Summary
Background
Global inequalities in access to health care are reflected in differences in cancer survival. The CONCORD programme was designed to assess worldwide differences and trends in population-based cancer survival. In this population-based study, we aimed to estimate survival inequalities globally for several subtypes of childhood leukaemia.
Methods
Cancer registries participating in CONCORD were asked to submit tumour registrations for all children aged 0–14 years who were diagnosed with leukaemia between Jan 1, 1995, and Dec 31, 2009, and followed up until Dec 31, 2009. Haematological malignancies were defined by morphology codes in the International Classification of Diseases for Oncology, third revision. We excluded data from registries from which the data were judged to be less reliable, or included only lymphomas, and data from countries in which data for fewer than ten children were available for analysis. We also excluded records because of a missing date of birth, diagnosis, or last known vital status. We estimated 5-year net survival (ie, the probability of surviving at least 5 years after diagnosis, after controlling for deaths from other causes [background mortality]) for children by calendar period of diagnosis (1995–99, 2000–04, and 2005–09), sex, and age at diagnosis (<1, 1–4, 5–9, and 10–14 years, inclusive) using appropriate life tables. We estimated age-standardised net survival for international comparison of survival trends for precursor-cell acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML).
Findings
We analysed data from 89 828 children from 198 registries in 53 countries. During 1995–99, 5-year age-standardised net survival for all lymphoid leukaemias combined ranged from 10·6% (95% CI 3·1–18·2) in the Chinese registries to 86·8% (81·6–92·0) in Austria. International differences in 5-year survival for childhood leukaemia were still large as recently as 2005–09, when age-standardised survival for lymphoid leukaemias ranged from 52·4% (95% CI 42·8–61·9) in Cali, Colombia, to 91·6% (89·5–93·6) in the German registries, and for AML ranged from 33·3% (18·9–47·7) in Bulgaria to 78·2% (72·0–84·3) in German registries. Survival from precursor-cell ALL was very close to that of all lymphoid leukaemias combined, with similar variation. In most countries, survival from AML improved more than survival from ALL between 2000–04 and 2005–09. Survival for each type of leukaemia varied markedly with age: survival was highest for children aged 1–4 and 5–9 years, and lowest for infants (younger than 1 year). There was no systematic difference in survival between boys and girls.
Interpretation
Global inequalities in survival from childhood leukaemia have narrowed with time but remain very wide for both ALL and AML. These results provide useful information for health policy makers on the effectiveness of health-care systems and for cancer policy makers to reduce inequalities in childhood cancer survival.
Funding
Canadian Partnership Against Cancer, Cancer Focus Northern Ireland, Cancer Institute New South Wales, Cancer Research UK, US Centers for Disease Control and Prevention, Swiss Re, Swiss Cancer Research foundation, Swiss Cancer League, and the University of Kentucky.
Introduction
Worldwide inequalities in health and health care are reflected in global differences in life expectancy and overall mortality in both adults and children,1 and the findings from several studies have highlighted global differences in cancer incidence2 and survival.3, 4 Diagnostic techniques and treatment for childhood leukaemia have improved since the 1990s. Access to these techniques and treatment has, however, been limited in some countries, partly by a shortage of resources.5
Leukaemias, a heterogeneous group of diseases of mostly unknown origin, are globally the most common malignancies in children (aged 0–14 years), except in Africa.6 Unlike in adults, acute lymphoid leukaemias are the commonest subtype in children (accounting for approximately 80% of cases), and acute myeloid leukaemia (AML) represents about 15% of cases. For both types, incidence varies widely with age; in lymphoid leukaemias, incidence is slightly higher in boys than girls, and in industrialised high-income countries (HIC).7 In low-income and middle-income countries (LMIC), where the population is young, the incidence of childhood leukaemias is lower than in HIC, but these diseases are still responsible for many deaths.2, 5
Research in context.
Evidence before this study
In 2015, the CONCORD-2 study initiated surveillance of survival trends for childhood leukaemia at a worldwide scale. Results from CONCORD-2 identified huge worldwide variation in 5-year net survival for children diagnosed with precursor-cell acute lymphoblastic leukaemia or lymphoma (ALL) during 1995-2009. Our analysis extends those results to cover survival trends for several subtypes of childhood leukaemia, grouped according to the third edition of the International Classification of Childhood Cancer.
Added value of this study
We included 89 828 children diagnosed with leukaemia during 1995–2009 in 53 countries. Despite substantial improvements in survival from childhood acute myeloid leukaemia (AML) in most countries during 1995–2009, huge international disparities in 5-year survival persisted up to 2009, matching those previously reported in the 2015 CONCORD-2 paper for childhood ALL. 5-year age-standardised net survival from AML (ie, the probability of surviving at least 5 years after diagnosis) was consistently lower than 5-year age-standardised net survival from ALL, but the difference narrowed in most countries since the early 2000s. Children aged 1–9 years at diagnosis had higher 5-year net survival than older or younger children, both for ALL and AML. Survival for older children (10–14 years) improved by 2009, but infants (aged <1 year) diagnosed with either ALL or AML still had the lowest 5-year net survival.
Implications of all the available evidence
Data obtained in the CONCORD programme provide a unique opportunity to explore disparities in survival from childhood leukaemia at an unprecedented scale. The results suggest that good access to health care and appropriate treatment have a clear population effect on survival for children with leukaemia. The findings support the need for continuing international efforts to improve worldwide access to appropriate cancer care for children. They can also be used to assess the effect of cancer strategies targeting childhood cancers.
Although cancer mortality trends provide a useful measure of the societal cancer burden, they depend on trends in both incidence and survival. Cancer survival is the probability that cancer patients survive up to a certain point after diagnosis. Observed survival and event-free survival are clinically important, but population-based net survival is the appropriate indicator for comparisons between populations. Net survival is the probability of surviving after controlling for mortality from other causes.
The CONCORD programme was designed to address the shortage of globally comparable data on population-based cancer survival.3, 4 Population-based cancer survival reflects several aspects of health care, from screening and early diagnosis, to access to effective treatment. This metric is increasingly used as a measure of the effectiveness of health-care systems in the management of cancer, and to assess the effectiveness of national cancer plans.8, 9 The second cycle of CONCORD (CONCORD-2)4 established global surveillance of population-based cancer survival for patients diagnosed with one of ten common cancers, or childhood leukaemia, during the 15-year period 1995–2009, using data from 279 population-based cancer registries in 67 countries. In this analysis, we examine worldwide trends in survival from precursor-cell acute lymphoblastic leukaemia (ALL) in children, by age and sex, alongside trends in survival from acute myeloid leukaemia (AML) and other types of childhood leukaemia.
Methods
Search strategy and selection criteria
Cancer registries participating in CONCORD-2 were asked to submit tumour registrations for all children (aged 0–14 years) diagnosed with a haematological malignancy between Jan 1, 1995, and Dec 31, 2009, including information about their vital status at Dec 31, 2009.4 Depending on the registry, patients were followed up actively, via direct investigation, or passively, using linkage to national or regional databases of death.4 Haematological malignancies were defined by morphology codes in the range 9590–9989 in the International Classification of Diseases for Oncology, third revision (ICD-O-3).10 215 registries in 60 countries submitted data on 126 830 children with a haematological malignancy. We excluded data from 13 registries from which the data were judged to be less reliable,4 or included only lymphomas (two), and data from countries for which fewer than 10 children were available for analysis (two). This left 124 015 records for children with a haematological malignancy.
We did standardised data cleaning in three phases, as detailed previously.4 Records that were ineligible (eg, for patients aged 15 years or older), inaccurate or inappropriate for survival analysis (eg, incoherent date sequence, or registration only from a death certificate or autopsy report) were excluded.4, 11 For patients with more than one record of a haematological malignancy diagnosed during 1995–2009, we kept only the record of the first malignancy. A few registries submitted records coded to earlier revisions of ICD-O or to the first revision of ICD-O-3. In agreement with these registries, we recoded those morphology codes to be compliant with ICD-O-3. Data from Sétif (Algeria), Arkhangelsk (Russia), Wrocław (Poland), and Northern Ireland (UK) were included after their data were recoded to ICD-O-3.
Data analysis
We estimated 5-year net survival with the Pohar-Perme estimator12 using the STNS command implemented in Stata 13. We used the life tables of background mortality rates by sex, single year of age, calendar year and—for USA, Israel, Malaysia, and New Zealand—by race or ethnic group, produced for CONCORD-2.13 For each country, we estimated net survival by calendar period of diagnosis (1995–99, 2000–04, and 2005–09). We used the classic cohort approach for children diagnosed during 1995–99 and 2000–04, because 5 years of follow-up data were available for all children. We used the period approach to predict 5-year survival for leukaemias diagnosed more recently (2005–09), as this approach allows for the prediction of survival where 5 years of follow-up are not yet available.14
Survival estimates for each country were based on data from a national registry or from one or several subnational registries. We excluded data from some regional registries from the pooled estimate for a given country if data quality or information about vital status were deemed unsatisfactory.4 Country estimates were flagged if data quality was considered less reliable.
We estimated 5-year survival by sex and age at diagnosis (<1, 1–4, 5–9, 10–14 years, inclusive). Exact age at diagnosis was calculated from the dates of birth and diagnosis. The rules adopted to impute missing components of dates have been described previously.4, 11 Age-standardised survival was calculated from three equally weighted age-specific estimates (0–4, 5–9, and 10–14 years).15 Data for age groups with fewer than ten patients were pooled with data for the adjacent age group; we then re-estimated survival for both age groups combined, and the pooled estimate was attributed to each age group.
Leukaemias were grouped according to the International Classification of Childhood Cancer (ICCC-3).16 We estimated survival for all lymphoid leukaemias combined (ICCC-3 group Ia), for acute myeloid leukaemias (AML; Ib), and for unspecified and other specified leukaemias (Ie) (appendix p 5). We also estimated survival separately for two subgroups of lymphoid leukaemia: precursor-cell lymphoid leukaemias (ALL; Ia1) and mature B-cell leukaemias (Ia2). We did not analyse survival for chronic myeloproliferative diseases (group Ic) or myelodysplastic syndrome and other myeloproliferative diseases (group Id).
Ethical approval for access to the data was obtained from the Ethics and Confidentiality Committee of the UK's statutory National Information Governance Board (now the Health Research Authority; ECC 3-04(i)/2011) and the UK National Health Service (NHS) Research Ethics Service (South-East; 11/LO/0331), and from other jurisdictions as required.4
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Of the 124 015 children who were considered for analysis, we excluded 1623 (1%) as ineligible, usually because of missing information on date of birth, diagnosis, or last known vital status (table 1). More than 75% of records from the Tunisia Central Registry were ineligible because of incomplete data. Overall, only 0·5% of records were excluded because the registration was based solely on a death certificate or an autopsy report. We excluded five patients with synchronous leukaemia and lymphoma, and 106 children whose leukaemia followed a lymphoma, also diagnosed during 1995–2009. We also excluded 2222 children with chronic myeloproliferative disease (ICCC-3 group Ic) and 2002 children with myelodysplastic syndrome or other myeloproliferative disease (Id). Lymphomas (27 609) were not included.
Table 1.
