Abstract
Water purification technologies such as microfiltration/ultrafiltration and reverse osmosis utilize porous membranes to remove suspended particles and solutes. These membranes, however, cause many drawbacks such as a high pumping cost and a need for periodic replacement due to fouling. Here we show an alternative membraneless method for separating suspended particles by exposing the colloidal suspension to CO2. Dissolution of CO2 into the suspension creates solute gradients that drive phoretic motion of particles. Due to the large diffusion potential generated by the dissociation of carbonic acid, colloidal particles move either away from or towards the gas–liquid interface depending on their surface charge. Using the directed motion of particles induced by exposure to CO2, we demonstrate a scalable, continuous flow, membraneless particle filtration process that exhibits low energy consumption, three orders of magnitude lower than conventional microfiltration/ultrafiltration processes, and is essentially free from fouling.
Water treatment processes mostly rely on the use of membranes and filters, which have high pumping costs and require periodic replacement. Here, the authors describe an efficient membraneless method that induces directed motion of suspended colloidal particles by exposing the suspension to CO2.
With global demand for clean water increasing, there is a continuing need to improve the performance of water treatment processes1,2. Membraneless separation of suspended particles is conventionally achieved by sedimentation, which relies on the gravitational force3. When particle suspensions are stable, for example, due to the particles being small, neutrally buoyant and highly charged, unassisted sedimentation is ineffective and additives are needed to induce aggregation (coagulation or flocculation4) and accelerate sedimentation. Therefore, inducing directed motion of colloidal particles in stable suspensions is critical to membraneless separation processes.
There are several ways to induce directed motion of colloidal particles such as using an external force, for example, electrostatic5, dielectric6, magnetic7, acoustic8, or optical9, inertial effects10 and so on. One less known but significant driving force is a chemical gradient, which causes diffusiophoresis11. Typically, diffusiophoresis is observed in liquid environments where chemical gradients are generated by contact between solutions with different solute concentrations12,13,14,15.
Here we show that diffusiophoresis of colloidal particles can be achieved by dissolution of a gas into a liquid, namely CO2 dissolution into water and its subsequent dissociation that produces ion concentration gradients. Using this principle, we show that the directed motion driven by dissolution of CO2 can be exploited to separate particles with very low energy consumption. Moreover, CO2 is abundant, biologically benign when dissolved in water, and can be easily separated. These features make CO2 dissolution a very promising means to clean water especially for the developing world, which requires water purification technologies that have low energy demands and are not chemically intense2.
Results
Gas-induced diffusiophoresis
Dissolved CO2 establishes an equilibrium with water through the overall reaction16,17
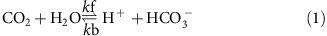 |
where kf and kb are the forward and backward reaction rate constants18, respectively. Reaction (1) implies that transient ion gradients can be created in aqueous particle suspensions when they are exposed to CO2 gas. Most significantly, the generated ions exhibit a large difference in their diffusivities19 (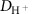 =9.3 × 10−9 m2 s−1 and
=9.3 × 10−9 m2 s−1 and 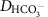 =1.2 × 10−9 m2 s−1), which may lead to strong particle diffusiophoresis due to a large diffusion potential (≈92 mV for a 100-fold concentration difference, whereas in ordinary salt gradients, the potential is 25 mV for Na+/Cl− and 0.1 mV for K+/Cl−).
=1.2 × 10−9 m2 s−1), which may lead to strong particle diffusiophoresis due to a large diffusion potential (≈92 mV for a 100-fold concentration difference, whereas in ordinary salt gradients, the potential is 25 mV for Na+/Cl− and 0.1 mV for K+/Cl−).
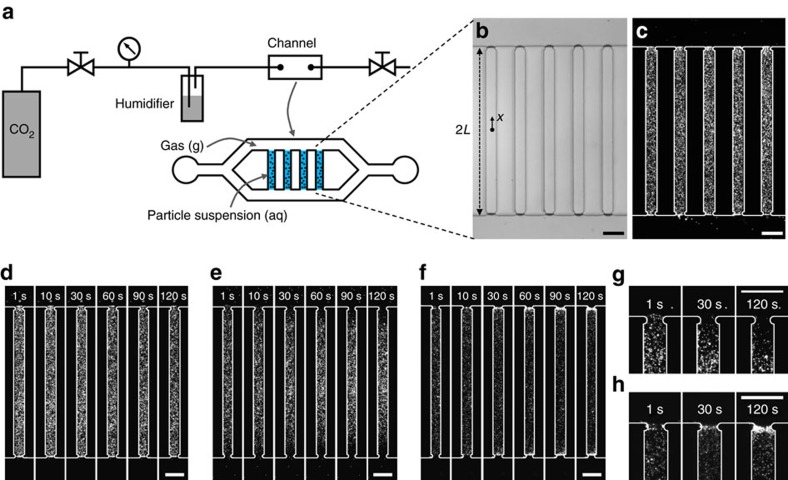
To test the hypothesis that exposure to CO2 can induce sufficient ion concentration gradients to drive particle motion, we developed a microfluidic system in which we create a stable gas–liquid interface (Fig. 1a, see Methods section). The microfluidic device, which is made out of ultraviolet-curable epoxy to prevent permeation of gases20, has two main channels bridged by multiple narrow pores that contain a colloidal suspension (Fig. 1b,c).
