Abstract
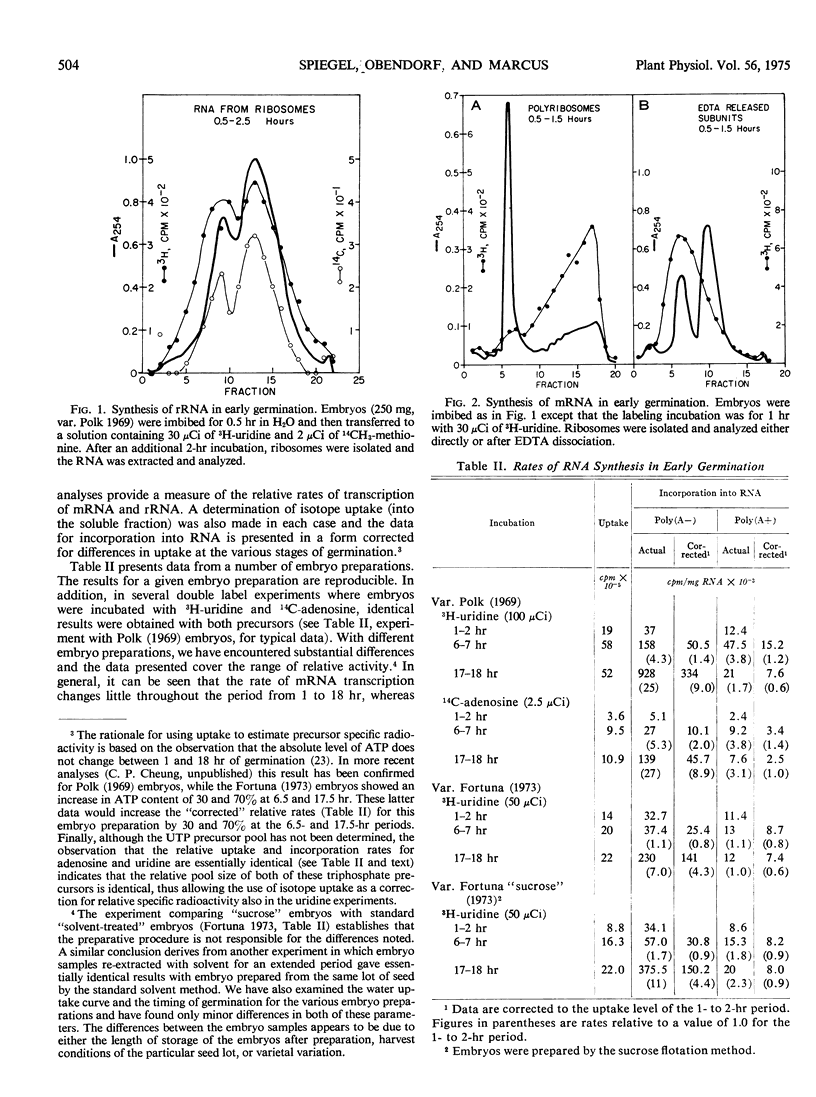
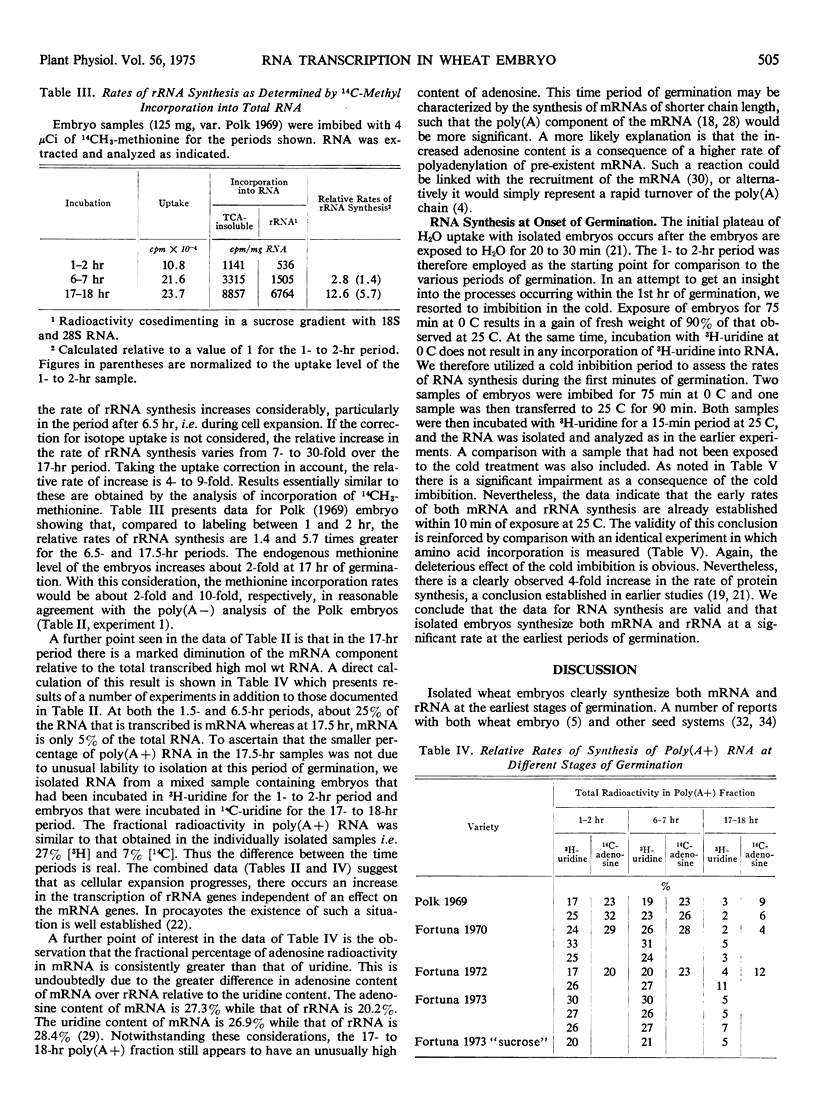
Germinating wheat embryos (Triticum aestivum L). synthesize both ribosomal and messenger RNA at the earliest times after the onset of germination. The rates of synthesis of these two RNAs are determined at various stages in germination by an analysis of newly synthesized radioactive RNA on oligo(dT)-cellulose. The rate of messenger RNA synthesis is essentially constant throughout 18 hours of germination, while that of ribosomal RNA synthesis increases steadily, particularly after the onset of cell expansion (6 hours), reaching at 16 to 18 hours, a rate of synthesis between 5- and 20-fold greater than that observed at the earliest stages. The net effect is a relative decrease in the fraction of transcribed high molecular weight RNA that is mRNA. Throughout the first 7 hours of germination, mRNA is 25 to 30% of the transcribed fraction, whereas by 16 to 18 hours it has declined to a level of 4 to 8%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Osborne D. J. Hormones in the translational control of early germination in wheat embryos. Nature. 1970 Jun 20;226(5251):1157–1160. doi: 10.1038/2261157a0. [DOI] [PubMed] [Google Scholar]
- Chen D., Schultz G., Katchalski E. Early ribosomal RNA transcription and appearance of cytoplasmic ribosomes during germination of the wheat embryo. Nat New Biol. 1971 May 19;231(20):69–72. doi: 10.1038/newbio231069a0. [DOI] [PubMed] [Google Scholar]
- Diez J., Brawerman G. Elongation of the polyadenylate segment of messenger RNA in the cytoplasm of mammalian cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4091–4095. doi: 10.1073/pnas.71.10.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanska M., Tomaszewski M., Grzelczak Z., Rejman E., Buchowicz J. Cascade activation of genome transcription in wheat. Nature. 