Data quality indicators for children aged 0–14 years diagnosed with leukaemia between 1995–2009: by continent and country
| Calendar period | Registries (n) | Patients submitted (n) | Eligible patients (n [%]) | DCO (n [%]) | Any haematological malignancy (n [% of eligible]) |
Leukaemia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Included in analyses (n [% of any haematological malignancy]) | Microscopically verified (n [%]) | Follow-up time (years) of alive patients* (median [IQR]) | Lymphoid leukaemia (n [%]) | Acute myeloid leukaemia (n [%]) | Unspecified and other specified leukaemia (n [%]) | ||||||||
| Africa | ·· | ·· | 373 | 223 (60%) | 1 (<1%) | 193 (87%) | 153 (79%) | 152 (99%) | 3·5 (0·5–4·7) | 111 (73%) | 33 (22%) | 9 (6%) | |
| Algerian registries | 2000–09 | 2 | 131 | 128 (98%) | 0 | 99 (77%) | 62 (63%) | 61 (98%) | 0·7 (0·2–3·0) | 37 (60%) | 20 (32%) | 5 (8%) | |
| Lesotho† | 1995–2009 | 1 | 27 | 27 (100%) | 0 | 27 (100%) | 27 (100%) | 27 (100%) | 6·6 (0·9–8·4) | 25 (93%) | 0 | 2 (7%) | |
| Libya (Benghazi) | 2003–04 | 1 | 23 | 23 (100%) | 1 (4%) | 22 (96%) | 21 (95%) | 21 (100%) | 4·9 (0·0–5·2) | 14 (67%) | 5 (24%) | 2 (10%) | |
| Tunisia (Central) | 1996–2007 | 1 | 192 | 45 (23%) | 0 | 45 (100%) | 43 (96%) | 43 (100%) | 1·7 (1·0–2·2) | 35 (81%) | 8 (19%) | 0 | |
| America (Central and South) | ·· | ·· | 5731 | 5722 (>99%) | 175 (3%) | 5488 (96%) | 5086 (93%) | 5063 (>99%) | 9·2 (7·3–11·5) | 4788 (94%) | 195 (4%) | 103 (2%) | |
| Argentina† | 2000–09 | 1 | 3671 | 3671 (100%) | 128 (3%) | 3496 (95%) | 3496 (100%) | 3496 (100%) | 6·5 (4·9–8·3) | 3496 (100%) | 0 | 0 | |
| Brazilian registries | 1996–2009 | 5 | 687 | 678 (99%) | 41 (6%) | 626 (92%) | 497 (79%) | 475 (96%) | 11·2 (9·8–13·1) | 387 (78%) | 79 (16%) | 31 (6%) | |
| Chilean registries | 1998–2008 | 2 | 116 | 116 (100%) | 0 | 116 (100%) | 96 (83%) | 96 (100%) | 9·2 (7·7–12·4) | 66 (69%) | 14 (15%) | 16 (17%) | |
| Colombia (Cali) | 1995–2009 | 1 | 383 | 383 (100%) | 0 | 382 (>99%) | 381 (>99%) | 381 (100%) | 6·8 (3·6–9·2) | 381 (100%) | 0 | 0 | |
| Ecuador (Quito) | 1995–2009 | 1 | 507 | 507 (100%) | 4 (1%) | 503 (99%) | 375 (75%) | 375 (100%) | 13·8 (11·5–17·3) | 295 (79%) | 67 (18%) | 13 (3%) | |
| Puerto Rico† | 2000–09 | 1 | 367 | 367 (100%) | 2 (1%) | 365 (99%) | 241 (66%) | 240 (>99%) | 7·4 (6·2–8·6) | 163 (68%) | 35 (15%) | 43 (18%) | |
| America (North) | ·· | ·· | 51 195 | 51 110 (>99%) | 144 (<1%) | 50 920 (>99%) | 36 970 (73%) | 36 156 (98%) | 10·7 (8·3–13·3) | 30 088 (81%) | 5894 (16%) | 988 (3%) | |
| Canada† | 1995–2009 | 13 | 5259 | 5200 (99%) | 13 (<1%) | 5186 (>99%) | 4152 (80%) | 3983 (96%) | 11·0 (8·6–13·5) | 3363 (81%) | 623 (15%) | 166 (4%) | |
| US registries | 1995–2009 | 38 | 45 936 | 45 910 (>99%) | 131 (<1%) | 45 734 (>99%) | 32 818 (72%) | 32 173 (98%) | 10·3 (8·0–13·1) | 26 725 (81%) | 5271 (16%) | 822 (3%) | |
| Asia | ·· | ·· | 17 371 | 17 176 (99%) | 74 (<1%) | 17 094 (>99%) | 12 552 (73%) | 12 309 (98%) | 9·6 (7·4–11·8) | 8553 (68%) | 3051 (24%) | 948 (8%) | |
| Chinese registries | 1995–2009 | 17 | 741 | 728 (98%) | 0 | 728 (100%) | 632 (87%) | 619 (98%) | 7·1 (6·3–8·2) | 309 (49%) | 149 (24%) | 174 (28%) | |
| Cyprus† | 2004–09 | 1 | 49 | 49 (100%) | 2 (4%) | 47 (96%) | 46 (98%) | 46 (100%) | 5·4 (5·1–5·8) | 35 (76%) | 10 (22%) | 1 (2%) | |
| India (Karunagappally) | 1995–2009 | 1 | 54 | 54 (100%) | 0 | 54 (100%) | 53 (98%) | 53 (100%) | 11·2 (7·4–14·8) | 43 (81%) | 8 (15%) | 2 (4%) | |
| Indonesia (Jakarta) | 2005–07 | 1 | 29 | 29 (100%) | 0 | 28 (97%) | 28 (100%) | 26 (93%) | ·· | 14 (50%) | 6 (21%) | 8 (29%) | |
| Israel† | 1995–2009 | 1 | 1880 | 1775 (94%) | 13 (1%) | 1759 (99%) | 1002 (57%) | 997 (>99%) | 10·3 (8·1–12·8) | 731 (73%) | 185 (18%) | 86 (9%) | |
| Japanese registries | 1995–2009 | 9 | 1729 | 1727 (>99%) | 46 (3%) | 1681 (97%) | 1441 (86%) | 1407 (98%) | 10·1 (6·2–11·3) | 969 (67%) | 414 (29%) | 58 (4%) | |
| Korea†‡ | 1995–2009 | 1 | 7569 | 7507 (99%) | 0 | 7507 (100%) | 5422 (72%) | 5279 (97%) | 11·5 (9·2–14·1) | 3583 (66%) | 1419 (26%) | 420 (8%) | |
| Malaysia (Penang) | 1995–2009 | 1 | 274 | 269 (98%) | 3 (1%) | 263 (98%) | 261 (99%) | 260 (>99%) | 9·4 (7·6–11·9) | 163 (62%) | 67 (26%) | 31 (12%) | |
| Mongolia† | 2005–09 | 1 | 47 | 42 (89%) | 0 | 41 (98%) | 41 (100%) | 31 (76%) | ·· | 25 (61%) | 8 (20%) | 8 (20%) | |
| Taiwan† | 1995–2009 | 1 | 3642 | 3642 (100%) | 0 | 3642 (100%) | 2641 (73%) | 2612 (99%) | 11·5 (9·2–14·0) | 1935 (73%) | 614 (23%) | 92 (3%) | |
| Thai registries | 1995–2009 | 3 | 537 | 537 (100%) | 6 (1%) | 531 (99%) | 469 (88%) | 463 (99%) | 10·8 (7·8–12·8) | 327 (70%) | 85 (18%) | 57 (12%) | |
| Turkey (Izmir) | 1995–2009 | 1 | 820 | 817 (>99%) | 4 (<1%) | 813 (>99%) | 516 (63%) | 516 (100%) | 9·2 (7·3–12·3) | 419 (81%) | 86 (17%) | 11 (2%) | |
| Europe | ·· | ·· | 45 127 | 44 003 (98%) | 165 (<1%) | 43 815 (>99%) | 31 797 (73%) | 31 256 (98%) | 11·0 (8·9–13·2) | 26 262 (83%) | 4856 (15%) | 679 (2%) | |
| Austria† | 1995–2009 | 2 | 1248 | 1209 (97%) | 0 | 1203 (>99%) | 826 (69%) | 823 (>99%) | 12·2 (9·5–14·4) | 681 (82%) | 122 (15%) | 23 (3%) | |
| Belarus† | 1995–2009 | 1 | 745 | 745 (100%) | 0 | 745 (100%) | 745 (100%) | 744 (>99%) | 12·6 (10·0–15·0) | 745 (100%) | 0 | 0 | |
| Belgium† | 2004–09 | 1 | 747 | 747 (100%) | 0 | 747 (100%) | 466 (62%) | 465 (>99%) | 8·0 (7·8–8·3) | 396 (85%) | 68 (15%) | 2 (<1%) | |
| Bulgaria† | 1995–2009 | 1 | 1079 | 1079 (100%) | 0 | 1079 (100%) | 714 (66%) | 678 (95%) | 13·0 (9·9–15·5) | 558 (78%) | 96 (13%) | 60 (8%) | |
| Croatia† | 1998–2009 | 1 | 651 | 651 (100%) | 0 | 651 (100%) | 446 (69%) | 446 (100%) | 9·9 (8·8–11·8) | 375 (84%) | 61 (14%) | 10 (2%) | |
| Denmark† | 1995–2009 | 1 | 695 | 695 (100%) | 0 | 695 (100%) | 680 (98%) | 660 (97%) | 10·4 (8·3–12·9) | 551 (81%) | 112 (16%) | 17 (3%) | |
| Estonia† | 1995–2008 | 1 | 160 | 160 (100%) | 1 (1%) | 159 (99%) | 99 (62%) | 99 (100%) | 11·9 (9·1–14·4) | 73 (74%) | 24 (24%) | 2 (2%) | |
| Finland† | 1995–2009 | 1 | 1007 | 1007 (100%) | 6 (1%) | 1001 (99%) | 728 (73%) | 726 (>99%) | 11·0 (8·5–13·5) | 593 (81%) | 101 (14%) | 34 (5%) | |
| France† | 1995–2009 | 1 | 10 619 | 10 032 (95%) | 0 | 10 030 (>99%) | 6875 (69%) | 6873 (>99%) | 9·4 (7·2–12·5) | 5611 (82%) | 1109 (16%) | 155 (2%) | |
| German registries | 1995–2009 | 13 | 3801 | 3781 (99%) | 96 (3%) | 3684 (97%) | 2661 (72%) | 2460 (92%) | 10·7 (8·4–13·4) | 2162 (81%) | 436 (16%) | 63 (2%) | |
| Iceland† | 1995–2009 | 1 | 36 | 36 (100%) | 0 | 36 (100%) | 36 (100%) | 36 (100%) | 10·9 (10·1–13·0) | 29 (81%) | 6 (17%) | 1 (3%) | |
| Ireland† | 1995–2009 | 1 | 817 | 797 (98%) | 2 (<1%) | 794 (>99%) | 571 (72%) | 570 (>99%) | 10·6 (8·2–13·2) | 458 (80%) | 99 (17%) | 14 (2%) | |
| Italian registries | 1995–2009 | 32 | 3955 | 3602 (91%) | 10 (<1%) | 3584 (>99%) | 2313 (65%) | 2247 (97%) | 9·7 (7·3–12·6) | 1930 (83%) | 336 (15%) | 47 (2%) | |
| Latvia† | 1995–2009 | 1 | 205 | 205 (100%) | 0 | 204 (>99%) | 196 (96%) | 194 (99%) | 13·4 (11·5–16·6) | 97 (49%) | 35 (18%) | 64 (33%) | |
| Lithuania† | 1995–2009 | 1 | 532 | 529 (99%) | 2 (<1%) | 527 (>99%) | 358 (68%) | 358 (100%) | 10·9 (9·1–13·6) | 301 (84%) | 53 (15%) | 4 (1%) | |
| Malta† | 1995–2009 | 1 | 92 | 92 (100%) | 0 | 92 (100%) | 61 (66%) | 60 (98%) | 10·8 (7·9–13·2) | 53 (87%) | 8 (13%) | 0 | |
| Netherlands† | 1995–2009 | 1 | 2860 | 2858 (>99%) | 3 (<1%) | 2855 (>99%) | 1988 (70%) | 1988 (100%) | 12·4 (10·1–15·0) | 1652 (83%) | 307 (15%) | 29 (1%) | |
| Norway† | 1995–2009 | 1 | 665 | 665 (100%) | 1 (<1%) | 664 (>99%) | 646 (97%) | 646 (100·0) | 10·8 (7·9–13·3) | 531 (82%) | 108 (17%) | 7 (1%) | |
| Poland (Wroclaw) | 1995–2009 | 1 | 205 | 205 (100%) | 0 | 203 (99%) | 120 (59%) | 120 (100%) | 10·2 (9·2–11·4) | 96 (80%) | 24 (20%) | 0 | |
| Portugal† | 1998–2009 | 4 | 872 | 855 (98%) | 0 | 854 (>99%) | 665 (78%) | 565 (85%) | 9·3 (8·2–10·9) | 511 (77%) | 134 (20%) | 20 (3%) | |
| Russia (Arkhangelsk) ‡ | 2000–09 | 1 | 60 | 60 (100%) | 1 (2%) | 59 (98%) | 59 (100%) | 59 (100%) | 10·7 (9·0–11·8) | 51 (86%) | 0 | 8 (14%) | |
| Slovakia† | 2000–07 | 1 | 426 | 423 (99%) | 6 (1%) | 417 (99%) | 284 (68%) | 284 (100%) | 9·7 (8·6–10·9) | 223 (79%) | 59 (21%) | 2 (1%) | |
| Slovenia† | 1995–2009 | 1 | 280 | 280 (100%) | 2 (1%) | 278 (99%) | 181 (65%) | 181 (100%) | 13·1 (10·9–15·2) | 147 (81%) | 33 (18%) | 1 (1%) | |
| Spanish registries | 1995–2009 | 11 | 1487 | 1479 (99%) | 9 (1%) | 1470 (99%) | 1152 (78%) | 1136 (99%) | 10·9 (8·6–13·3) | 997 (87%) | 123 (11%) | 32 (3%) | |
| Sweden† | 1995–2009 | 1 | 1162 | 1162 (100%) | 0 | 1162 (100%) | 1145 (99%) | 1145 (100%) | 11·1 (8·2–13·6) | 984 (86%) | 154 (13%) | 7 (1%) | |
| Switzerland† | 1995–2009 | 1 | 853 | 852 (>99%) | 1 (<1%) | 851 (>99%) | 813 (96%) | 812 (>99%) | 11·2 (9·2–13·2) | 684 (84%) | 119 (15%) | 10 (1%) | |
| UK† | 1995–2009 | 3 | 9868 | 9797 (99%) | 25 (<1%) | 9771 (>99%) | 6969 (71%) | 6881 (99%) | 12·5 (10·0–14·9) | 5773 (83%) | 1129 (16%) | 67 (1%) | |
| Oceania | ·· | ·· | 4218 | 4158 (99%) | 6 (<1%) | 4151 (>99%) | 3270 (79%) | 3143 (96%) | 11·2 (9·1–13·9) | 2672 (82%) | 544 (17%) | 54 (2%) | |
| Australian registries | 1995–2009 | 6 | 3537 | 3477 (98%) | 2 (<1%) | 3474 (>99%) | 2607 (75%) | 2480 (95%) | 9·7 (7·5–12·4) | 2145 (82%) | 424 (16%) | 38 (1%) | |
| New Zealand† | 1995–2009 | 1 | 681 | 681 (100%) | 4 (1%) | 677 (99%) | 663 (98%) | 663 (100%) | 12·8 (10·7–15·4) | 527 (79%) | 120 (18%) | 16 (2%) | |
| Total | ·· | 198 | 124 015 | 122 392 (99%) | 565 (<1%) | 121 661 (99%) | 89 828 (74%) | 88 079 (98%) | ·· | 72 474 (81%) | 14 573 (16%) | 2781 (3%) | |
Calendar period shows the maximum time span covered by the data. DCO=patients diagnosed from autopsy or Death Certificate Only (% of eligible patients). Microscopic verification=cytology or histology, or morphological verification unspecified (% of patients included in analyses).