Figure 1. Motion of colloidal particles upon exposure of aqueous suspensions to gas.
(a) Experimental set-up for creating a liquid column with a stable gas–liquid interface. (b) Bright-field and (c) corresponding fluorescence microscopy images of suspension-filled channels exposed to gas, where the length of the channel is 2L=800 μm. The width and the height of the channels are 60 and 40 μm, respectively. The ends of the pores are slightly contracted to effectively fix the liquid columns. (d) Image sequence showing particles (polystyrene, diameter 0.5 μm) exposed to N2. (e,f) Image sequences showing directed motion of particles with (e) negative (polystyrene, diameter 0.5 μm) and (f) positive (amine-functionalized polystyrene, diameter 1 μm) surface charge exposed to CO2. (g,h) Close-up images of the time evolution of the (g) negatively charged and (h) positively charged particles near the gas–liquid interface during exposure to CO2. The gas pressures in all cases are 136 kPa. All scale bars are 100 μm.
Upon exposure to CO2 gas (pressure 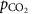 =136 kPa), negatively charged particles (polystyrene, diameter≈0.5 μm, zeta potential≈−70 mV) immediately migrate away from the two gas–liquid interfaces (Fig. 1e,g, Supplementary Movie 1). In contrast (Fig. 1d, Supplementary Movie 2), no appreciable motion of these particles, other than Brownian motion, is observed upon exposure to N2 gas (
=136 kPa), negatively charged particles (polystyrene, diameter≈0.5 μm, zeta potential≈−70 mV) immediately migrate away from the two gas–liquid interfaces (Fig. 1e,g, Supplementary Movie 1). In contrast (Fig. 1d, Supplementary Movie 2), no appreciable motion of these particles, other than Brownian motion, is observed upon exposure to N2 gas (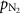 =136 kPa). Based on our hypothesis, the observed particle migration is mainly induced by the diffusion potential due to H+ and
=136 kPa). Based on our hypothesis, the observed particle migration is mainly induced by the diffusion potential due to H+ and 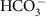 . This assertion is supported by experiments with positively charged particles (amine-functionalized polystyrene, diameter≈1 μm, zeta potential≈60 μm) that migrate toward the gas–liquid interfaces upon exposure to CO2, forming clouds of particles near these interfaces (Fig. 1f,h, Supplementary Movie 3).
. This assertion is supported by experiments with positively charged particles (amine-functionalized polystyrene, diameter≈1 μm, zeta potential≈60 μm) that migrate toward the gas–liquid interfaces upon exposure to CO2, forming clouds of particles near these interfaces (Fig. 1f,h, Supplementary Movie 3).
