot represents an individual mouse. Bars indicate mean with six mice/group/experiment (n = 6). Ctrl; PBS control. Statistical analysis was performed using analysis of variance with Tukey's test, *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
In this study, we have demonstrated that low‐dose priming with inhaled allergen produces a Th2 response equal to high‐dose priming. However, single low‐dose priming failed to induce specific IgE and IgG1 while repeated low‐dose immunization induced allergen‐specific IgG1 and IgE. This is the first study to demonstrate that the distinct elements of the allergic response are dependent on the priming dose of respiratory allergen.
The use of very small amounts of allergen for priming has been examined before. Small amounts of OVA allergen without adjuvant failed to induce a specific antibody response in most mouse strains except the high responder BALB/c mouse strain.20 However, previous reports have shown that small amounts of antigen (0·1–1·0 μg) of OVA and benzylpenicilloyl conjugated antigens with any adjuvant such as Al(OH)3 or Bordetella pertussis could elicit humoral immune responses in some strains of mice and rats.21, 22, 23 They have shown that low‐dose allergen priming needed any adjuvant to induce an antibody response. Especially repeated small‐dose OVA (0·1 μg) plus Al(OH)3 by intra‐peritoneal immunization induced persistent IgE synthesis, while low‐dose OVA without adjuvant failed to provoke any antibody response.22 Interestingly, challenge with a larger dose (10 μg) elicited a progressively diminishing antibody titre, whereas with a smaller dose the antibody response was maintained.23 Hence, antigen dose has a profound influence on antibody production. However, none of these reports showed whether priming dose effected Th2 cells, Th2 cytokines, or other immune cells.
Previously, polarization of helper CD4+ T‐cell subtypes was found to be affected by antigen dose. Sakai et al. reported that priming 10 μg of OVA in an asthma mouse model could induce Th2 cytokines and an IgE response and stronger eosinophilic inflammation than that with 1000 μg of OVA.24 They suggested that high‐dose sensitization attenuated Th2 response by increasing the levels of Th1 cytokines. In vitro studies using T‐cell receptor transgenic mice demonstrated that naive CD4+ T cells cultured with low concentrations of antigenic peptide such as OVA peptide,25 pigeon cytochrome c 26 and tobacco hornworm moth cytochrome c 27 induced a Th2 response (GATA‐3, IL‐4), whereas stimulation with high concentrations of peptide abrogated GATA‐3 expression and induced IFN‐γ expression. In contrast, Hosken et al. showed that both high and very low doses of OVA peptide induced IL‐4‐producing CD4+ T cells cultured with antigen‐presenting cells in vitro whereas midrange peptide doses shifted the response to IFN‐γ‐producing CD4+ T cells.28 Constant et al. concluded that it was controversial whether high or low doses of antigen are best suited to induce a Th2 immune response.29
In the study presented here we have demonstrated that low‐dose priming produced comparable Th2 cytokines and histological findings in BAL and lung tissue to high‐dose sensitization. The low‐dose model induced larger or similar amounts of IL‐5, IL‐13 and IL‐33 compared with the high‐dose model and equal Th2 inflammation. However, low‐dose priming failed to induce allergen‐specific antibodies. Therefore, we hypothesize that low‐dose priming may not have been able to recruit sufficient B cells and this was supported by the observation that more B cells were present in BALF with high‐dose priming.
Recently, ILC2s have been shown to be important players in the lung immune response at an early stage of the development of Th2 immunity. ILC2s are reported to produce IL‐5 and IL‐13 in response to IL‐33 without antigen‐specific T cells and acquired immunity.30 In mice, influenza virus, ozone and protease all activated ILC2s and resulted in a Th2 response in a T‐cell‐independent manner31, 32, 33 and some experiments have shown that the Th2 response to house dust mite or Alternaria was induced by ILC2s.34, 35, 36 Gold et al. reported that ILC2‐deficient mice exhibited lower levels of house dust mite‐specific IgE and IgG1 than wild‐type mice.34 They found that ILC2s played an important role in priming adaptive immunity, but those priming doses were > 10 μg of allergen. In the present study, we demonstrated that more ILC2s were present in the lungs of mice sensitized with the lower dose of BTE whereas IL‐4‐producing CD4+ T cells were more strongly elevated in lung and MLN by priming with the higher dose of allergen.
Regarding the induction of IL‐4 levels in BALF and respiratory tissues, there were different results between those detected by the ELISA and flow cytometry using 4get mice in low‐ and high‐dose priming models. ELISA for the detection of IL‐4 levels may have been insufficiently sensitive or may have been influenced by consumption of this cytokine in the cultures.37 Therefore, we used the more sensitive and robust IL‐4 reporter transgenic mice for evaluation of the IL‐4 response in respiratory tissues.
