Abstract
Lifestyle diseases, which are associated with nutrition, account for 30% of elderly requiring long-term care. To increase health expectancy among Japan’s rapidly aging population, we investigated the nutritional status and body composition of elderly adults living in a region subject to heavy snowfall, to identify pertinent health indicators. The dietary habits of 288 local residents aged ≥50 years were analyzed using body composition and a brief-type self-administered diet history questionnaire. Body mass index of all residents was normal. Basal metabolic rate (BMR) and muscle mass were reduced in the older group. Dietary habits did not differ with age among men, but older women had significantly higher dietary intake. BMR and muscle mass declined with age, even when dietary intake was sustained. Despite sufficient dietary intake, independently living older adults demonstrate less efficient use of food with age. Interventions to reduce excessive sodium and protein intake are required.
Keywords: body composition, health expectancy, independently living older adults, nutritional status
Introduction
Japan’s population is rapidly aging. The 2015 national census revealed that 26% of the population (ca. 33.8 million people) is 65 years or older and 12.5% (ca. 15.7 million people) is 75 years or older (Ministry of Internal Affairs and Communications, 2015). The proportion of the population who are 65 years or older is higher in Japan than in other major industrialized nations, such as Italy (22.4%) and Germany (21.2%). Japan currently has the world’s highest life expectancy (United Nations, 2015). The demographic shift has resulted from a concurrent decrease in birth and death rates, leading to an increased average life expectancy. The life expectancy averages in Japan are 80.5 and 86.8 years for men and women, respectively (Ministry of Health, Labour, and Welfare, 2015). Health expectancy ages are 71.2 years for men and 74.2 years for women. Since the World Health Organization began advocating increasing health expectancy in 2000, interest in how to increase the length of time an individual can live in good health, rather than simply increasing the length of life, has increased. Because health expectancy is defined as the length of time a person can live without being constrained by health problems, the difference between average life expectancy and health expectancy represents the “unhealthy period” during which there are constraints to daily living. In 2013, in Japan, that difference was approximately 10 years. To increase health expectancy, it is necessary to understand the health status and living habits of healthy residents in local communities.
In 2015, the principal causes of death in Japan were malignancy, cardiovascular disease, pneumonia, cerebrovascular disease, and accidents for men, and malignancy, cardiovascular disease, senility, cerebrovascular disease, and pneumonia in women (Ministry of Health, Labour, and Welfare, 2015). Prevention of lifestyle diseases, which underlie some of the principal causes of death, is important. In addition, asthenia, dementia, and falls are often the reasons for the loss of ability of later-stage elderly to live independently, and become certified as “yō-kaigo” (in need of long-term care). According to a health promotion program of the Ministry of Health, Labour, and Welfare for the 21st century, 30% of the need for long-term care in Japan arises from diseases of lifestyle, including cerebrovascular disease (Ministry of Health, Labour, and Welfare, 2010). However, 50% of this need is due to dementia, age-related asthenia, arthropathy, fractures, and falls (Ministry of Health, Labour, and Welfare, 2010). Nutrition is associated with lifestyle diseases, fractures, and falls (Curtis & Safford, 2012). Therefore, to increase health expectancy, understanding the nutritional status of individuals after middle age, in which lifestyle-related diseases develop, is important.
Typical factors causing poor nutrition among older adults in Japan are social factors, psychological factors, aging, and illness (Kuzuya, 2012). In addition, overnutrition is directly related to living habits and leads to lifestyle diseases, such as obesity, diabetes mellitus, dyslipidemia, and hypertension (Hozawa et al., 2008). However, there is debate about whether nutrition has the same significant impact on mortality among older adults, especially the later-stage elderly, as it does among younger adults. Older adults tend to accumulate visceral fat and have a higher prevalence of metabolic syndrome. However, it has been reported that, for older adults, the effect of metabolic syndrome on cardiovascular disease–related mortality is insignificant (Hildrum, Mykletun, Dahl, & Midthjell, 2009). Furthermore, the effect of blood cholesterol levels and obesity on mortality diminishes with age (Prospective Studies Collaboration et al., 2007; Tamakoshi et al., 2010). A study conducted in the West reported that overnutrition, particularly obesity (body mass index [BMI] ≥30 kg/m2), was associated with frailty (Blaum, Xue, Michelon, Semba, & Fried, 2005). This study showed that, of the five criteria used to diagnose frailty, a small percentage of the obese group met the criterion for weight loss; however, a high percentage met the criteria for slowness (walking speed), low physical activity, and weakness (grip strength). Whether these data apply to the Japanese population requires further empirical testing, because the number of Japanese older adults who qualify as obese is extremely small.