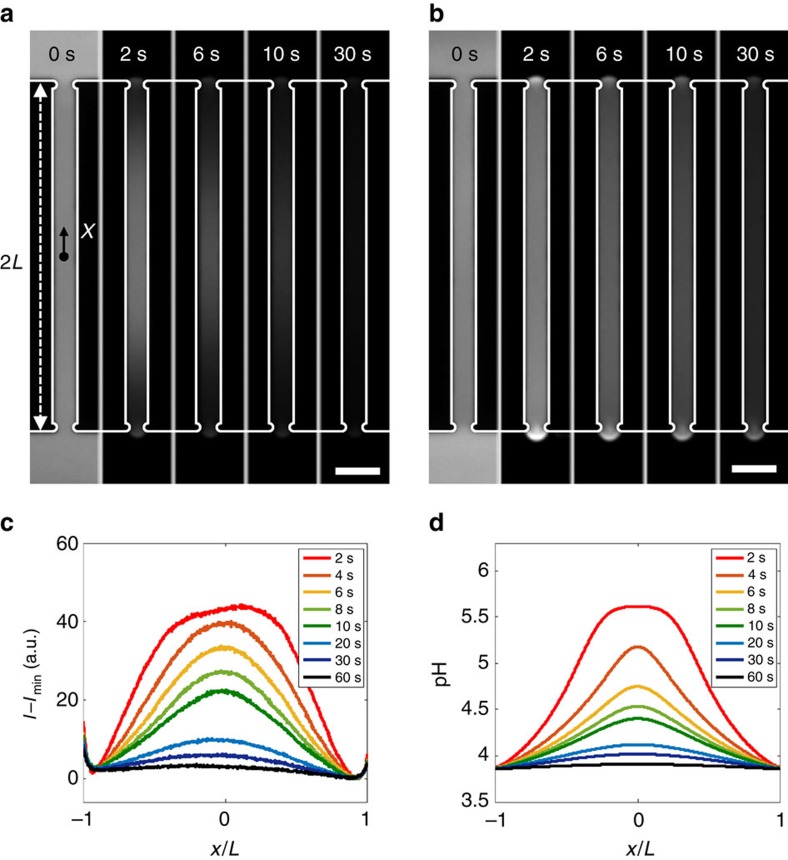
The presence of ion concentration gradients, responsible for driving the motion of particles, was confirmed by observing the response of liquid columns filled with pH indicator solution to gas exposure. We used Oregon Green fluorescent dye (0.1 mM) as the pH indicator due to its linear dependence of fluorescence intensity on pH over a broad pH range21. As the water is exposed to CO2 at 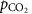 =136 kPa, the fluorescence intensity immediately decreases near the gas–liquid interfaces, and then the decreasing intensity propagates toward the middle of the column, indicating that pH decreases from the interfaces inward (Fig. 2a). The high intensity evident near the gas–liquid interface is due to the curvature of the meniscus. In contrast to CO2, exposure to N2 at the same pressure does not produce spatial variations in fluorescence intensity, but only a gradual decrease of fluorescence intensity due to photobleaching (Fig. 2b).
=136 kPa, the fluorescence intensity immediately decreases near the gas–liquid interfaces, and then the decreasing intensity propagates toward the middle of the column, indicating that pH decreases from the interfaces inward (Fig. 2a). The high intensity evident near the gas–liquid interface is due to the curvature of the meniscus. In contrast to CO2, exposure to N2 at the same pressure does not produce spatial variations in fluorescence intensity, but only a gradual decrease of fluorescence intensity due to photobleaching (Fig. 2b).
Figure 2. pH change in water upon exposure to gas.
(a,b) Image sequences showing fluorescence intensity in aqueous Oregon Green solution (0.1 mM) exposed to (a) CO2 and (b) N2, both at a pressure of 136 kPa. (c) Fluorescence intensity (I) distribution, reported as I−Imin to account for photobleaching, along the centre of the channel shown in a. Imin is the minimum intensity in the channel. (d) Computed pH distribution in water exposed to CO2 at 136 kPa. All scale bars are 100 μm.
Solving coupled diffusion-reaction equations predicts the transient pH distribution under exposure to CO2. The dissolved CO2 and ion concentrations are coupled through the net forward rate of reaction (1) r=kfcc−kb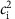 , where cc(x,t) is the concentration of dissolved CO2, and ci(x,t) gives the concentrations of both generated ions, H+ and
, where cc(x,t) is the concentration of dissolved CO2, and ci(x,t) gives the concentrations of both generated ions, H+ and 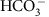 , which are effectively equal due to local charge neutrality22,23. Then, solving the diffusion-reaction equations ∂cc/∂t=Dc∇2cc−r and ∂ci/∂t=Di∇2ci+r yields the transient ion concentration distribution, where Dc is the diffusivity of CO2 and Di is the ambipolar diffusivity of the ions. The calculated pH distribution (Fig. 2d) shows reasonable agreement with the observed fluorescence intensity profile (Fig. 2c). As the pH decreases from 5.6 (the pH of water in equilibrium with the atmospheric CO2 concentration,
, which are effectively equal due to local charge neutrality22,23. Then, solving the diffusion-reaction equations ∂cc/∂t=Dc∇2cc−r and ∂ci/∂t=Di∇2ci+r yields the transient ion concentration distribution, where Dc is the diffusivity of CO2 and Di is the ambipolar diffusivity of the ions. The calculated pH distribution (Fig. 2d) shows reasonable agreement with the observed fluorescence intensity profile (Fig. 2c). As the pH decreases from 5.6 (the pH of water in equilibrium with the atmospheric CO2 concentration, 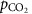 =40 kPa) down to 3.8 for
=40 kPa) down to 3.8 for 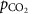 =136 kPa, a nearly 100-fold difference in ion concentrations occurs within the column.
