Abstract
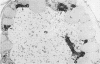
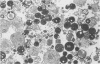
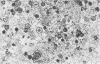
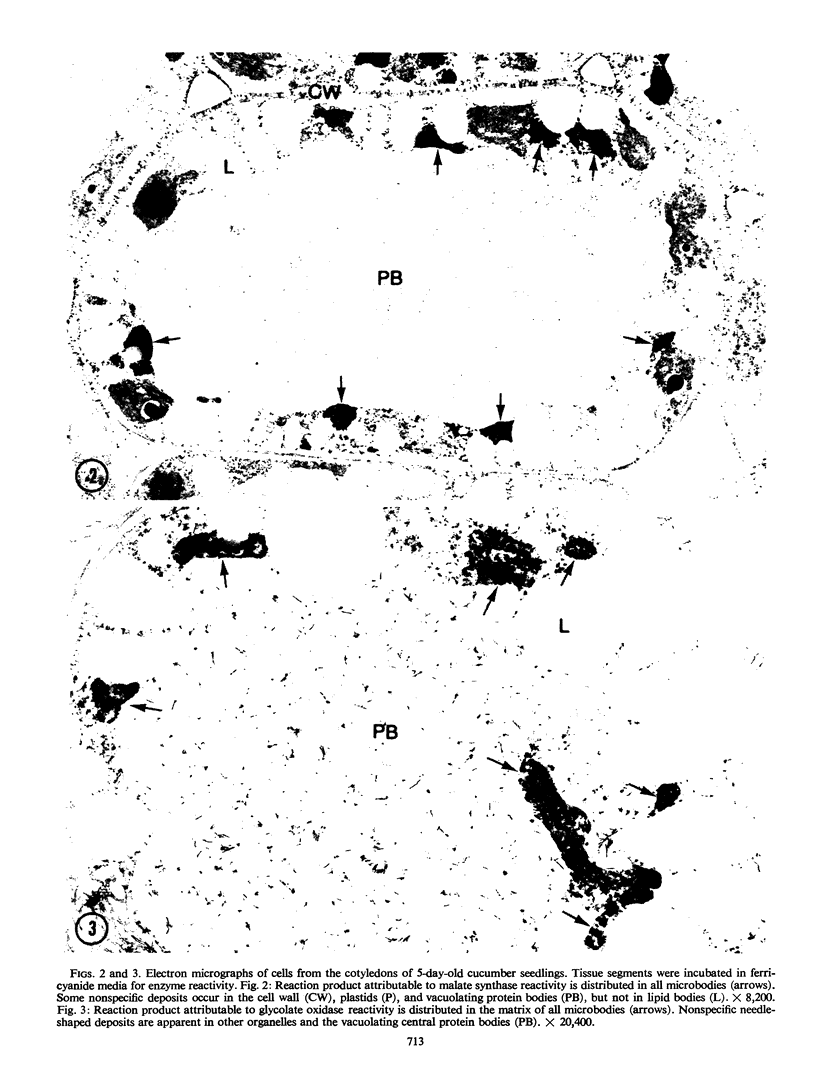
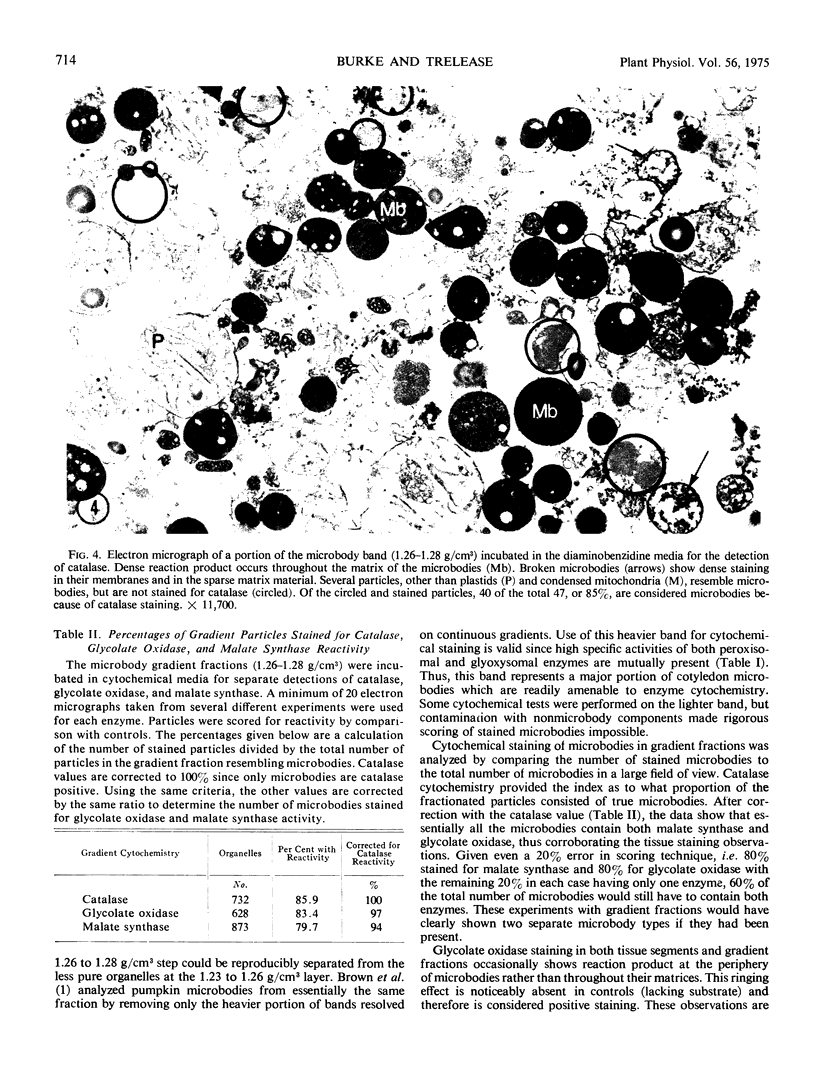
The cytochemical localizations of malate synthase (glyoxysomal marker) and glycolate oxidase (peroxisomal marker) have been examined in cotyledon segments and sucrose-gradient fractions from germinated cucumber (Cucumis sativus L.) seedlings. The seedlings were grown in the dark for 4 days, transferred to 4 hours of continuous light, then returned to the dark for 24 hours. Under these conditions, high specific activities for both glyoxysomal and peroxisomal enzymes are maintained in cotyledon homogenates and microbody-enriched fractions. Electron cytochemistry of the marker enzymes reveals that all or virtually all the microbodies observed in cotyledonary cells and sucrose-gradient fractions contain both enzymes. The staining in gradient fractions was determined from scoring a minimum of 600 photographed microbodies for each enzyme. After correcting for the number of particles stained for catalase reactivity (representing true microbodies), 94 and 97% of the microbodies were found stained for malate synthase and glycolate oxidase activity, respectively.