1973 Aug 24;244(5417):507–509. doi: 10.1038/244507a0. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Holleman J. M., Key J. L. Inactive and protein precursor pools of amino acids in the soybean hypocotyl. Plant Physiol. 1967 Jan;42(1):29–36. doi: 10.1104/pp.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Jendrisak J., Becker W. M. Isolation, purification and characterization of RNA polymerases from wheat germ. Biochim Biophys Acta. 1973 Aug 10;319(1):48–54. doi: 10.1016/0005-2787(73)90039-7. [DOI] [PubMed] [Google Scholar]
- Johri M. M., Varner J. E. Characterization of rapidly labeled ribonucleic acid from dwarf peas. Plant Physiol. 1970 Mar;45(3):348–357. doi: 10.1104/pp.45.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan C. O., App A. A., Still C. C. The presence of polyadenylate sequences in the ribonucleic acid of a higher plant. Biochem Biophys Res Commun. 1973 Jul 17;53(2):588–595. doi: 10.1016/0006-291x(73)90702-x. [DOI] [PubMed] [Google Scholar]
- Marcus A., Efron D., Weeks D. P. The wheat embryo cell-free system. Methods Enzymol. 1974;30:749–754. doi: 10.1016/0076-6879(74)30073-0. [DOI] [PubMed] [Google Scholar]
- Marcus A., Feeley J., Volcani T. Protein Synthesis in Imbibed Seeds III. Kinetics of Amino Acid Incorporation Ribosome Activation, and Polysome Formation. Plant Physiol. 1966 Sep;41(7):1167–1172. doi: 10.1104/pp.41.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. Seed germination and the capacity for protein synthesis. Symp Soc Exp Biol. 1969;23:143–160. [PubMed] [Google Scholar]