Median follow-up of patients diagnosed during 1995–2004, and IQR.
National coverage—the data are derived from a population-based cancer registry (registries) covering the entire country.
Korea: Republic of Korea; Russia: Russian Federation.
Data quality was generally very high, and a high proportion of diagnoses were reported with a specific morphology. Improvements in diagnosis and registration are illustrated in Latvia by the drop in the number of unspecified and other specified leukaemias (group Ie) between 1995–99 and 2005–09.
We focused our analyses on 89 828 children (73·8% of all haematological malignancies) from 198 registries in 53 countries who were diagnosed with lymphoid leukaemia (ICCC-3 group Ia), acute myeloid leukaemia (Ib), or unspecified or other specified leukaemia (Ie).
For children diagnosed during 1995–2004 who were not known to have died, the median follow-up was at least 5 years in all participating countries in North, Central, and South America, Europe, Asia, and Oceania. In Africa, the median follow-up of surviving children diagnosed up to 2004 was 0·7 years (IQR 0·2–3·0) in the two Algerian registries and 1·7 years (1·0–2·2) in the Tunisia Central Registry, although the maximum follow-up was 8·9 and 13·3 years, respectively.
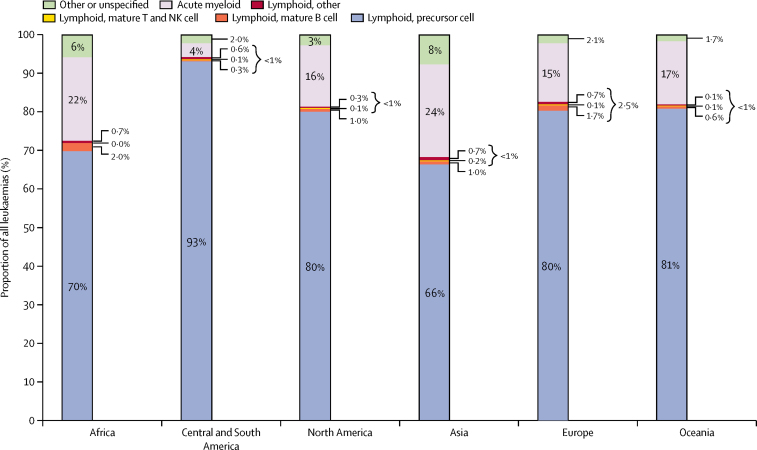
All lymphoid leukaemias combined represented 81% of leukaemias, AML 16%, and unspecified and other specified leukaemias the remaining 3% (table 1). In Lesotho, no AML was registered during 1995–2009. Information from the cancer registries in Belarus, Argentina, and Colombia (Cali) was only available for lymphoid leukaemias. Precursor-cell ALL was by far the most common type of lymphoid leukaemia, but some registries submitted data on rarer types, such as mature B-cell (mostly Burkitt leukaemia), mature T and Natural-Killer (NK) cell leukaemias (figure 1). Due to the rarity of mature T-cell and NK-cell leukaemia (n=94, ICCC-3 group Ia3) and unspecified lymphoid leukaemias (n=446, Ia4), we did not estimate survival separately for these morphological groups. In the data from Indonesia (Jakarta), 17 Chinese registries, and Latvia, more than 25% of the childhood leukaemias were coded as unspecified or other specified leukaemia (ICCC-3 group Ie).
Figure 1.
Distribution (%) of leukaemia subtypes in children diagnosed during 1995–2009 and included in survival analyses, by continent
Leukaemias were classified according to the third edition of the International Classification of Childhood Cancer.
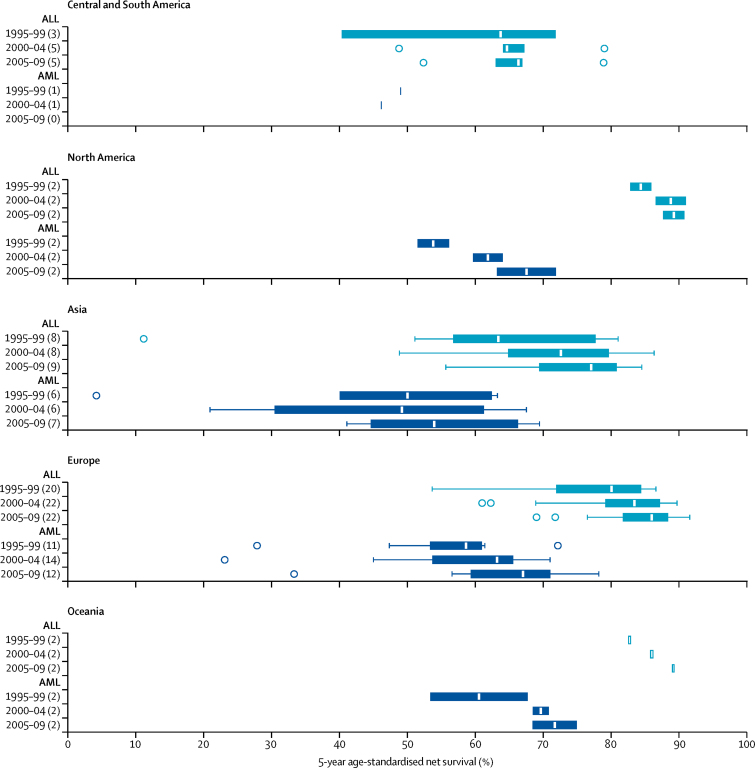
During 1995–99, 5-year age-standardised net survival for all lymphoid leukaemias combined ranged from 10·6% (95% CI 3·1–18·2) in the Chinese registries to 86·8% (81·6–92·0) in Austria (table 2). This wide range in survival narrowed over time, with survival in 2005–09 ranging from 52·4% (42·8–61·9) in Cali, Colombia, to 91·6% (89·5–93·6) in the German registries. Survival from precursor-cell ALL was very close to that of all lymphoid leukaemias combined, with similar variation (figure 2). The greatest absolute difference in survival between all lymphoid leukaemias and ALL was noted in Iceland (80·9% and 90·1%, respectively) but these estimates were not age-standardised because of small numbers. Survival from precursor-cell ALL increased between 1995–99 and 2005–09 in most countries (figure 3).
Table 2.
5-year age-standardised net survival in children aged 0–14 years diagnosed with leukaemia
|
Lymphoid leukaemia |
Acute myeloid leukaemia (ICCC-3 group Ib) |
Unspecified & other leukaemias (ICCC-3 group Ie) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All lymphoid (Ia) |
Precursor cell (Ia1) |
Mature B cell (Ia2) |
|||||||||
| n | Net survival (%), 95% CI | n | Net survival (%), 95% CI | n | Net survival (%), 95% CI | n | Net survival (%), 95% CI | n | Net survival (%), 95% CI | ||
| Africa | |||||||||||
| Algerian registries | |||||||||||
| 2000–04 | 19 | 21·6%*(0·0–45·6) | 19 | 21·6%*(0·0–45·6) | .. | .. | 13 | 23·9%*(0·0–48·5) | 3 | .. | |
| 2005–09 | 18 | .. | 17 | .. | .. | .. | 7 | .. | 2 | .. | |
| Lesotho† | |||||||||||
| 1995–2009 | 25 | 43·0%‡(20·9–65·1) | 22 | 39·5%‡(16·3–62·7) | 3 | .. | .. | .. | 2 | .. | |
| Libya (Benghazi) | |||||||||||
| 2003–04 | 14 | 70·2% (43·4–96·9) | 14 | 70·2% (43·4–96·9) | .. | .. | 5 | .. | 2 | .. | |
| Tunisia (Central) | |||||||||||
| 1996–99 | 20 | 46·1%*(13·2–79·1) | 20 | 46·1%*(13·2–79·1) | .. | .. | 3 | .. | .. | .. | |
| 2000–04 | 6 | .. | 6 | .. | .. | .. | 2 | .. | .. | .. | |
| 2005–07 | 9 | .. | 9 | .. | .. | .. | 3 | .. | .. | .. | |
| America (Central and South) | |||||||||||
| Argentina† | |||||||||||
| 2000–04 | 1785 | 64·6% (62·3–67·0) | 1785 | 64·6% (62·3–67·0) | .. | .. | .. | .. | .. | .. | |
| 2005–09 | 1711 | 66·9% (64·4–69·3) | 1711 | 66·9% (64·4–69·3) | .. | .. | .. | .. | .. | .. | |
| Brazilian registries | |||||||||||
| 1996–99 | 67 | 72·9% (61·8–84·0) | 64 | 71·8% (60·0–83·6) | .. | .. | 13 | 38·6% (13·8–63·5) | 4 | .. | |
| 2000–04 | 172 | 67·9% (60·5–75·3) | 168 | 67·1% (59·6–74·7) | .. | .. | 41 | 46·1% (32·1–60·1) | 16 | 75·5% (55·0–96·0) | |
| 2005–09 | 148 | 66·2% (58·5–73·9) | 132 | 66·4% (58·4–74·3) | 10 | 80·3%‡(48·7–100·0) | 25 | 53·1% (36·9–69·4) | 11 | 55·8% (31·7–79·9) | |
| Chilean registries | |||||||||||
| 1998–99 | 17 | 41·2% (19·1–63·3) | 17 | 41·2% (19·1–63·3) | .. | .. | 3 | .. | .. | .. | |
| 2000–04 | 21 | 71·5% (52·7–90·3) | 21 | 71·5% (52·7–90·3) | .. | .. | 2 | .. | 12 | 100·0% | |
| 2005–08 | 28 | 83·9%§(73·3–94·5) | 23 | 77·5% (60·5–94·6) | .. | .. | 9 | .. | 4 | .. | |
| Colombia (Cali) | |||||||||||
| 1995–99 | 125 | 40·7% (31·6–49·9) | 124 | 40·4% (31·2–49·6) | .. | .. | .. | .. | .. | .. | |
| 2000–04 | 137 | 48·4% (39·4–57·4) | 136 | 48·8% (39·7–57·8) | .. | .. | .. | .. | .. | .. | |
| 2005–09 | 119 | 52·4% (42·8–61·9) | 117 | 52·4% (42·8–61·9) | .. | .. | .. | .. | .. | .. | |
| Ecuador (Quito) | |||||||||||
| 1995–99 | 85 | 64·3% (54·2–74·4) | 81 | 63·7% (53·3–74·1) | .. | .. | 33 | 49·0%§(35·9–62·1) | 5 | .. | |
| 2000–04 | 112 | 63·5% (54·2–72·8) | 110 | 64·1% (54·7–73·6) | .. | .. | 18 | 50·2% (28·2–72·1) | 6 | .. | |
| 2005–09 | 98 | 62·5% (53·5–71·5) | 95 | 63·1% (54·0–72·1) | .. | .. | 16 | 59·3% (36·8–81·7) | 2 | .. | |
| Puerto Rico† | |||||||||||
| 2000–04 | 79 | 79·4% (70·5–88·2) | 73 | 79·0% (69·7–88·3) | 3 | .. | 17 | 53·0% (30·3–75·8) | 34 | 72·4%§(60·8–83·9) | |
| 2005–09 | 84 | 78·7% (69·5–87·9) | 81 | 78·9% (69·4–88·4) | 3 | .. | 18 | 48·1% (26·4–69·8) | 9 | .. | |
| America (North) | |||||||||||
| Canada† | |||||||||||
| 1995–99 | 1156 | 86·0% (83·6–88·4) | 1134 | 85·9% (83·4–88·3) | 11 | 81·9% (60·2–100·0) | 235 | 56·1% (49·5–62·7) | 53 | 68·0% (55·2–80·8) | |
| 2000–04 | 1092 | 91·0% (89·0–93·0) | 1074 | 91·0% (89·0–93·0) | 10 | 90·0% (72·4–100·0) | 188 | 64·0% (57·0–70·9) | 51 | 72·7% (60·6–84·9) | |
| 2005–09 | 1115 | 90·5% (88·4–92·6) | 1097 | 90·7% (88·6–92·9) | 14 | 80·0% (60·5–99·6) | 200 | 71·8% (65·1–78·5) | 62 | 83·1% (72·9–93·3) | |
| US registries | |||||||||||
| 1995–99 | 7801 | 82·9% (81·9–83·9) | 7670 | 82·9% (81·9–83·9) | 73 | 77·0% (67·7–86·4) | 1574 | 51·5% (48·9–54·1) | 241 | 68·8% (62·4–75·2) | |
| 2000–04 | 9025 | 86·5% (85·7–87·4) | 8842 | 86·6% (85·8–87·5) | 128 | 86·5% (80·1–92·9) | 1799 | 59·7% (57·3–62·1) | 285 | 63·8% (57·7–69·8) | |