In Japan, in 2010, there were 2.15 doctors for every 1,000 people, which is significantly fewer than the average of 3.00 for the member countries of the Organisation for Economic Cooperation and Development. Furthermore, the distribution of doctors among communities is uneven, with few doctors in mountainous areas and remote regions (Annual Health, Labour and Welfare Report, 2012-2013). In Hokkaido, a large island in northern Japan, the life expectancy averages are 78.3 and 85.8 years and health expectancy averages are 70.0 and 73.2 years for men and women, respectively. These differences are similar to the national averages. There are more medically underserved areas in Hokkaido than anywhere else in Japan; however, this is improving. Moreover, access to medical facilities in this region can be constrained during the winter, due to snowfall.
Given the importance of exploring possible measures to maintain health in such areas, a telehealth consultation system was designed and empirically tested for the promotion and maintenance of health (Shimoda et al., 2015). It was demonstrated that health consultations can be successfully conducted using information and communication technology when patients are far from medical facilities. Moreover, the content of those consultations was shown to be diverse. However, the lives and nutritional status of people living in these kinds of regions with heavy snowfall are yet to be studied. The weather may be a barrier to physical activity, but this hypothesis has not yet been assessed objectively. The estimated magnitudes for the various effects were modest, ranging from ca. 1% to ca. 20%. Thus, for an average individual taking ca. 10,000 steps/day, weather-dependent changes in physical activity could involve 2,000 steps/day (Chan, Ryan, & Tudor-Locke, 2006). Therefore, we studied a sample of healthy older adults living in a region prone to heavy snowfall to assess their nutritional status and body composition to promote the health of local residents and to obtain pertinent indicators.
Method
Participants
The participants consisted of 409 community residents who attended health examinations at a drugstore between January and March 2016. Data from 288 residents aged ≥50 years were used for this analysis.
Measurements and Survey Items
Medical history and living habits
Participants completed a self-administered questionnaire that asked about their medical history (previous illnesses and treatment), exercise habits (frequency per week engaging in moderate exercise for ≥10 min), and bowel habits (number of stools passed per week); they were also asked to provide a self-rated health assessment (“healthy,” “more or less healthy,” “not very healthy,” and “not healthy”). For the analysis, these responses were grouped into two categories: “healthy” and “not healthy.”
Height and body composition
Height was measured using a digital height gauge (AD-6400; A&D Medical, Tokyo, Japan). Body composition was measured using a body composition scale (Well Scan900; Canon, Osaka, Japan).
Blood pressure and grip strength
Blood pressure was measured using an automatic blood pressure monitor (HBP-T1055-N; Omron, Kyoto, Japan). Grip strength was measured twice on the right and left using the Jamar Hydraulic Crush Strength Meter (Fabrication Enterprises, Inc., White Plains, NY, USA).
Self-drawing of blood samples
Under the supervision of a pharmacist, the participants used a simple blood testing kit (Eiken Chemical Co., Tokyo, Japan) to draw a sample of their own blood. Each blood sample was collected into a specimen tube that came with the kit and was analyzed for total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, creatinine, urea nitrogen, uric acid, and hemoglobin A1c.
Nutritional status
Participants completed the brief-type self-administered diet history questionnaire (BDHQ). This is a brief version of the diet history questionnaire that retains the features of the original questionnaire, but simplifies its structure and makes the processing of the answers and data more convenient. The questionnaire can be printed on both sides of a single A3-size page of paper and takes an average of 15 min to complete. The BDHQ was developed for use in large-scale nutritional epidemiology studies and in studies in which nutrition is of secondary interest. Using a nutritional value calculation program, the dietary intake of approximately 30 types of nutrients and 50 types of foods can be calculated. The questionnaire’s validity has been demonstrated (Kobayashi et al., 2012).
Statistical Analysis
The data obtained from the questionnaires were stratified into three groups according to age: 50 to 64 years (Group X), 65 to 74 years (Group Y), and 75 years or older (Group Z). Significance was tested using the chi-square test and ANOVA. Results are shown as mean ± standard deviation. JMP 12.2.0 software was used to perform statistical analyses. The level of statistical significance was 5%.
Results
Responses were obtained from 71 men and 217 women who were 50 to 89 years of age. As shown in Table 1, in the self-reported health assessment, 68.8%, 70.6%, and 52.4% of men in Groups X, Y, and Z, respectively, considered themselves healthy, as did 67.6%, 74.1%, and 44.0%, respectively, of women. There were no significant differences between the three groups in terms of self-rated health assessment. The majority (≥70%) of both men and women habitually exercised 1 to 2 days per week. There were no significant group differences in terms of bowel habits. Table 2 shows the data from the blood tests. There were no significant differences for the groups among men. However, among women, there were significant group differences in levels of creatinine, urea nitrogen, and uric acid.