Finally, we determined whether repeated low‐dose exposure to BTE could induce an antibody response. In contrast with a previous study using epicutaneous exposure38 to very‐low‐dose allergen where mice could not produce antibodies, we could induce BTE‐specific antibodies after repeated intranasal exposure to low doses of antigen. Prolonged allergen exposure has been reported to be associated with both persistence1 and suppression39, 40 of allergic inflammation. In these experiments repeat doses used were > 10 μg of a mite allergen on each occasion.1, 40 We demonstrated that repeated low‐dose exposure could induce antibodies. It is possible that continuously elevated ILC2s might support a Th2 response by secretion of Th2 cytokines34, 35, 36 or enhanced CD4+ T‐cell proliferation.41 Hence, low‐dose mite allergen priming produced a Th2 response equal to the high‐dose sensitization model. However, low‐dose priming failed to elicit specific antibody unless administered repeatedly.
Disclosures
This work was supported by the National Medical Research Council (NMRC) (Grant No. NMRC/1321/2012). The authors declare no other financial or commercial conflict of interest.
Supporting information
Figure S1. Expressions of Foxp3 in respiratory tissues are not significantly different between low‐ and high‐dose priming.
Figure S2. The levels of total immunoglobulin subtypes in serum are not significantly different between low‐ and high‐dose priming except for IgG2a.
Figure S3. The interferon‐γ level from mediastinal lymph node cells in high‐dose priming is significantly higher than that in low‐dose priming.
Acknowledgements and contributions
We would like to thank Shu Shin La, Neil Quanwei Tay and Nayana Prabhu for helpful discussions. KF, YLC, KSHW, QZ, DCPL and KHL performed the experimental work, analysed the data and drafted the manuscript. GHT and PEH contributed to the laboratory work and data analysis. DMK, principal investigator, conceived the study design, wrote the grant, analysed data and drafted the manuscript. KF and DMK wrote the manuscript.
References
- 1. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ et al Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004; 169:378–85. [DOI] [PubMed] [Google Scholar]
- 2. Fattouh R, Pouladi MA, Alvarez D, Johnson JR, Walker TD, Goncharova S et al House dust mite facilitates ovalbumin‐specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med 2005; 172:314–21. [DOI] [PubMed] [Google Scholar]
- 3. Lundy SK, Berlin AA, Lukacs NW. Interleukin‐12‐independent down‐modulation of cockroach antigen‐induced asthma in mice by intranasal exposure to bacterial lipopolysaccharide. Am J Pathol 2003; 163:1961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR et al Allergen‐induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci U S A 1997; 94:1344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daan de Boer J, Roelofs JJ, de Vos AF, de Beer R, Schouten M, Hommes TJ et al Lipopolysaccharide inhibits Th2 lung inflammation induced by house dust mite allergens in mice. Am J Respir Cell Mol Biol 2013; 48:382–99. [DOI] [PubMed] [Google Scholar]
- 6. Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med 2013; 19:977–9. [DOI] [PubMed] [Google Scholar]
- 7. Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010; 376:835–43. [DOI] [PubMed] [Google Scholar]
- 8. Yeoh SM, Kuo IC, Wang DY, Liam CK, Sam CK, De Bruyne JA et al Sensitization profiles of Malaysian and Singaporean subjects to allergens from Dermatophagoides pteronyssinus and Blomia tropicalis . Int Arch Allergy Immunol 2003; 132:215–20. [DOI] [PubMed] [Google Scholar]
- 9. Kidon MI, Chiang WC, Liew WK, Lim SH, See Y, Goh A et al Sensitization to dust mites in children with allergic rhinitis in Singapore: does it matter if you scratch while you sneeze? Clin Exp Allergy 2005; 35:434–40. [DOI] [PubMed] [Google Scholar]
- 10. Tsai JJ, Wu HH, Shen HD, Hsu EL, Wang SR. Sensitization to Blomia tropicalis among asthmatic patients in Taiwan. Int Arch Allergy Immunol 1998; 115:144–9. [DOI] [PubMed] [Google Scholar]
- 11. Nelson RP Jr, DiNicolo R, Fernandez‐Caldas E, Seleznick MJ, Lockey RF, Good RA. Allergen specific IgE levels and mite allergen exposure in children with acute asthma first seen in an emergency department and in non‐asthmatic control subjects. J Allergy Clin Immunol 1996; 98:258–63. [DOI] [PubMed] [Google Scholar]
- 12. Caraballo L, Puerta L, Fernandez‐Caldas E, Lockey RF, Martinez B. Sensitization to mite allergens and acute asthma in a tropical environment. J Invest Allergol Clin Immunol 1998; 8:281–4. [PubMed] [Google Scholar]
- 13. Arruda LK, Vailes LD, Platts‐Mills TA, Fernandez‐Caldas E, Montealegre F, Lin KL et al Sensitization to Blomia tropicalis in patients with asthma and identification of allergen Blo t 5. Am J Respir Crit Care Med 1997; 155:343–50. [DOI] [PubMed] [Google Scholar]
- 14. Manolio TA, Barnes KC, Naidu RP, Levett PN, Beaty TH, Wilson AF. Correlates of sensitization to Blomia tropicalis and Dermatophagoides pteronyssinus in asthma in Barbados. Int Arch Allergy Immunol 2003; 131:119–26. [DOI] [PubMed] [Google Scholar]