=136 kPa, a nearly 100-fold difference in ion concentrations occurs within the column.
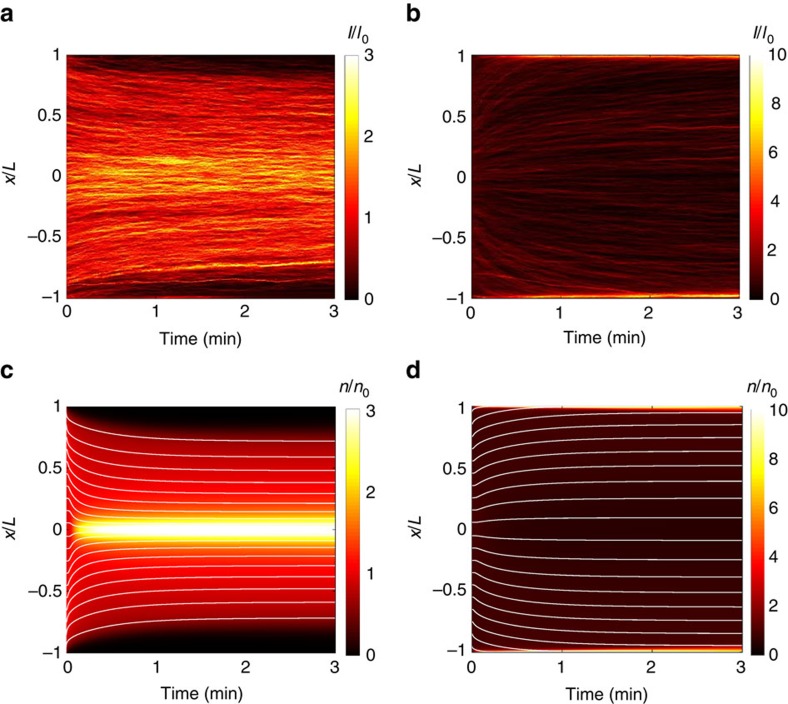
This chemical gradient generated by dissolution of CO2 induces particle migration via diffusiophoresis. The particle diffusiophoretic velocity is expressed as up=Γp∇lnci, where the prefactor Γp is the diffusiophoretic mobility, which is a measure of the strength of diffusiophoresis based on surface-solute interactions11. By solving an additional advection–diffusion equation for the particles, we obtain the particle dynamics driven by the dissolution of CO2 (Supplementary Discussion). The one-dimensional transport model captures the key dynamics that are consistent with the experimental observations for both the negatively (Fig. 3a,c) and positively charged particles (Fig. 3b,d), where the negatively charged particles move away from the gas–liquid interface and subsequently focus to the centre of the column; motion is in the opposite direction for positively charged particles. From calculations at other conditions than those of these experiments, we note that the minimum CO2 pressure needed for inducing appreciable particle motion is much lower than 136 kPa, but it must be sufficiently higher than the atmospheric partial pressure of CO2 (see Supplementary Fig. 1, Supplementary Table 2, and Supplementary Discussion).
Figure 3. Spatio-temporal evolution of particle distributions upon exposure to CO2.
(a,b) Experimental and (c,d) computed plots for (a,c) negatively charged (polystyrene, diameter 0.5 μm) and (b,d) positively charged particles (amine-functionalized polystyrene, diameter 1 μm) exposed to CO2 at 136 kPa. I and n represent fluorescence intensity and particle concentration, respectively. Subscript 0 indicates a quantity at t=0 s. The experimental plots are obtained by averaging the intensity over the width of the channel. The white curves in (c,d) indicate the trajectories of individual particles obtained by integrating their diffusiophoretic velocities and neglecting Brownian motion. The computations for (d) account for the presence of a low concentration of ionic solutes in the positive particle suspensions (see Supplementary Fig. 2 and Supplementary Discussion).