| 2005–09 | 9899 | 87·7% (86·9–88·5) | 9735 | 87·7% (86·9–88·5) | 132 | 88·7% (83·0–94·5) | 1898 | 63·3% (60·9–65·6) | 296 | 69·8% (64·2–75·4) | |
| Asia | |||||||||||
| Chinese registries | |||||||||||
| 1995–99 | 29 | 10·6%§(3·1–18·2) | 28 | 11·1%§(3·3–19·0) | .. | .. | 27 | 4·2%§(0·0–8·6) | 23 | 8·7% (0·0–18·9) | |
| 2000–04 | 98 | 45·6% (36·3–54·9) | 84 | 48·8% (38·8–58·8) | .. | .. | 61 | 20·9% (10·1–31·7) | 69 | 15·0% (6·7–23·2) | |
| 2005–09 | 182 | 69·2% (61·6–76·8) | 151 | 69·4% (61·1–77·8) | 10 | 68·7%‡(40·8–96·6) | 61 | 41·1% (27·8–54·4) | 82 | 26·0% (15·9–36·0) | |
| Cyprus† | |||||||||||
| 2004–09 | 35 | 83·0%‡ (70·9–95·0) | 34 | 80·8%‡ (68·9–92·6) | 1 | .. | 10 | 60·1%‡(32·2–88·0) | 1 | .. | |
| India (Karunagappally) | |||||||||||
| 1995–99 | 17 | 59·3% (36·7–81·9) | 17 | 59·3% (36·7–81·9) | .. | .. | 1 | .. | .. | .. | |
| 2000–04 | 14 | 57·4% (32·6–82·1) | 14 | 57·4% (32·6–82·1) | .. | .. | 4 | .. | .. | .. | |
| 2005–09 | 12 | 80·2% (60·3–100·0) | 12 | 80·2% (60·3–100·0) | .. | .. | 3 | .. | 2 | .. | |
| Indonesia (Jakarta) | |||||||||||
| 2005–07 | 14 | 44·3% (13·4–75·3) | 14 | 44·3% (13·4–75·3) | .. | .. | 6 | .. | 8 | .. | |
| Israel† | |||||||||||
| 1995–99 | 192 | 81·4% (74·9–87·8) | 188 | 81·0% (74·5–87·6) | 2 | .. | 40 | 63·2%§(51·6–74·9) | 27 | 74·1% (57·9–90·3) | |
| 2000–04 | 264 | 86·3% (81·6–91·1) | 258 | 86·3% (81·5–91·1) | 4 | .. | 67 | 67·5% (56·6–78·4) | 27 | 77·8% (62·5–93·2) | |
| 2005–09 | 275 | 84·4% (79·6–89·1) | 271 | 84·5% (79·7–89·3) | 1 | .. | 78 | 66·2% (56·2–76·3) | 32 | 89·2%§(80·7–97·7) | |
| Japanese registries | |||||||||||
| 1995–99 | 294 | 78·0% (72·9–83·1) | 294 | 78·0% (72·9–83·1) | .. | .. | 122 | 62·4% (53·7–71·0) | 25 | 60·1% (41·4–78·8) | |
| 2000–04 | 409 | 78·0% (73·2–82·9) | 406 | 77·9% (73·1–82·8) | 2 | .. | 181 | 61·2% (53·5–68·9) | 24 | 87·5% (74·6–100·0) | |
| 2005–09 | 266 | 82·5% (78·0–86·9) | 259 | 82·2% (77·6–86·8) | 4 | .. | 111 | 69·4% (62·0–76·9) | 9 | .. | |
| Korea†;¶ | |||||||||||
| 1995–99 | 1191 | 63·1% (60·2–66·0) | 1171 | 63·2% (60·3–66·1) | .. | .. | 482 | 40·1% (35·6–44·5) | 167 | 44·2% (36·8–51·6) | |
| 2000–04 | 1221 | 73·0% (70·3–75·6) | 1197 | 73·2% (70·5–75·8) | .. | .. | 504 | 49·7% (45·4–54·1) | 128 | 55·4% (46·9–63·9) | |
| 2005–09 | 1171 | 76·4% (73·9–78·9) | 1137 | 77·1% (74·5–79·6) | 43 | 65·2%‡ (52·9–77·5) | 433 | 53·9% (49·3–58·6) | 125 | 63·9% (55·7–72·1) | |
| Malaysia (Penang) | |||||||||||
| 1995–99 | 51 | 77·3% (65·5–89·1) | 51 | 77·3% (65·5–89·1) | .. | .. | 22 | 68·3% (49·3–87·2) | 1 | .. | |
| 2000–04 | 53 | 82·8%§(74·2–91·3) | 51 | 81·3%§(72·1–90·4) | .. | .. | 23 | 74·0% (56·5–91·5) | 5 | .. | |
| 2005–09 | 59 | 70·1% (58·4–81·9) | 57 | 69·5% (57·4–81·5) | 1 | .. | 22 | 49·1% (27·8–70·5) | 25 | 76·4% (58·5–94·4) | |
| Mongolia† | |||||||||||
| 2005–09 | 25 | 18·7% (0·0–40·8) | 24 | 19·5% (0·0–42·5) | .. | .. | 8 | .. | 8 | .. | |
| Taiwan† | |||||||||||
| 1995–99 | 630 | 62·8% (58·8–66·9) | 601 | 62·5% (58·4–66·6) | 18 | 72·3% (52·3–92·4) | 195 | 40·8% (33·8–47·8) | 52 | 50·8% (38·2–63·3) | |
| 2000–04 | 682 | 72·2% (68·5–76·0) | 665 | 72·0% (68·2–75·8) | 15 | 80·0% (60·5–99·6) | 214 | 48·6% (41·8–55·5) | 22 | 77·3% (60·3–94·4) | |
| 2005–09 | 623 | 77·6% (74·1–81·2) | 610 | 78·1% (74·6–81·7) | 11 | 62·1% (39·2–85·0) | 205 | 55·6% (48·8–62·3) | 18 | 63·9% (43·2–84·7) | |
| Thai registries | |||||||||||
| 1995–99 | 102 | 51·1% (39·4–62·8) | 102 | 51·1% (39·4–62·8) | .. | .. | 18 | 21·8% (2·1–41·5) | 20 | 52·0% (28·7–75·4) | |
| 2000–04 | 120 | 58·9%(49·2–68·5) | 120 | 58·9% (49·2–68·5) | .. | .. | 31 | 30·5%§(18·0–43·0) | 20 | 37·3% (16·6–58·1) | |
| 2005–09 | 105 | 55·0% (45·4–64·7) | 102 | 55·6% (46·0–65·3) | .. | .. | 36 | 44·7%§(31·8–57·5) | 17 | 57·0% (31·5–82·5) | |
| Turkey (Izmir) | |||||||||||
| 1995–99 | 118 | 62·8% (53·0–72·7) | 116 | 63·5% (53·7–73·4) | 2 | .. | 34 | 59·2%§(46·1–72·4) | 2 | .. | |
| 2000–04 | 135 | 71·0% (62·6–79·3) | 131 | 70·9% (62·5–79·3) | 3 | .. | 24 | 31·2% (13·1–49·4) | 1 | .. | |
| 2005–09 | 166 | 73·6% (66·3–81·0) | 161 | 74·2% (66·8–81·7) | 4 | .. | 28 | 50·6%§(35·7–65·4) | 8 | .. | |
| Europe | |||||||||||
| Austria† | |||||||||||
| 1995–99 | 242 | 86·8% (81·6–92·0) | 240 | 86·6% (81·3–91·9) | .. | .. | 47 | 60·1%§(49·1–71·1) | 7 | .. | |
| 2000–04 | 213 | 90·1% (85·7–94·5) | 208 | 89·7% (85·2–94·3) | 1 | .. | 40 | 65·0%§(53·4–76·5) | 12 | 66·7% (41·4–92·1) | |
| 2005–09 | 226 | 91·1% (86·9–95·3) | 221 | 91·4% (87·2–95·7) | 3 | .. | 35 | 72·6%§(61·2–84·1) | 4 | .. | |
| Belarus† | |||||||||||
| 1995–99 | 286 | 74·3% (69·1–79·6) | 282 | 74·7% (69·4–79·9) | .. | .. | .. | .. | .. | .. | |
| 2000–04 | 241 | 77·5% (72·0–83·0) | 235 | 78·4% (72·9–83·9) | .. | .. | .. | .. | .. | .. | |
| 2005–09 | 218 | 88·1% (83·4–92·7) | 209 | 88·3% (83·6–93·0) | 17 | 74·8%‡(54·0–95·7) | .. | .. | .. | .. | |
| Belgium† | |||||||||||
| 2004–09 | 396 | 87·2%‡ (82·9–91·4) | 386 | 86·9%‡ (82·6–91·3) | 10 | 90·0%‡(72·4–100·0) | 68 | 56·6%‡ (43·5–69·6) | 2 | .. | |
| Bulgaria† | |||||||||||
| 1995–99 | 184 | 57·0% (49·8–64·2) | 166 | 57·6% (50·0–65·2) | .. | .. | 28 | 27·8%§(15·7–39·9) | 37 | 13·5%§(5·6–21·3) | |
| 2000–04 | 159 | 61·8% (53·9–69·6) | 154 | 62·3% (54·3–70·3) | .. | .. | 35 | 23·1%§(12·7–33·5) | 17 | 29·4% (9·6–49·3) | |
| 2005–09 | 215 | 72·0% (65·3–78·8) | 215 | 71·8% (64·9–78·6) | .. | .. | 33 | 33·3% (18·9–47·7) | 6 | .. | |
| Croatia† | |||||||||||
| 1998–99 | 51 | 68·7% (56·9–80·6) | 44 | 69·8%§(59·2–80·4) | .. | .. | 13 | 46·2% (20·7–71·8) | 2 | .. | |
| 2000–04 | 177 | 84·1% (77·6–90·6) | 160 | 81·6% (74·2–89·0) | .. | .. | 20 | 65·0% (44·8–85·3) | 4 | .. | |
| 2005–09 | 147 | 85·6% (79·8–91·5) | 146 | 85·7% (79·8–91·7) | 17 | 94·1%‡(83·3–100·0) | 28 | 55·9% (37·6–74·2) | 4 | .. | |
| Denmark† | |||||||||||
| 1995–99 | 166 | 85·9% (79·7–92·0) | 163 | 85·6% (79·4–91·8) | 3 | .. | 32 | 59·4% (42·9–75·9) | 4 | .. | |
| 2000–04 | 212 | 84·2% (78·6–89·8) | 209 | 84·5% (78·9–90·1) | 2 | .. | 42 | 69·1% (55·3–82·9) | 10 | 70·0% (43·3–96·8) | |
| 2005–09 | 173 | 87·4% (81·9–93·0) | 171 | 87·2% (81·5–92·9) | 2 | .. | 38 | 68·9% (54·5–83·3) | 3 | .. | |
| Estonia† | |||||||||||
| 1995–99 | 29 | 53·6%§(40·0–67·3) | 29 | 53·6%§(40·0–67·3) | .. | .. | 7 | .. | 2 | .. | |
| 2000–04 | 31 | 61·0%§(47·8–74·2) | 31 | 61·0%§(47·8–74·2) | .. | .. | 11 | 36·4% (10·3–62·6) | .. | .. | |
| 2005–08 | 13 | 75·4% (57·0–93·7) | 13 | 75·4% (57·0–93·7) | .. | .. | 6 | .. | .. | .. | |
| Finland† | |||||||||||
| 1995–99 | 193 | 82·3% (76·3–88·4) | 193 | 82·3% (76·3–88·4) | .. | .. | 37 | 78·4% (65·4–91·5) | 4 | .. | |
| 2000–04 | 192 | 84·8% (78·1–91·4) | 191 | 84·7% (78·0–91·4) | .. | .. | 29 | 65·4%§(52·1–78·8) | 12 | 58·4% (32·0–84·8) | |
| 2005–09 | 208 | 82·0% (75·4–88·5) | 205 | 81·8% (75·2–88·4) | 2 | .. | 35 | 69·2% (54·3–84·2) | 18 | 66·9% (45·5–88·4) | |
| France† | |||||||||||
| 1995–99 | 1806 | 82·4% (80·4–84·3) | 1728 | 82·4% (80·4–84·4) | 78 | 78·7% (69·2–88·2) | 380 | 60·2% (55·1–65·3) | 36 | 52·8% (36·8–68·8) | |
| 2000–04 | 1883 | 88·0% (86·3–89·6) | 1793 | 88·1% (86·4–89·8) | 89 | 86·0% (78·7–93·3) | 392 | 62·4% (57·7–67·1) | 48 | 59·8% (46·1–73·5) | |
| 2005–09 | 1922 | 89·2% (87·6–90·8) | 1828 | 89·2% (87·6–90·9) | 93 | 88·8% (82·2–95·4) | 337 | 69·4% (64·5–74·2) | 71 | 64·9% (53·0–76·8) | |