- 15. Kuo IC, Cheong N, Trakultivakorn M, Lee BW, Chua KY. An extensive study of human IgE cross‐reactivity of Blo t 5 and Der p 5. J Allergy Clin Immunol 2003; 111:603–9. [DOI] [PubMed] [Google Scholar]
- 16. Chan SL, Ong TC, Gao YF, Tiong YS, de Wang Y, Chew FT et al Nuclear magnetic resonance structure and IgE epitopes of Blo t 5, a major dust mite allergen. J Immunol 2008; 181:2586–96. [DOI] [PubMed] [Google Scholar]
- 17. Zhou Q, Ho AW, Schlitzer A, Tang Y, Wong KH, Wong FH et al GM‐CSF‐licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis . J Immunol 2014; 193:496–509. [DOI] [PubMed] [Google Scholar]
- 18. Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD et al Fas‐positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med 2006; 203:1173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuda H, Suda T, Sato J, Nagata T, Koide Y, Chida K et al α‐Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am J Respir Cell Mol Biol 2005; 33:22–31. [DOI] [PubMed] [Google Scholar]
- 20. Holt PG, Rose AH, Batty JE, Turner KJ. Induction of adjuvant‐independent IgE responses in inbred mice: primary, secondary, and persistent IgE responses to ovalbumin and ovomucoid. Int Arch Allergy Immunol 1981; 65:42–50. [DOI] [PubMed] [Google Scholar]
- 21. Levine BB, Vaz NM. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. Int Arch Allergy 1970; 39:156–71. [DOI] [PubMed] [Google Scholar]
- 22. Vaz EM, Vaz NM, Levine BB. Persistent formation of reagins in mice injected with low doses of ovalbumin. Immunology 1971; 21:11–5. [PMC free article] [PubMed] [Google Scholar]
- 23. Jarrett EE, Hall E. Regulation of ongoing IgE antibody responses with minute doses antigen. Eur J Immunol 1981; 11:520–3. [DOI] [PubMed] [Google Scholar]
- 24. Sakai K, Yokoyama A, Kohno N, Hiwada K. Effect of different sensitizing doses of antigen in a murine model of atopic asthma. Clin Exp Immunol 1999; 118:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ise W, Totsuka M, Sogawa Y, Ametani A, Hachimura S, Sato T et al Naive CD4+ T cells exhibit distinct expression patterns of cytokines and cell surface molecules on their primary responses to varying doses of antigen. J Immunol 2002; 168:3242–50. [DOI] [PubMed] [Google Scholar]
- 26. Yamane H, Zhu J, Paul WE. Independent roles for IL‐2 and GATA‐3 in stimulating naive CD4+ T cells to generate a Th2‐inducing cytokine environment. J Exp Med 2005; 202:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med 1995; 182:1591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor‐α β‐transgenic model. J Exp Med 1995; 182:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol 1997; 15:297–322. [DOI] [PubMed] [Google Scholar]
- 30. Yu S, Kim HY, Chang YJ, DeKruyff RH, Umetsu DT. Innate lymphoid cells and asthma. J Allergy Clin Immunol 2014; 133:943–50. [DOI] [PubMed] [Google Scholar]
- 31. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE et al Innate lymphoid cells mediate influenza‐induced airway hyper‐reactivity independently of adaptive immunity. Nat Immunol 2011; 12:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Q, Ge MQ, Kokalari B, Redai IG, Wang X, Kemeny DM et al Group 2 innate lymphoid cells mediate ozone‐induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2016; 137:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity 2012; 36:451–63. [DOI] [PubMed] [Google Scholar]
- 34. Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C et al Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2‐inducing allergen exposures. J Allergy Clin Immunol 2014; 133:1142–8. [DOI] [PubMed] [Google Scholar]
- 35. Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y et al Pulmonary innate lymphoid cells are major producers of IL‐5 and IL‐13 in murine models of allergic asthma. Eur J Immunol 2012; 42:1106–16. [DOI] [PubMed] [Google Scholar]
- 36. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL‐33‐responsive lineage‐ CD25+ CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 2012; 188:1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ewen C, Baca‐Estrada ME. Evaluation of interleukin‐4 concentration by ELISA is influenced by the consumption of IL‐4 by cultured cells. J Interferon Cytokine Res 2001; 21:39–43. [DOI] [PubMed] [Google Scholar]
- 38. Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2‐like response with high IgE production in mice. J Immunol 1996; 156:4077–82. [PubMed] [Google Scholar]
- 39. Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol 2007; 36:573–84. [DOI] [PubMed] [Google Scholar]
- 40. Bracken SJ, Adami AJ, Szczepanek SM, Ehsan M, Natarajan P, Guernsey LA et al Long‐term exposure to house dust mite leads to the suppression of allergic airway disease despite persistent lung inflammation. Int Arch Allergy Immunol 2015; 166:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 2014; 69:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expressions of Foxp3 in respiratory tissues are not significantly different between low‐ and high‐dose priming.
Figure S2. The levels of total immunoglobulin subtypes in serum are not significantly different between low‐ and high‐dose priming except for IgG2a.
Figure S3. The interferon‐γ level from mediastinal lymph node cells in high‐dose priming is significantly higher than that in low‐dose priming.