Membraneless water filtration
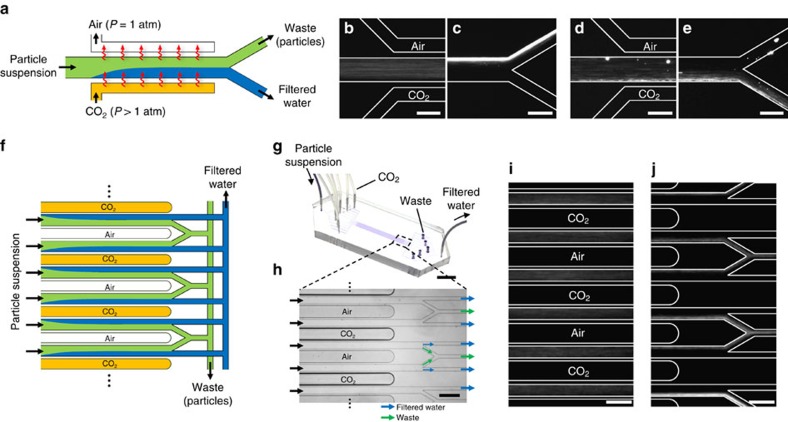
The observed colloid dynamics suggest an alternate route to separating particles without the use of membranes or filters, which is by exposing the colloidal suspension to CO2 to concentrate the particles locally as in field-flow fractionation24,25. A continuous flow particle filtration device is illustrated in Fig. 4a. A colloidal suspension flows through a straight channel in a gas permeable material, in this case, polydimethylsiloxane (PDMS)26,27. Separated by a thin wall, a CO2 gas channel passes parallel to the main flow channel. The gas permeates through the wall and dissolves into the flow stream, inducing particle motion transverse to the flow direction; the direction of motion depends on the particles' surface charge. An air channel on the other side of the wall prevents saturation of CO2 in the suspension, thus sustaining a greater concentration gradient and enabling further migration of particles by diffusiophoresis. After all of the particles have been concentrated at one side of the channel, they are separated by splitting the flow near the outlet.
Figure 4. Continuous-flow membraneless water filtration using dissolution of CO2.
(a) Schematic of the water filtration process. The channel material (PDMS) allows permeation of CO2 gas into the particle suspension, inducing directed particle motion normal to the flow direction. (b–e) Fluorescence microscopy images of (b,c) negatively and (d,e) positively charged particles migrating transverse to the flow direction. Images are taken near the (b,d) entrance and (c,e) end of the flow channel, where the channel length is 3 cm. PDMS walls (30 μm wide) separate the parallel gas and flow channels. The CO2 channel is kept at a pressure of 170 kPa whereas the air channel is left open to the atmosphere. The air channel prevents saturation of CO2 in the suspension, thus enhancing particle migration transverse to the flow direction. (f) Schematic of a scalable water filtration process based on the unit device shown in a. (g) Photograph of water filtration device having 10 parallel flow channels in a monolithic PDMS block. (h) Bright-field microscopy image of the microfluidic channels near the outlets. The CO2 channels are kept at 136 kPa whereas the air channels are left open to the atmosphere. (i,j) Fluorescence microscopy images of the negatively charged particles near the (i) entrance and (j) end of the flow channel. The scale bars are (b–e) 100 μm, (g) 1 cm and (h–j) 200 μm.
The removal of colloidal particles using our method is presented in Fig. 4b–e, where the device is made out of standard PDMS. Negatively charged polystyrene particles, which are initially distributed uniformly (Fig. 4b), migrate away from the wall that is adjacent to the CO2 channel as they flow downstream, and are eventually separated from the main stream (Fig. 4c). Positively charged particles can also be filtered using the same principle (Fig. 4d,e). The particle removal rate in terms of log reduction value is estimated to be 5.3 (see Methods and Supplementary Fig. 3), which is comparable to values for conventional microfiltration and ultrafiltration techniques28.
Since the demonstrated method does not involve any filters, its energy consumption is low compared to conventional filtration methods. Energy dissipation is reduced because the fluid may flow through a channel that is much wider than the particles instead of passing through pores that are smaller than the particles. Based on the current channel geometry (width × height × length=0.1 × 0.02 × 30 mm3) and flow rate (2 μl h-1), the pressure drop across the channel is Δp≈0.2 kPa. Assuming 50% recovery of water from the suspension, the energy consumption is estimated as 0.12 mW h l−1, at least three orders of magnitude lower than the energy consumed in typical microfiltration and ultrafiltration processes29,30 (0.1∼20 W h l−1). The energy efficiency can be further enhanced by optimizing the channel geometry such as increasing the channel height to reduce the pressure drop for a given flow speed.
Furthermore, the proposed device is easily scalable due to its simple design, for example by using parallel flow channels (Fig. 4f). Such a design allows easy scaling-up in both the in-plane and out-of-plane directions due to the isotropic permeability of gas in PDMS31. As a proof of concept, a 10-fold upscaled device made out of a monolithic PDMS block is shown in Fig. 4g–j. Likewise, a million-fold upscaling, which is feasible using standard microfabrication techniques, would purify water at ≈50 l per day. The dissolved CO2 in the output streams can be removed by equilibration with air (see for example, ref. 27). The waste stream could pass then through the same process multiple times to further concentrate the particle stream and extract additional water.