| German registries | |||||||||||
| 1995–99 | 481 | 86·3% (83·0–89·6) | 468 | 86·5% (83·1–89·8) | .. | .. | 107 | 61·4% (52·3–70·5) | 27 | 74·1% (57·9–90·3) | |
| 2000–04 | 661 | 87·3% (84·7–90·0) | 642 | 87·1% (84·4–89·8) | .. | .. | 139 | 71·0% (63·2–78·8) | 16 | 81·3% (62·8–99·8) | |
| 2005–09 | 1020 | 91·6% (89·5–93·6) | 989 | 91·6% (89·5–93·6) | 39 | 93·7%‡ (85·7–100·0) | 190 | 78·2% (72·0–84·3) | 20 | 74·3% (53·0–95·5) | |
| Iceland† | |||||||||||
| 1995–99 | 9 | .. | 9 | .. | .. | .. | .. | .. | 1 | .. | |
| 2000–04 | 9 | .. | 9 | .. | .. | .. | 2 | .. | .. | .. | |
| 2005–09 | 11 | 80·9% (58·1–100·0) | 10 | 90·1% (72·4–100·0) | 1 | .. | 4 | .. | .. | .. | |
| Ireland† | |||||||||||
| 1995–99 | 149 | 78·9% (71·5–86·3) | 146 | 79·0% (71·6–86·5) | 1 | .. | 29 | 65·6% (48·7–82·5) | 3 | .. | |
| 2000–04 | 146 | 82·9% (76·6–89·2) | 145 | 82·8% (76·5–89·2) | 1 | .. | 35 | 60·0% (44·1–76·0) | 7 | .. | |
| 2005–09 | 163 | 84·7% (78·3–91·1) | 162 | 84·7% (78·3–91·1) | 1 | .. | 35 | 65·8% (51·0–80·7) | 4 | .. | |
| Italian registries | |||||||||||
| 1995–99 | 677 | 83·8% (80·7–86·9) | 661 | 83·7% (80·6–86·9) | .. | .. | 109 | 60·9% (51·7–70·1) | 16 | 75·1% (54·6–95·5) | |
| 2000–04 | 740 | 82·4% (79·1–85·7) | 725 | 82·5% (79·1–85·8) | .. | .. | 124 | 66·7% (58·5–74·9) | 18 | 77·8% (59·2–96·5) | |
| 2005–09 | 513 | 87·9% (85·0–90·9) | 507 | 88·0% (85·0–90·9) | 24 | 76·7%‡(59·0–94·3) | 103 | 68·9% (60·4–77·4) | 13 | 77·2% (55·3–99·2) | |
| Latvia† | |||||||||||
| 1995–99 | 16 | 50·2% (26·7–73·6) | 15 | 46·8% (22·8–70·9) | .. | .. | 15 | 40·1% (16·6–63·5) | 43 | 69·6%§(58·4–80·9) | |
| 2000–04 | 36 | 91·8% (82·9–100·0) | 35 | 91·6% (82·5–100·0) | .. | .. | 11 | 45·5% (18·1–72·9) | 19 | 63·3% (42·2–84·3) | |
| 2005–09 | 45 | 77·0%§(66·5–87·6) | 45 | 76·5%§(65·8–87·2) | .. | .. | 9 | .. | 2 | .. | |
| Lithuania† | |||||||||||
| 1995–99 | 103 | 59·4% (49·3–69·6) | 102 | 59·2% (49·0–69·4) | .. | .. | 12 | 41·8% (15·9–67·7) | 3 | .. | |
| 2000–04 | 112 | 73·7% (64·9–82·5) | 106 | 74·3% (65·3–83·4) | 2 | .. | 27 | 22·3% (7·3–37·2) | .. | .. | |
| 2005–09 | 86 | 68·2% (58·1–78·4) | 86 | 69·0% (58·4–79·6) | .. | .. | 14 | 44·0% (20·1–67·9) | 1 | .. | |
| Malta† | |||||||||||
| 1995–99 | 19 | 63·3% (42·3–84·3) | 19 | 63·3% (42·3–84·3) | .. | .. | .. | .. | .. | .. | |
| 2000–04 | 16 | 81·4% (62·9–99·8) | 16 | 81·4% (62·9–99·8) | .. | .. | 4 | .. | .. | .. | |
| 2005–09 | 18 | 83·1% (66·0–100·0) | 17 | 82·5% (64·9–100·0) | .. | .. | 4 | .. | .. | .. | |
| Netherlands† | |||||||||||
| 1995–99 | 529 | 81·1% (77·2–84·9) | 527 | 81·0% (77·1–84·9) | 2 | .. | 92 | 58·6% (48·0–69·2) | 12 | 75·1% (51·8–98·5) | |
| 2000–04 | 586 | 84·0% (80·6–87·4) | 582 | 84·0% (80·6–87·4) | 1 | .. | 109 | 53·7% (43·7–63·8) | 11 | 81·9% (60·2–100·0) | |
| 2005–09 | 537 | 86·2% (82·9–89·6) | 533 | 86·2% (82·9–89·6) | 4 | .. | 106 | 58·6% (48·2–69·0) | 6 | .. | |
| Norway† | |||||||||||
| 1995–99 | 180 | 79·2% (71·6–86·9) | 177 | 79·1% (71·4–86·8) | 2 | .. | 42 | 54·3% (39·6–69·0) | 3 | .. | |
| 2000–04 | 182 | 87·7% (82·5–93·0) | 178 | 87·7% (82·3–93·1) | 4 | .. | 37 | 65·5%§(53·1–77·9) | 2 | .. | |
| 2005–09 | 169 | 89·7% (84·5–94·9) | 169 | 89·7% (84·5–94·9) | .. | .. | 29 | 67·3% (50·1–84·5) | 2 | .. | |
| Poland (Wroclaw) | |||||||||||
| 2000–04 | 33 | 68·9%§(56·6–81·2) | 33 | 68·9%§(56·6–81·2) | .. | .. | 8 | .. | .. | .. | |
| 2005–09 | 63 | 80·9% (70·8–90·9) | 62 | 80·9% (70·8–90·9) | 1 | .. | 16 | 66·7% (43·6–89·9) | .. | .. | |
| Portugal† | |||||||||||
| 1998–99 | 45 | 66·0%§(54·3–77·6) | 45 | 66·0%§(54·3–77·6) | .. | .. | 17 | 53·0% (30·2–75·8) | 1 | .. | |
| 2000–04 | 257 | 79·3% (73·8–84·8) | 238 | 79·2% (73·5–84·8) | .. | .. | 65 | 53·3% (41·7–65·0) | 7 | .. | |
| 2005–09 | 209 | 84·0% (78·8–89·2) | 191 | 83·7% (78·1–89·2) | 21 | 94·5%‡(84·2–100·0) | 52 | 60·4% (48·6–72·3) | 12 | 69·7% (42·5–96·8) | |
| Russia (Arkhangelsk)¶ | |||||||||||
| 2000–04 | 27 | 55·7% (37·3–74·0) | 23 | 52·2% (32·4–72·1) | 1 | .. | .. | .. | 6 | .. | |
| 2005–09 | 24 | 74·8% (57·7–92·0) | 23 | 73·1% (55·0–91·2) | .. | .. | .. | .. | 2 | .. | |
| Slovakia† | |||||||||||
| 2000–04 | 132 | 79·4% (72·1–86·7) | 131 | 79·3% (72·0–86·6) | .. | .. | 35 | 45·0% (30·0–59·9) | 2 | .. | |
| 2005–07 | 91 | 79·1% (70·4–87·7) | 89 | 78·3% (69·5–87·2) | .. | .. | 24 | 46·7% (23·2–70·3) | .. | .. | |
| Slovenia† | |||||||||||
| 1995–99 | 50 | 83·4%§(74·4–92·3) | 50 | 83·4%§(74·4–92·3) | .. | .. | 7 | .. | .. | .. | |
| 2000–04 | 49 | 89·7%§(81·8–97·6) | 49 | 89·7%§(81·8–97·6) | .. | .. | 11 | 63·7% (36·8–90·5) | .. | .. | |
| 2005–09 | 48 | 75·8%§(65·4–86·2) | 43 | 81·3% (69·7–92·9) | 5 | .. | 15 | 79·5% (59·5–99·5) | 1 | .. | |
| Spanish registries | |||||||||||
| 1995–99 | 296 | 74·4% (68·9–79·9) | 290 | 74·1% (68·5–79·7) | .. | .. | 38 | 47·3%§(34·7–59·9) | 17 | 58·9% (36·4–81·4) | |
| 2000–04 | 319 | 81·6% (77·0–86·1) | 308 | 81·9% (77·3–86·5) | .. | .. | 39 | 64·0% (50·0–78·0) | 10 | 70·0% (43·3–96·8) | |
| 2005–09 | 382 | 83·7% (79·5–87·9) | 362 | 84·2% (80·0–88·4) | 30 | 80·0%‡ (67·4–92·7) | 46 | 60·2% (47·2–73·2) | 5 | .. | |
| Sweden† | |||||||||||
| 1995–99 | 368 | 84·2% (79·8–88·7) | 344 | 85·0% (80·5–89·5) | 2 | .. | 50 | 72·2%§(61·6–82·7) | .. | .. | |
| 2000–04 | 333 | 85·9% (81·7–90·0) | 313 | 86·8% (82·6–90·9) | .. | .. | 56 | 56·3%§(45·8–66·8) | 3 | .. | |
| 2005–09 | 283 | 84·5% (80·1–88·9) | 230 | 85·5% (80·9–90·1) | 2 | .. | 48 | 65·9%§(55·5–76·4) | 4 | .. | |
| Switzerland† | |||||||||||
| 1995–99 | 224 | 86·0% (80·9–91·1) | 220 | 85·6% (80·3–90·8) | .. | .. | 42 | 53·4% (38·7–68·1) | .. | .. | |
| 2000–04 | 229 | 87·6% (82·8–92·4) | 222 | 87·2% (82·3–92·1) | .. | .. | 43 | 53·7%§(42·3–65·2) | 6 | .. | |
| 2005–09 | 231 | 87·9% (83·3–92·6) | 225 | 88·3% (83·7–92·9) | 14 | 100·0%‡(100·0–100·0) | 34 | 75·2%§(64·0–86·3) | 4 | .. | |
| UK† | |||||||||||
| 1995–99 | 1896 | 79·1% (77·0–81·2) | 1871 | 79·2% (77·0–81·3) | .. | .. | 371 | 58·6% (53·4–63·8) | 27 | 70·5% (53·6–87·3) | |
| 2000–04 | 1976 | 85·9% (84·2–87·7) | 1954 | 86·0% (84·3–87·8) | .. | .. | 390 | 65·6% (60·7–70·5) | 18 | 55·6% (33·7–77·5) | |
| 2005–09 | 1901 | 89·3% (87·7–90·9) | 1893 | 89·2% (87·6–90·8) | 51 | 77·8%‡ (66·4–89·1) | 368 | 68·1% (63·2–73·1) | 22 | 76·3% (58·4–94·1) | |
| Oceania | |||||||||||
| Australian registries | |||||||||||
| 1995–99 | 685 | 82·8% (79·6–86·0) | 682 | 82·9% (79·7–86·1) | .. | .. | 125 | 53·4% (44·6–62·2) | 13 | 46·2% (20·7–71·7) | |
| 2000–04 | 833 | 86·0% (83·3–88·7) | 825 | 86·2% (83·4–88·9) | .. | .. | 170 | 70·8% (63·8–77·8) | 16 | 68·9% (47·0–90·8) | |
| 2005–09 | 627 | 88·8% (86·0–91·6) | 617 | 89·0% (86·2–91·8) | 17 | 82·0%‡(64·0–99·9) | 129 | 68·5% (60·3–76·6) | 9 | .. | |
| New Zealand† | |||||||||||
| 1995–99 | 168 | 82·8% (76·4–89·3) | 167 | 82·6% (76·0–89·1) | 1 | .. | 42 | 67·6%§(56·3–78·9) | 5 | .. | |
| 2000–04 | 183 | 85·2% (79·3–91·2) | 182 | 85·8% (79·9–91·7) | 1 | .. | 44 | 68·5%§(57·7–79·4) | 6 | .. | |
| 2005–09 | 176 | 89·3% (83·8–94·8) | 176 | 89·3% (83·8–94·8) | .. | .. | 34 | 74·9%§(63·7–86·1) | 5 | .. | |
Data stratified by continent, country, and calendar period of diagnosis (1995–99, 2000–04, and 2005–09). Underlined estimates are not age-standardised. ICCC-3= International Classification of Childhood Cancer, 3rd edition.