- Obendorf R. L., Marcus A. Rapid Increase in Adenosine 5'-Triphosphate during Early Wheat Embryo Germination. Plant Physiol. 1974 May;53(5):779–781. doi: 10.1104/pp.53.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. I. Kinetics of incorporation of nucleosides into nucleotide pools and pool sizes during growth cycle. J Cell Physiol. 1971 Apr;77(2):213–240. doi: 10.1002/jcp.1040770212. [DOI] [PubMed] [Google Scholar]
- Sen S., Payne P. I., Osborne D. J. Early ribonucleic acid synthesis during the germination of rye (Secale cereale) embryos and the relationship to early protein synthesis. Biochem J. 1975 Jun;148(3):381–387. doi: 10.1042/bj1480381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. A comparison between heterogeneous nuclear RNA and polysomal messenger RNA in HeLa cells by RNA-DNA hybridization. J Cell Biol. 1970 Mar;44(3):467–475. doi: 10.1083/jcb.44.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Walle C. Polyadenylic sequences in plant RNA. FEBS Lett. 1973 Aug 1;34(1):31–34. doi: 10.1016/0014-5793(73)80696-9. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Regulation of RNA synthesis in plant cell culture: delayed synthesis of ribosomal RNA during transition from the stationary phase to active growth. Dev Biol. 1973 Jan;30(1):104–114. doi: 10.1016/0012-1606(73)90050-x. [DOI] [PubMed] [Google Scholar]
- Walbot V., Capdevila A., Dure L. S., 3rd Action of 3'd adenosine (cordycepin) and 3'd cytidine on the translation of the stored mRNA of cotton cotyledons. Biochem Biophys Res Commun. 1974 Sep 9;60(1):103–110. doi: 10.1016/0006-291x(74)90178-8. [DOI] [PubMed] [Google Scholar]
- Walton D. C. Germination of phaseolus vulgaris I. Resumption of axis growth. Plant Physiol. 1966 Feb;41(2):298–302. doi: 10.1104/pp.41.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. F., Holler B. W. Methylation of 45 s ribosomal RNA precursor in HeLa cells. J Mol Biol. 1967 Jan 28;23(2):149–161. doi: 10.1016/s0022-2836(67)80023-8. [DOI] [PubMed] [Google Scholar]