The results from these studies provide pertinent information toward understanding the succession from glyoxysomal to peroxisomal metabolism in cotyledons of fatty seedlings. The coexistence of two separate microbody types functioning at different stages of development apparently is not the case. The localizations of both marker enzymes within one microbody type strongly suggest that the metabolic transition involves a change in enzyme complement within an ongoing population of microbodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Lord J. M., Merrett M. J. Fractionation of the proteins of plant microbodies. Biochem J. 1974 Dec;144(3):559–566. doi: 10.1042/bj1440559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand A. R. Ultrastructural localization of L-alpha-hydroxy acid oxidase in rat liver perioxisomes. Histochemistry. 1975;41(3):195–206. doi: 10.1007/BF00497683. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Rechcigl M., Jr Microbodies and related particles. Morphology, biochemistry, and physiology. Int Rev Cytol. 1969;(Suppl):1–296. [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitka T. K., Talibi G. G. Cytochemical localization by ferricyanide reduction of -hydroxy acid oxidase activity in peroxisomes of rat kidney. Histochemie. 1971;27(2):137–158. doi: 10.1007/BF00284956. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Burke J. J. Cytochemical localization of malate synthase in glyoxysomes. J Cell Biol. 1974 Feb;60(2):483–495. doi: 10.1083/jcb.60.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Plant microbodies. J Histochem Cytochem. 1973 Nov;21(11):958–962. doi: 10.1177/21.11.958. [DOI] [PubMed] [Google Scholar]