Estimate judged as less reliable.
National coverage—the data are derived from a population-based cancer registry (registries) covering the entire country.
Estimate based on merging data for 2 (or all 3) calendar periods.
Age-standardised estimate computed by pooling two age-specific estimates and re-estimation.
Korea: Republic of Korea; Russia: Russian Federation.
Figure 2.
Age-standardised 5-year net survival (%) for children diagnosed with acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML) during 1995–2009
The number of countries for which survival estimates are shown in each box-plot is given in parentheses. Box-plots in light blue are for ALL (group Ia1 according to the third edition of the International Classification of Childhood Cancer [ICCC-3]), and dark blue for AML (ICCC-3 group Ib). The vertical line inside each box denotes the median survival value, and the box shows the IQR between the lower and upper quartiles. The extreme limits of the box-plot are 1·5 times the IQR below the lower quartile and above the upper quartile. Open circles indicate outlier values, outside this range Survival estimates for African countries are not shown because they were either not standardised or less reliable.
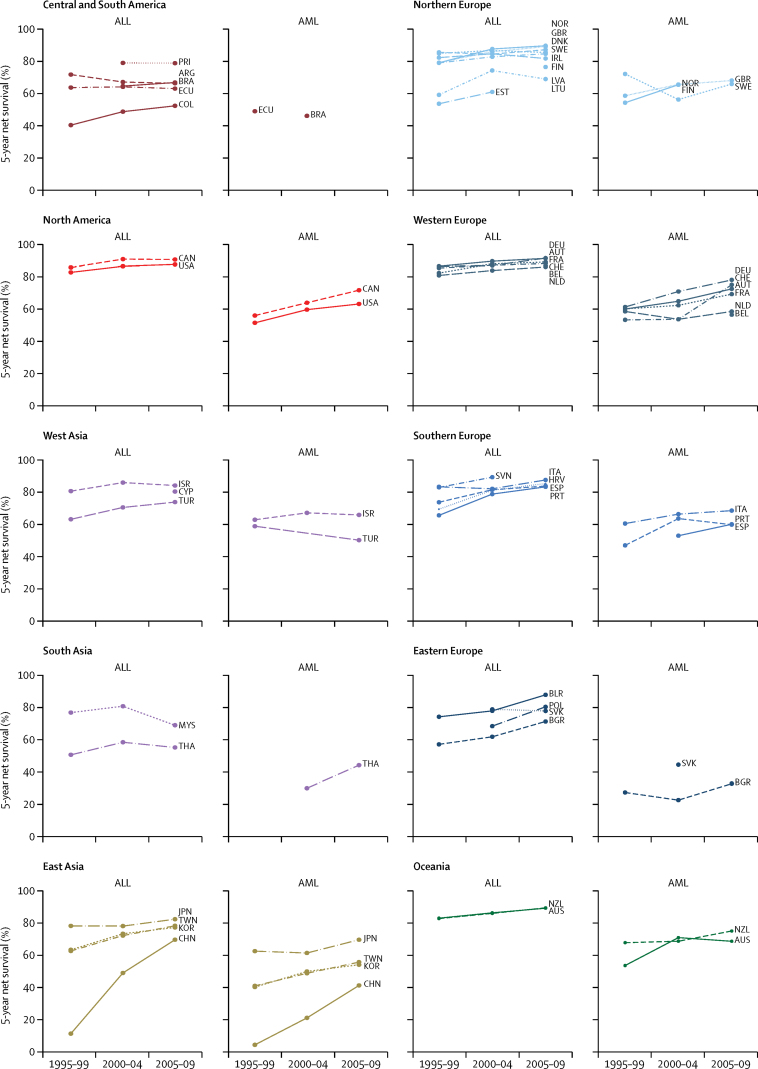
Figure 3.
Trends in age-standardised 5-year net survival (%) for children diagnosed with acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML), during 1995–1999, 2000–2004, and 2005–2009
Countries have been grouped into ten geographical regions. Survival estimates for African countries are not shown because they were either not standardised or less reliable. ALL: group Ia1 according to the third edition of the International Classification of Childhood Cancer (ICCC-3). AML: ICCC-3 group Ib. ARG=Argentina. AUS=Australia. AUT=Austria. BEL=Belgium. BGR=Bulgaria. BLR=Belarus. BRA=Brazil. CAN=Canada. CHE=Switzerland. CHN=China. COL=Colombia. CYP=Cyprus. DEU=Germany. DNK=Denmark. ECU=Ecuador. ESP=Spain. EST=Estonia. FIN=Finland. FRA=France. GBR=United Kingdom. HRV=Croatia. IRL=Ireland. ISR=Israel. ITA=Italy. JPN=Japan. KOR=Republic of Korea. LTU=Lithuania. LVA=Latvia. MYS=Malaysia. NLD=Netherlands. NOR=Norway. NZL=New Zealand. POL=Poland. PRI=Puerto Rico. PRT=Portugal. SVK=Slovakia. SVN=Slovenia. SWE=Sweden. TWN=Taiwan. THA=Thailand. TUR=Turkey. USA=United States of America.
We estimated survival for mature B-cell leukaemia for 17 countries. Many of these estimates were based on a pooled analysis for children diagnosed throughout 1995–2009, because the number of patients diagnosed in each 5-year period was small. In France and the US registries, 5-year age-standardised net survival from mature B-cell leukaemia was lower than that of precursor-cell ALL in 1995–99, but the difference had disappeared by 2005–09.
5-year age-standardised net survival for AML was consistently lower than that for ALL (table 2, figure 2). Age-standardised survival for AML in 1995–99 ranged from 4·2% (0·0–8·6) in the Chinese registries to 72·2% (61·6–82·7) in Sweden, and from 33·3% (18·9–47·7) in Bulgaria to 78·2% (72·0–84·3) in German registries for 2005–09. In most countries, survival from childhood AML increased quite remarkably over time (figure 3). Age-standardised 5-year survival for unspecified and other specified leukaemia (group Ie) showed wide variation, ranging from 13·5% (Bulgaria) to 69·6% (Latvia) in 1995–99, and from 26·0% (Chinese registries) to 89·2% (Israel) in 2005–09.
Survival from ALL in infants (aged <1 year) was much lower than for older children, including those aged 10–14 years (appendix pp 6–14). By contrast, survival for infants with AML in many countries was close to that for children aged 10–14 years (appendix pp 15–22). Children aged 1–4 and 5–9 years had the highest survival for both ALL and AML. The difference in survival between children with ALL aged 10–14 and those aged 1–4 and 5–9 years fell progressively between 1995–99 and 2005–09 in most countries. The pattern was not so clear for AML. Survival from AML and ALL was often slightly higher for girls than for boys, but this pattern was not consistent across all countries (appendix pp 6–22).
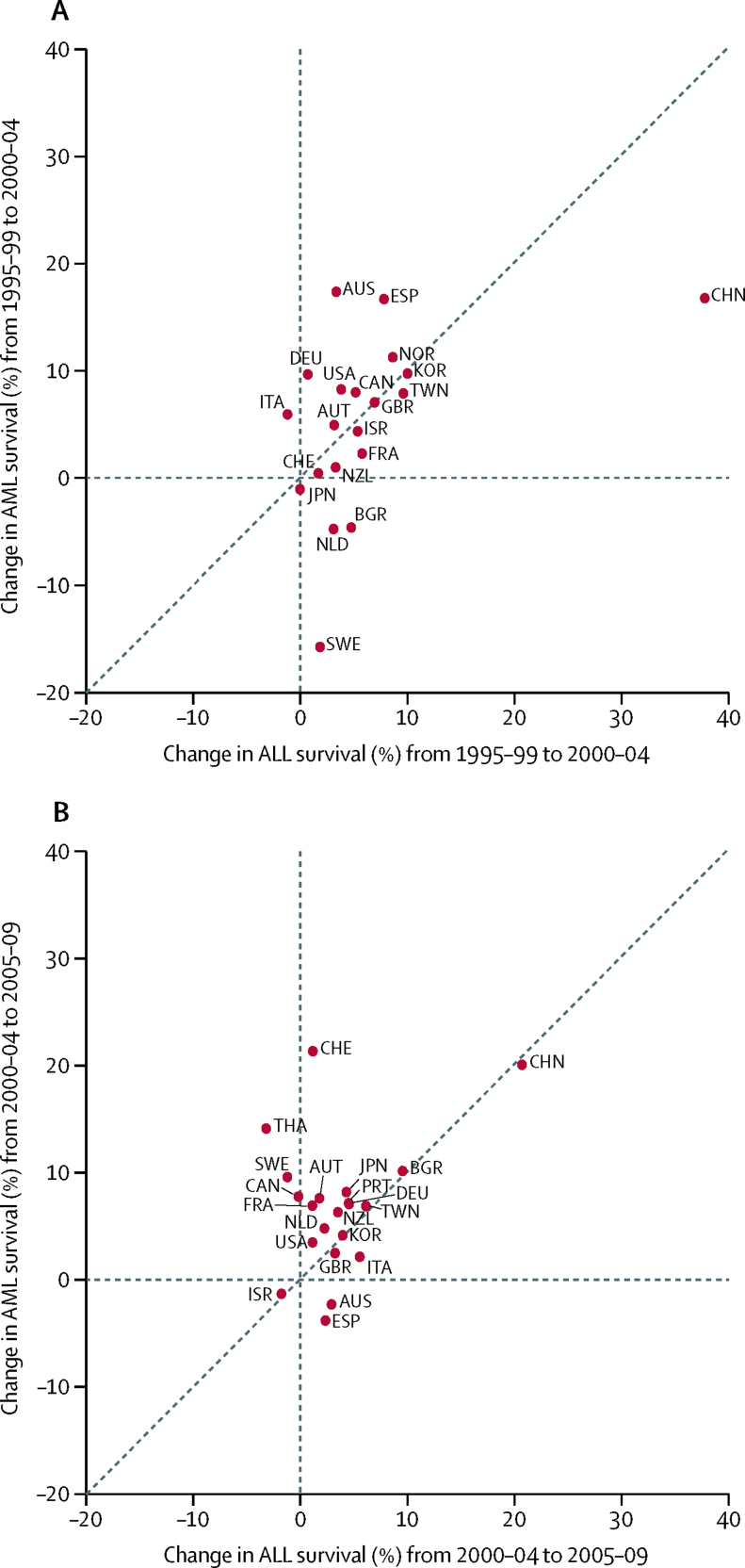
Survival trends for precursor-cell ALL and AML were not markedly different between 1995–99 and 2000–04 (figure 4); however, between 2000–04 and 2005–09, survival from AML increased more than survival from ALL in most countries (figure 4B), particularly in Thailand and Switzerland.