Discussion
By exploiting the dissolution of CO2 as a way to drive particle motion, we have demonstrated a membraneless method that is capable of separating the types of particles that would otherwise form a stable suspension. Due to their small size, surface charge, and the absence of dissolved ions to screen the charge (that is, a large Debye length), the particles we separate would be difficult to remove via sedimentation. The proposed technique is easily scalable and shows a three order of magnitude lower energy consumption than conventional filtration systems, suggesting the technique could be particularly beneficial in both the developing and developed worlds2. We suggest that the proposed technique could be used either to replace microfiltration and ultrafiltration or benefit such conventional filtration processes by mitigating membrane fouling. Also, considering that most bioparticles are charged32, the proposed method can be utilized to remove bacteria and viruses without chlorination or ultraviolet treatment2,30.
Efficient scaling-up of the process to significant flow rates requires optimization of the channel dimensions, the flow rate in each channel, the split ratio, and the number of parallel channels. While frictional losses can be reduced by using a wider or taller channel, increasing the width decreases the magnitude of the concentration gradient. Reduced gradients and lower residence times due to higher flow rates can be compensated by using longer channels at the expense of increasing the required energy input. Longer and narrower channels can also be used to overcome reductions in the diffusiophoretic driving force in applications with sufficient background salt concentrations. To assess the extent of the decrease in the diffusiophoretic driving force with increasing salt concentrations, further experiments with controlled amounts of common salts as well as representative samples with typical mineral contents from industrial and natural water sources would be valuable. Long-term experiments are also needed to assess fouling, such as by proteins or bacterial extracellular polymeric substances.
Our demonstration of particle transport driven by CO2 dissolution is not limited to particle separation, but may also explain the mechanisms underlying some applications that involve dissolution of CO2, such as the stabilization of bubbles via particle assembly at their surfaces33 and the enhancement of froth flotation through the use of CO2 bubbles34. These applications require collection of particles at gas–liquid interfaces, and Fig. 1h demonstrates that even hydrophilic particles can be collected at a gas–liquid interface from a dilute suspension.
Methods
Liquid column experiments
The microfluidic device for the liquid column experiments shown in Fig. 1 was made out of ultraviolet-curable epoxy (NOA81, Norland Products) using the microfluidic sticker technique35. The channels were 40 μm thick. Humidified gas is fed through the microfluidic channel, which is initially filled with colloidal suspension. The gas pushes out all the suspension in the main channels, leaving behind columns of colloid in the pores that bridge the two main channels, thus creating a stable gas–liquid interface. This is facilitated by small constrictions at the ends of the pores which serve to break the liquid columns at the right place. Once the suspension is flushed out, the outlet is closed to stop the gas flow, which may otherwise interrupt the particle motion. The gas pressure is monitored and controlled by a pressure regulator (Type-10, Bellofram) equipped with a digital pressure gauge (DPG1000B, Omega). The gas pressure of 136 kPa (5 psi above atmospheric pressure) was chosen to allow flow through the device to an outlet at atmospheric pressure. The colloidal particles (polystyrene, Bangs Laboratories; amine-functionalized polystyrene, Sigma-Aldrich) were prepared by dilution in deionized water (Milli-Q, Millipore) to a volume fraction of 0.01%. pH visualization experiments were conducted in the same manner. The pH indicator was prepared by diluting fluorescent dye (Oregon Green 488, Thermo Fisher Scientific) in deionized water at 0.1 mM. The colloidal particles and the dye were observed with an inverted fluorescence microscope (DMI4000B, Leica). The zeta potentials of the colloidal particles were measured using Zetasizer Nano-ZS (Malvern Instruments).
Water filtration experiments
The microfluidic device for the water filtration experiments shown in Fig. 4 was made out of PDMS (Sylgard 184 Elastomer Kit, Dow Corning) using a conventional soft lithography technique. The monomer and the cross linker were mixed at a weight ratio of 10:1. A syringe pump (PhD Ultra, Harvard Apparatus) was used to flow the colloidal suspensions through the filtration device. For the positively charged particle experiments, in order to prevent adhesion of amine-functionalized polystyrene particles to the channel walls, the PDMS was modified with amine by flowing a 1 vol% aqueous solution of 3-aminopropyltrimethoxysilane (Sigma-Aldrich) through the PDMS channel for 20 min followed by rinsing with deionized water for 10 min prior to experiments36.