Figure 4.
Change (absolute difference, %) in age-standardised 5-year net survival for acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML), between (A) 1995–99 and 2000–04 and (B) between 2000–04 and 2005–09
Each datapoint represents one of the participating countries. Datapoints above the diagonal indicate that survival from AML increased more than survival from ALL between the two calendar periods. Countries are represented only if 5-year age-standardised estimates were available for ALL and for AML in successive calendar periods. ALL: group Ia1 according to the third edition of the International Classification of Childhood Cancer (ICCC-3). AML: ICCC-3 group Ib. AUS=Australia. AUT=Austria. BGR=Bulgaria. CAN=Canada. CHE=Switzerland. CHN=China. DEU=Germany. ESP=Spain. FRA=France. GBR=United Kingdom. ISR=Israel. ITA=Italy. JPN=Japan. KOR=Republic of Korea. NLD=Netherlands. NOR=Norway. NZL=New Zealand. PRT=Portugal. SWE=Sweden. THA=Thailand. TWN=Taiwan. USA=United States of America.
Discussion
This study provides the largest population-based comparison of survival from childhood leukaemia. It covers trends over 15 years between 1995–2009 in 53 countries, of which 17 were classified by the World Bank as low-income or middle-income countries (LMIC) in 2011. The results highlight the very wide international differences in 5-year net survival for children with acute lymphoid leukaemia and for children with myeloid leukaemia. 5-year net survival has been increasing for both precursor-cell ALL and AML, but it remains consistently higher for precursor-cell ALL than for AML; this worldwide pattern is consistent with previous studies in various regions of the world.7, 17, 18, 19, 20 For many LMICs, analyses and interpretation are limited by sparse data. Several survival estimates could not be age-standardised. However, our results show that, overall, survival has been increasing for ALL and for AML in most countries during 1995–2009, including both high-income and LMICs.
5-year survival for both ALL and AML was very high in Germany and Austria. This might be attributable to the very tight adherence of paediatric haematologists and oncologists in those countries to the protocols and trials of the BFM Group (Berlin, Frankfurt, Muenster), within the framework of a national paediatric cancer registry and reference laboratories, imaging review and tumour boards.21
In most countries, the difference in survival between ALL and AML tended to narrow over the period 1995–2009. This happened both in countries where survival from ALL was very high throughout 1995–2009, and in countries where survival from ALL was low in 1995–99, but increased up to 2005–09. The greater improvement in 5-year survival from AML than from ALL might be related to recent improvements in the care of childhood AML. Our results might reflect the effect of better diagnostic characterisation, risk stratification and subsequent adaptation of treatment, and restriction of indications to cranial radiotherapy and haemopoietic stem-cell transplantation.22
Net survival from AML seemed to approach the level of survival from precursor-cell ALL earlier in some countries than in others. This suggests that improvements in clinical practice have not been implemented in all countries at the same time. In Switzerland, there was a large increase in survival from AML between 1995–2004 and 2005–09, by which time it approached the level of survival from ALL. In Thailand, national protocols were introduced for childhood leukaemia in 2006. This may have contributed to the marked improvement in survival from AML, but did not lead to substantial improvements in survival for ALL.23 In China, despite the impressive increases in survival from AML and ALL throughout 1995–2009, there was no reduction in the difference in survival between ALL and AML.
This large study offered a unique opportunity to examine age-standardised trends in population-based survival from some of the rarer childhood leukaemias, such as mature B-cell leukaemias, mostly Burkitt's leukaemia. In France and the USA, where survival from mature B-cell leukaemia could be age-standardised, the most recent estimates showed that survival from mature B-cell leukaemia was close to that of precursor-cell ALL. A degree of misclassification between precursor-cell ALL and Burkitt's leukaemia is likely because of their common historical classification and the remaining ambiguity in coding, but this is unlikely to explain the increasing survival trends for mature B-cell leukaemia in France and the USA.
Leukaemia survival has been reported as higher in girls than in boys, both in Europe from 1970 up to the 1990s,24 and still recently in North America,18 but this is not a consistent feature worldwide.
As expected, age at diagnosis was an important determinant of 5-year survival: children diagnosed with precursor-cell ALL aged 1–4 years consistently had the best prognosis. Survival from leukaemia in infants (under 1 year) was usually lower than at older ages. Survival was lower for infants with precursor-cell ALL than for infants with AML. This might be because unfavourable prognostic factors such as MLL gene rearrangements are more frequent in infants with ALL than AML. The new coding rules to individualise ALLs and AMLs with specific genetic rearrangements should enable analysis of more specific subgroups in the future.25
Interpretation might be restricted by changes in the clinical definition of leukaemias and lymphomas over time, and differences in coding between registries. The classifications for grouping the types of leukaemia and lymphoma have also changed over decades. ICCC-3 was proposed in 2005,16 and, in 2010, the HAEMACARE Working Group proposed a classification for haematological malignancies in both adults and children.26 Results shown here were based on ICCC-3, which is commonly used for childhood cancer surveillance.7, 17 Using the HAEMACARE classification, we noted similar results for precursor-cell ALL and AML, but some differences for Burkitt's leukaemia, probably due to the broader grouping defined by HAEMACARE (data not shown). We did not have data to show the effect on survival estimates of including endemic Burkitt's lymphoma in Africa in that group.
Children are more likely to be diagnosed and registered with a poorly specified type of leukaemia in settings where access to a pathologist is less than optimal. This could have clinical implications, with children not gaining access to the most appropriate treatment for their particular leukaemia.27 Where the estimates for this poorly specified group of leukaemias could be age-standardised, they indicate that in high-income countries, survival was between that of precursor-cell ALL and AML.
For the Chinese registries, where the proportion of unspecified and other specified leukaemias remained higher than 25% throughout 1995–2009, survival for those leukaemias was lower than that for AML. This suggests uncertain diagnosis and insufficient or inappropriate treatment. Implementation of the recent resource-stratified guidelines for the management of childhood ALL in Asia should lead to better diagnostic characterisation, more appropriate treatment and higher survival.28 In Colombia, the low survival estimate for lymphoid leukaemia (52%) suggests the need for further investigation, since childhood cancer became a national priority in 2010.
Leukaemia is the most common malignancy in children in most countries,6 and we used population-based data, but comparisons of survival by age and type of leukaemia were sometimes limited by low numbers, especially in small populations. Some age-standardised survival estimates have wide CIs. This was particularly true for AML and mature B-cell leukaemia, and for all types of leukaemia in infants. Survival estimates for Tunisian and Algerian registries were less reliable than for other countries. We used the age range 0–14 years for our analysis because this range has been the standard in childhood cancer studies for many years. Adolescents and young adults are increasingly being treated under paediatric protocols, but our choice was agreed with 100 collaborators during a 2-day meeting of the CONCORD Working Group (Cork, Ireland, 2012), at which the protocol was finalised. Detailed analyses of survival for adolescents, young adults, and older adults with leukaemia are under preparation for publication elsewhere.
Other studies of childhood cancer survival have not used net survival. The US SEER programme presents relative survival.18 The European programmes, EUROCARE17 and ACCIS,7 present observed survival, because background mortality in children does not vary greatly between European populations. The CONCORD-2 study has worldwide coverage, so we estimated net survival, because infant mortality varied particularly widely between participating countries.4 For example, infant mortality rates in males covered a 25-fold range in 2007, from 3·0 per 1000 livebirths in Finland to 82·5 per 1000 livebirths in Lesotho.13
Population-based cancer registry data include all or nearly all cases of a given malignancy in each registry's jurisdiction. By contrast with the best achievable survival estimates obtained from patients included in clinical trials, population-based survival estimates reflect the survival of all cancer patients in the population, irrespective of socioeconomic status and disease features. They reflect the overall effectiveness of the health system, from parents' perception of how to respond to symptoms suggestive of malignancy in their child, as well as the efficiency of referral, the quality of investigation and treatment, and the resourcing and organisation of the health service.
In many high-income countries, the incidence of ALL is rising by an average of about 1% every year.7, 18 Whether this is due to a true increase, improved registration, or both, is still debated.29 It is unlikely that improved diagnosis of the less aggressive forms of leukaemia can explain the very widespread rises in survival that we report. In many countries, childhood cancer treatment is provided in specialised centres, which makes cancer registration for children easier than for adults, although active follow-up can be more challenging in children and adolescents. Population-based cancer registry data might still be restricted by underdiagnosis and under-registration of cancer patients, both of which are difficult to quantify. This might be a more important issue for participating registries in the 17 LMICs, but it is nevertheless important to capture the available information as a guide to policy.
There is still room for improvement in the management of childhood leukaemia in many countries. In some LMICs, even the most basic treatment for leukaemia,28, 30 or pain relief, was still not consistently available until recently.31 Abandonment of treatment is also a major issue in some settings.32, 33 5-year survival for children with precursor-cell lymphoblastic leukaemia can be as high as 90%, and up to 80% for children with AML, but in some countries, survival remains below 60% for both types of leukaemia. Interventions that have been proven to improve outcomes in childhood malignancy include enrolment in clinical trials, international collaboration, and treatment guidelines.5, 34, 35 Wider implementation of these initiatives, together with mobilisation of additional resources, especially in poorer countries, would be likely to improve the delivery of effective treatments, and to reduce worldwide inequalities in survival.8, 9, 28, 36, 37
Acknowledgments
Acknowledgments
This work was supported by the Canadian Partnership Against Cancer, Cancer Focus Northern Ireland, Cancer Institute New South Wales, Cancer Research UK (C1336/A16148), US Centers for Disease Control and Prevention (CDC: 12FED03123, ACO12036), Swiss Re, Swiss Cancer Research Foundation, Swiss Cancer League, and the University of Kentucky (3049024672-12-568).
Contributors
AB, CA, and MPC designed the analyses for this study. CAS, JC, DCS, RM-G, RP-B, CEK, and MFGM contributed to data acquisition. AB, RH, HC, DS, MPC, and CA had access to all raw data. AB, RH, HC, DS, MPC, and CA contributed to the data preparation, quality control and analyses, and checked the results. AB drafted the initial report. All authors contributed to the data interpretation, critically revised the manuscript, and approved the version to be published. All members of the CONCORD Working Group had access to the results and contributed to interpretation of the findings.
Declaration of interests
We declare no competing interests.
Contributor Information
Audrey Bonaventure, Email: concord@lshtm.ac.uk.
CONCORD Working Group:
S Bouzbid, M Hamdi-Chérif, Z Zaidi, E Bah, R Swaminathan, SH Nortje, MM El Mistiri, S Bayo, B Malle, SS Manraj, R Sewpaul-Sungkur, Fabowale, OJ Ogunbiyi, D Bradshaw, NIM Somdyala, DC Stefan, M Abdel-Rahman, L Jaidane, M Mokni, I Kumcher, F Moreno, MS González, EA Laura, SB Espinola, GH Calabrano, B Carballo Quintero, R Fita, DA Garcilazo, PL Giacciani, MC Diumenjo, WD Laspada, MA Green, MF Lanza, SG Ibañez, CA Lima, E Lobo de Oliveira, C Daniel, C Scandiuzzi, PCF De Souza, CD Melo, K Del Pino, C Laporte, MP Curado, JC de Oliveira, CLA Veneziano, DB Veneziano, TS Alexandre, AS Verdugo, G Azevedo e Silva, JC Galaz, JA Moya, DA Herrmann, S Vargas, VM Herrera, CJ Uribe, LE Bravo, NE Arias-Ortiz, DM Jurado, MC Yépez, YH Galán, P Torres, F Martínez-Reyes, ML Pérez-Meza, L Jaramillo, R Quinto, P Cueva, JG Yépez, CR Torres-Cintrón, G Tortolero-Luna, R Alonso, E Barrios, C Nikiforuk, L Shack, AJ Coldman, RR Woods, G Noonan, D Turner, E Kumar, B Zhang, FR McCrate, S Ryan, H Hannah, RAD Dewar, M MacIntyre, A Lalany, M Ruta, L Marrett, DE Nishri, C McClure, KA Vriends, C Bertrand, R Louchini, KI Robb, H Stuart-Panko, S Demers, S Wright, JT George, X Shen, JT Brockhouse, DK O'Brien, KC Ward, L Almon, J Bates, R Rycroft, L Mueller, C Phillips, H Brown, B Cromartie, AG Schwartz, F Vigneau, JA MacKinnon, B Wohler, AR Bayakly, CA Clarke, SL Glaser, D West, MD Green, BY Hernandez, CJ Johnson, D Jozwik, ME Charlton, CF Lynch, B Huang, TC Tucker, D Deapen, L Liu, MC Hsieh, XC Wu, K Stern, ST Gershman, RC Knowlton, J Alverson, GE Copeland, DB Rogers, D Lemons, LL Williamson, M Hood, GM Hosain, JR Rees, KS Pawlish, A Stroup, C Key, C Wiggins, AR Kahn, MJ Schymura, G Leung, C Rao, L Giljahn, B Warther, A Pate, M Patil, SS Schubert, JJ Rubertone, SJ Slack, JP Fulton, DL Rousseau, TA Janes, SM Schwartz, SW Bolick, DM Hurley, J Richards, MA Whiteside, LM Nogueira, K Herget, C Sweeney, J Martin, S Wang, DG Harrelson, MB Keitheri Cheteri, S Farley, AG Hudson, R Borchers, L Stephenson, JR Espinoza, HK Weir, BK Edwards, N Wang, L Yang, JS Chen, GH Song, XP Gu, P Zhang, HM Ge, DL Zhao, JH Zhang, FD Zhu, JG Tang, Y Shen, J Wang, QL Li, XP Yang, J Dong, W Li, LP Cheng, JG Chen, QH Huang, SQ Huang, GP Guo, K Wei, WQ Chen, H Zeng, AV Demetriou, P Pavlou, WK Mang, KC Ngan, R Swaminathan, AC Kataki, M Krishnatreya, PA Jayalekshmi, P Sebastian, SD Sapkota, Y Verma, A Nandakumar, E Suzanna, L Keinan-Boker, BG Silverman, H Ito, H Nakagawa, M Hattori, Y Kaizaki, H Sugiyama, M Utada, K Katayama, H Narimatsu, S Kanemura, T Koike, I Miyashiro, M Yoshii, I Oki, A Shibata, T Matsuda, O Nimri, A Ab Manan, N Bhoo Pathy, O Chimedsuren, S Tuvshingerel, AHM Al Khater, MM El Mistiri, H Al-Eid, KW Jung, YJ Won, CJ Chiang, MS Lai, K Suwanrungruang, S Wiangnon, K Daoprasert, D Pongnikorn, SL Geater, H Sriplung, S Eser, CI Yakut, M Hackl, H Mühlböck, W Oberaigner, AA Zborovskaya, OV Aleinikova, K Henau, L Van Eycken, N Dimitrova, Z Valerianova, M Šekerija, M Zvolský, G Engholm, H Storm, K Innos, M Mägi, N Malila, K Seppä, J Jégu, M Velten, E Cornet, X Troussard, AM Bouvier, J Faivre, AV Guizard, V Bouvier, G Launoy, P Arveux, M Maynadié, M Mounier, E Fournier, AS Woronoff, M Daoulas, J Clavel, S Le Guyader-Peyrou, A Monnereau, B Trétarre, M Colonna, A Cowppli-Bony, F Molinié, S Bara, D Degré, O Ganry, B Lapôtre-Ledoux, P Grosclaude, J Estève, F Bray, M Piñeros, F Sassi, R Stabenow, A Eberle, C Erb, A Nennecke, J Kieschke, E Sirri, H Kajueter, K Emrich, SR Zeissig, B Holleczek, N Eisemann, A Katalinic, H Brenner, RA Asquez, V Kumar, EJ Ólafsdóttir, L Tryggvadóttir, H Comber, PM Walsh, H Sundseth, E Devigili, G Mazzoleni, A Giacomin, F Bella, M Castaing, A Sutera, G Gola, S Ferretti, D Serraino, A Zucchetto, R Lillini, M Vercelli, S Busco, F Pannozzo, S Vitarelli, P Ricci, C Pascucci, M Autelitano, C Cirilli, M Federico, M Fusco, MF Vitale, M Usala, R Cusimano, W Mazzucco, M Michiara, P Sgargi, MM Maule, C Sacerdote, R Tumino, E Di Felice, M Vicentini, F Falcini, L Cremone, M Budroni, R Cesaraccio, ML Contrino, F Tisano, AC Fanetti, S Maspero, G Candela, T Scuderi, MA Gentilini, S Piffer, S Rosso, L Sacchetto, A Caldarella, F La Rosa, F Stracci, P Contiero, G Tagliabue, AP Dei Tos, M Zorzi, R Zanetti, P Baili, F Berrino, G Gatta, M Sant, R Capocaccia, R De Angelis, E Liepina, A Maurina, G Smailyte, D Agius, N Calleja, S Siesling, O Visser, S Larønningen, B Møller, A Dyzmann-Sroka, M Trojanowski, S Gózdz, R Mezyk, M Gradalska-Lampart, AU Radziszewska, JA Didkowska, U Wojciechowska, J Blaszczyk, K Kepska, M Bielska-Lasota, K Kwiatkowska, G Forjaz, RA Rego, J Bastos, MA Silva, L Antunes, MJ Bento, A Mayer-da-Silva, A Miranda, D Coza, AI Todescu, MY Valkov, J Adamcik, C Safaei Diba, M Primic-Žakelj, T Žagar, J Stare, E Almar, A Mateos, JR Quirós, J Bidaurrazaga, N Larrañaga, JM Díaz García, AI Marcos, R Marcos-Gragera, ML Vilardell Gil, E Molina, MJ Sánchez, P Franch Sureda, M Ramos Montserrat, MD Chirlaque, C Navarro, EE Ardanaz, CC Moreno-Iribas, R Fernández-Delgado, R Peris-Bonet, J Galceran, S Khan, M Lambe, B Camey, C Bouchardy, M Usel, SM Ess, C Herrmann, JL Bulliard, M Maspoli-Conconi, H Frick, CE Kuehni, M Schindler, A Bordoni, A Spitale, A Chiolero, I Konzelmann, SI Dehler, KL Matthes, J Rashbass, CA Stiller, D Fitzpatrick, A Gavin, F Bannon, RJ Black, DH Brewster, DW Huws, C White, P Finan, C Allemani, A Bonaventure, H Carreira, MP Coleman, V Di Carlo, R Harewood, K Liu, M Matz, L Montel, M Nikšic, B Rachet, N Sanz, D Spika, R Stephens, M Peake, MFG Murphy, E Chalker, L Newman, D Baker, MJ Soeberg, J Aitken, C Scott, BC Stokes, A Venn, H Farrugia, GG Giles, T Threlfall, D Currow, H You, J Hendrix, C Lewis, MRDO Latorre, and LF Tanaka
Supplementary Material
References
- 1.Wang H, Liddell CA, Coates MM. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:957–979. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:e359–e386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Weir HK, Carreira H. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magrath I, Steliarova-Foucher E, Epelman S. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14:e104–e116. doi: 10.1016/S1470-2045(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Kramárová E, Draper GJ, editors. International incidence of childhood cancer, vol II (IARC Scientific Publications 144) International Agency for Research on Cancer; Lyon: 1998. [Google Scholar]
- 7.Coebergh JW, Reedijk AM, de Vries E. Leukaemia incidence and survival in children and adolescents in Europe during 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2019–2036. doi: 10.1016/j.ejca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Union for International Cancer Control . World Cancer Declaration 2013. UICC; Geneva: 2013. http://www.uicc.org/world-cancer-declaration (accessed March 11, 2015). [Google Scholar]
- 9.European Society for Paediatric Oncology . The SIOPE strategic plan: a European cancer plan for children and adolescents. SIOP Europe; 2015. [Google Scholar]
- 10.Fritz AG, Percy C, Jack A, editors. International classification of diseases for oncology (ICD-O) 3rd edn. World Health Organization; Geneva: 2000. [Google Scholar]
- 11.Li R, Abela L, Moore J. Control of data quality for population-based cancer survival analysis. Cancer Epidemiol. 2014;38:314–320. doi: 10.1016/j.canep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Pohar Perme M, Henderson R, Stare J. An approach to estimation in relative survival regression. Biostatistics. 2009;10:136–146. doi: 10.1093/biostatistics/kxn021. [DOI] [PubMed] [Google Scholar]
- 13.Spika D, Rachet B, Bannon F. Life tables for the CONCORD-2 study. CONCORD Central Analytic Team; London: 2015. http://csg.lshtm.ac.uk/tools-analysis/ (accessed Sept 24, 2016). [Google Scholar]
- 14.Brenner H, Gefeller O. Deriving more up-to-date estimates of long-term patient survival. J Clin Epidemiol. 1997;50:211–216. doi: 10.1016/s0895-4356(97)00280-1. [DOI] [PubMed] [Google Scholar]
- 15.Stiller CA, Bunch KJ. Trends in survival for childhood cancer in Britain diagnosed 1971–85. Br J Cancer. 1990;62:806–815. doi: 10.1038/bjc.1990.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, 3rd edn. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 17.Gatta G, Botta L, Rossi S. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 18.Howlader N, Noone AM, Krapcho M, editors. SEER cancer statistics review, 1975–2012. National Cancer Institute; Bethesda: 2015. [Google Scholar]
- 19.Ajiki W, Tsukuma H, Oshida A. Survival rates of childhood cancer patients in Osaka, Japan. Jpn J Clin Oncol. 2004;34:50–54. doi: 10.1093/jjco/hyh003. [DOI] [PubMed] [Google Scholar]
- 20.Baade PD, Youlden DR, Valery PC. Population-based survival estimates for childhood cancer in Australia during the period 1997–2006. Br J Cancer. 2010;103:1663–1670. doi: 10.1038/sj.bjc.6605985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossig C, Juergens H, Schrappe M. Effective childhood cancer treatment: the impact of large scale clinical trials in Germany and Austria. Pediatr Blood Cancer. 2013;60:1574–1581. doi: 10.1002/pbc.24598. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Schrappe M, Ribeiro R, Niemeyer CM. Childhood and adolescent lymphoid and myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2004;1:118–145. doi: 10.1182/asheducation-2004.1.118. [DOI] [PubMed] [Google Scholar]
- 23.Seksarn P, Wiangnon S, Veerakul G, Chotsampancharoen T, Kanjanapongkul S, Chainansamit S-O. Outcome of childhood acute lymphoblastic leukemia treated using the thai national protocols. Asian Pac J Cancer Prev. 2015;16:4609–4614. doi: 10.7314/apjcp.2015.16.11.4609. [DOI] [PubMed] [Google Scholar]
- 24.Coebergh JWW, Capocaccia R, Gatta G, Magnani C, Stiller CA. Childhood cancer survival in Europe 1978–92: the EUROCARE study. Eur J Cancer. 2001;37:671–672. doi: 10.1016/s0959-8049(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 25.Fritz AG, Percy C, Jack A, editors. International classification of diseases for oncology (ICD-O) first revision of 3rd edn. World Health Organization; Geneva: 2013. [Google Scholar]
- 26.Sant M, Karjalainen-Lindsberg ML, Maynadié M. Manual for coding and reporting haematological malignancies. Tumori. 2010;96:i–A32. [PubMed] [Google Scholar]
- 27.Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, Stiller C, Coebergh JW. Cancer in children and adolescents in Europe: developments over 20 years and future challenges. Eur J Cancer. 2006;42:2183–2190. doi: 10.1016/j.ejca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Yeoh AE, Tan D, Li CK, Hori H, Tse E, Pui CH. Management of adult and paediatric acute lymphoblastic leukaemia in Asia: resource-stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol. 2013;14:e508–e523. doi: 10.1016/S1470-2045(13)70452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah A, Coleman MP. Increasing incidence of childhood leukaemia: a controversy re-examined. Br J Cancer. 2007;97:1009–1012. doi: 10.1038/sj.bjc.6603946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Union for International Cancer Control Childhood Cancer. http://www.uicc.org/programmes/chica/issue (accessed Nov 11, 2015).
- 31.WHO . Guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. World Health Organization; Geneva: 2012. [PubMed] [Google Scholar]
- 32.Gupta S, Yeh S, Martiniuk A. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2555–2564. doi: 10.1016/j.ejca.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Mostert S, Arora RS, Arreola M. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol. 2011;12:719–720. doi: 10.1016/S1470-2045(11)70128-0. [DOI] [PubMed] [Google Scholar]
- 34.Pui CH, Yang JJ, Hunger SP. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchard-Jones K, Pieters R, Reaman GH. Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol. 2013;14:e95–103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 36.National Cancer Institute . An analysis of the National Cancer Institute's investment in pediatric cancer research. US Department of Health and Human Services, National Institutes of Health; 2013. [Google Scholar]
- 37.Sullivan R, Kowalczyk JR, Agarwal B. New policies to address the global burden of childhood cancers. Lancet Oncol. 2013;14:e125–e135. doi: 10.1016/S1470-2045(13)70007-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.