Log reduction value measurement
Owing to the low flow rate (2 μl h−1) and high fluorescence intensity of the particles, we are able to count the number of individual particles passing through the filtrate stream (Supplementary Fig. 3). During 5 min, out of ∼2.2 × 107 total particles only 104 pass through the filtrate stream, which corresponds to a log reduction value of 5.3.
Simulations
The coupled reaction-diffusion equations for the CO2 (Supplementary equation (2)) and ion concentrations (Supplementary equation (8)) together with the advection–diffusion equation (Supplementary equation (9)) for the particle concentration were solved in MATLAB using pdepe. Exploiting symmetry, solutions were computed for x∈[0, L] using a fine grid with refinement at x=0 and x=L to resolve gradients in concentration. The particle trajectories xp(t) were computed by interpolating the pre-computed ion concentration solution and numerically integrating dxp/dt=udp(xp(t), t) for several uniformly spaced initial positions using ode113 also in MATLAB. Additional details are provided in the Supplementary Discussion. The model parameters are listed in Supplementary Table 1.
Data availability
The data that support the findings of this study are available from the authors upon reasonable request.
Additional information
How to cite this article: Shin, S. et al. Membraneless water filtration using CO2. Nat. Commun. 8, 15181 doi: 10.1038/ncomms15181 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables, Supplementary Discussions and Supplementary References
Suspension of negative particles exposed to carbon dioxide. Negatively charged polystyrene particles suspended in water migrate away from the gas-liquid interface when the suspension is exposed to carbon dioxide.
Suspension of negative particles exposed to nitrogen. When a suspension of negatively charged polystyrene particles in water is exposed to nitrogen, only Brownian motion of the particles is observed.
Suspension of positive particles exposed to carbon dioxide. Positively charged, aminefunctionalized, polystyrene particles suspended in water accumulate near the gas-liquid interface when the suspension is exposed to carbon dioxide.
Acknowledgments
We acknowledge support from Unilever Research. We thank Bhargav Rallabandi and Suin Shim for valuable discussions. O.S. thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) for a postdoctoral fellowship.
Footnotes
P.B.W. discloses a substantive (>$10,000) stock holding in Unilever PLC. The authors have filed a provisional patent application related to this work. The remaining authors declare no competing financial interests.
Author contributions All authors conceived the project. S.S. performed the experiments. O.S. performed the simulations. All authors discussed the results and wrote the paper.
References
- UNICEF. Progress on Sanitation And Drinking Water: 2015 Update and MDG Assessment World Health Organization (2015). [Google Scholar]
- Shannon M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). [DOI] [PubMed] [Google Scholar]
- Rushton A., Ward A. S. & Holdich R. G. Solid-Liquid Filtration and Separation Technology 2nd edn (Wiley-VCH, 2000).
- Berg J. C. An Introduction to Interfaces & Colloids World Scientific (2010). [Google Scholar]
- Radko S. P. & Chrambach A. Separation and characterization of sub-μm-and-μm-sized particles by capillary zone electrophoresis. Electrophoresis 23, 1957–1972 (2002). [DOI] [PubMed] [Google Scholar]
- Gascoyne P. R. C. & Vykoukal J. Particle separation by dielectrophoresis. Electrophoresis 23, 1973–1983 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijs M. A. M. Magnetic bead handling on-chip: new opportunities for analytical applications. Microfluid. Nanofluid. 1, 22–40 (2004). [Google Scholar]
- Shi J., Huang H., Stratton Z., Huang Y. & Huang T. J. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab. Chip. 9, 3354–3359 (2009). [DOI] [PubMed] [Google Scholar]
- Tkachenko G. & Brasselet E. Optofluidic sorting of material chirality by chiral light. Nat. Commun. 5, 3577 (2014). [DOI] [PubMed] [Google Scholar]
- Di Carlo D., Irimia D., Tompkins R. G. & Toner M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl Acad. Sci. USA 104, 18892–18897 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L. Colloid transport by interfacial forces. Ann. Rev. Fluid Mech. 21, 61–99 (1989). [Google Scholar]
- Abécassis B., Cottin-Bizonne C., Ybert C., Ajdari A. & Bocquet L. Boosting migration of large particles by solute contrasts. Nat. Mater. 7, 785–789 (2008). [DOI] [PubMed] [Google Scholar]
- Kar A., Chiang T.-Y., Rivera I. O., Sen A. & Velegol D. Enhanced transport into and out of dead-end pores. ACS Nano 9, 746–753 (2015). [DOI] [PubMed] [Google Scholar]
- Shin S. et al. Size-dependent control of colloid transport via solute gradients in dead-end channels. Proc. Natl Acad. Sci. USA 113, 257–261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velegol D., Garg A., Guha R., Kar A. & Kumar M. Origins of concentration gradients for diffusiophoresis. Soft Matter 12, 4686–4703 (2016). [DOI] [PubMed] [Google Scholar]
- Ho C. & Sturtevant J. M. The kinetics of the hydration of carbon dioxide at 25°. J. Biol. Chem. 238, 3499–3501 (1963). [PubMed] [Google Scholar]
- Gibbons B. H. & Edsall J. T. Rate of hydration of carbon dioxide and dehydration of carbonic acid at 25°. J. Biol. Chem. 238, 3502–3507 (1963). [PubMed] [Google Scholar]
- Jolly W. L. Modern Inorganic Chemistry 2nd edn (McGraw-Hill, 1991).
- Haynes W. M. (ed.). CRC Handbook of Chemistry and Physics 96th edn (CRC Press, 2015).
- Paustian J. S., Azevedo R. N., Lundin S.-T. B., Gilkey M. J. & Squires T. M. Microfluidic microdialysis: spatiotemporal control over solution microenvironments using integrated hydrogel membrane microwindows. Phys. Rev. X 3, 041010 (2013). [Google Scholar]
- Hayward R., Saliba K. J. & Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J. Cell Sci. 119, 1016–1025 (2006). [DOI] [PubMed] [Google Scholar]
- Jackson J. L. Charge neutrality in electrolytic solutions and the liquid junction potential. J. Phys. Chem. 78, 2060–2064 (1974). [Google Scholar]
- Probstein R. F. Physicochemical Hydrodynamics: An Introduction John Wiley & Sons (2005). [Google Scholar]
- Giddings J. C. A new separation concept based on a coupling of concentration and flow nonuniformities. Sep. Sci. 1, 123–125 (1966). [Google Scholar]
- Giddings J. C., Yang F. J. & Myers M. N. Criteria for concentration field-flow fractionation. Sep. Sci. 12, 381–393 (1977). [Google Scholar]
- Merkel T. C., Bondar V. I., Nagai K., Freeman B. D. & Pinnau I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 38, 415–434 (2000). [Google Scholar]
- de Jong J., Verheijden P. W., Lammertink R. G. H. & Wessling M. Generation of local concentration gradients by gas-liquid contacting. Anal. Chem. 80, 3190–3197 (2008). [DOI] [PubMed] [Google Scholar]
- Hai F. I., Riley T., Shawkat S., Magram S. F. & Yamamoto K. Removal of pathogens by membrane bioreactors: a review of the mechanisms, influencing factors and reduction in chemical disinfectant dosing. Water 6, 3603–3630 (2014). [Google Scholar]
- Cheryan M. Ultrafiltration and Microfiltration Handbook CRC press (1998). [Google Scholar]
- Au K.-K. Water Treatment and Pathogen Control: Process Efficiency in Achieving Safe Drinking-Water IWA Publishing (2004). [Google Scholar]
- Sattler K. D. Handbook of Nanophysics: Functional Nanomaterials CRC Press (2010). [Google Scholar]
- Wilson W. W., Wade M. M., Holman S. C. & Champlin F. R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43, 153–164 (2001). [DOI] [PubMed] [Google Scholar]
- Park J. I. et al. A microfluidic approach to chemically driven assembly of colloidal particles at gas-liquid interfaces. Angew. Chem. Int. Ed. 121, 5404–5408 (2009). [DOI] [PubMed] [Google Scholar]
- Dai Z., Fornasiero D. & Ralston J. Influence of dissolved gas on bubble-particle heterocoagulation. J. Chem. Soc. Faraday Trans. 94, 1983–1987 (1998). [Google Scholar]
- Bartolo D., Degré G., Nghe P. & Studer V. Microfluidic stickers. Lab. Chip. 8, 274–279 (2008). [DOI] [PubMed] [Google Scholar]
- Lee N. Y. & Chung B. H. Novel poly(dimethylsiloxane) bonding strategy via room temperature ‘chemical gluing'. Langmuir 25, 3861–3866 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables, Supplementary Discussions and Supplementary References
Suspension of negative particles exposed to carbon dioxide. Negatively charged polystyrene particles suspended in water migrate away from the gas-liquid interface when the suspension is exposed to carbon dioxide.
Suspension of negative particles exposed to nitrogen. When a suspension of negatively charged polystyrene particles in water is exposed to nitrogen, only Brownian motion of the particles is observed.
Suspension of positive particles exposed to carbon dioxide. Positively charged, aminefunctionalized, polystyrene particles suspended in water accumulate near the gas-liquid interface when the suspension is exposed to carbon dioxide.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.