Table 1.
Characteristics of Participants Stratified by Age and Sex.
| Men |
Women |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group |
X |
Y |
Z |
p | X |
Y |
Z |
p | ||||||
| Age (years) | 50-64 |
65-74 |
75+ |
50-64 |
65-74 |
75+ |
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| n | 16 | 22.5 | 34 | 47.9 | 21 | 29.6 | 111 | 51.2 | 81 | 37.3 | 25 | 11.5 | ||
| Health assessment | ||||||||||||||
| Healthy | 11 | 68.8 | 24 | 70.6 | 11 | 52.4 | ns | 75 | 67.6 | 60 | 74.1 | 11 | 44.0 | ns |
| Not healthy | 1 | 6.3 | 7 | 20.6 | 5 | 23.8 | 17 | 15.3 | 7 | 8.6 | 4 | 16.0 | ||
| No response | 4 | 25.0 | 3 | 8.8 | 5 | 23.8 | 19 | 17.1 | 14 | 17.3 | 10 | 40.0 | ||
| Exercise habits | ||||||||||||||
| 0 days | 2 | 12.5 | 0 | 0.0 | 0 | 0.0 | ns | 4 | 3.6 | 2 | 2.5 | 1 | 4.0 | ns |
| 1-2 days | 12 | 75.0 | 31 | 91.2 | 17 | 81.0 | 93 | 83.8 | 72 | 88.9 | 19 | 76.0 | ||
| 3+ days | 0 | 0.0 | 1 | 2.9 | 1 | 4.8 | 0 | 0.0 | 1 | 1.2 | 0 | 0.0 | ||
| No response | 2 | 12.5 | 2 | 5.9 | 3 | 14.3 | 14 | 12.6 | 6 | 7.4 | 5 | 20.0 | ||
| Bowel movements | ||||||||||||||
| Less than 4/week | 0 | 0.0 | 4 | 11.8 | 4 | 19.0 | ns | 13 | 11.7 | 9 | 11.1 | 5 | 20.0 | ns |
| 4+/week | 13 | 81.3 | 29 | 85.3 | 15 | 71.4 | 89 | 80.2 | 66 | 81.5 | 17 | 68.0 | ||
| No response | 3 | 18.8 | 1 | 2.9 | 2 | 9.5 | 9 | 8.1 | 6 | 7.4 | 3 | 12.0 | ||
| Medical history | ||||||||||||||
| Malignant tumors | 0 | 0.0 | 2 | 2.8 | 2 | 2.8 | — | 2 | 0.9 | 3 | 1.4 | 1 | 0.5 | — |
| Stroke | 0 | 0.0 | 4 | 5.6 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | ||
| Heart attack | 0 | 0.0 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Angina | 0 | 0.0 | 0 | 0.0 | 4 | 5.6 | 2 | 0.9 | 1 | 0.5 | 0 | 0.0 | ||
| Arrhythmia | 0 | 0.0 | 2 | 2.8 | 1 | 1.4 | 2 | 0.9 | 3 | 1.4 | 2 | 0.9 | ||
| High blood pressure | 4 | 5.6 | 12 | 16.9 | 5 | 7.0 | 17 | 7.8 | 19 | 8.8 | 8 | 3.7 | ||
| Dyslipidemia | 1 | 1.4 | 5 | 7.0 | 6 | 8.5 | 20 | 9.2 | 16 | 7.4 | 8 | 3.7 | ||
| Diabetes | 0 | 0.0 | 3 | 4.2 | 3 | 4.2 | 4 | 1.8 | 3 | 1.4 | 1 | 0.5 | ||
| COPD | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Tuberculosis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Kidney disease | 0 | 0.0 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Bone fractures | 3 | 4.2 | 4 | 5.6 | 1 | 1.4 | 9 | 4.1 | 7 | 3.2 | 6 | 2.8 | ||
| Dementia | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
Note. COPD = chronic obstructive pulmonary disease. p values were derived using McNemar’s test.
Table 2.
Blood Test Results of Participants Stratified by Age and Sex.
| Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
X |
Y |
Z |
X |
Y |
Z |
|||
| Age (years) | 50-64 | 65-74 | 75+ | p | 50-64 | 65-74 | 75+ | p | |
| Total cholesterol (mg/dl) | M | 196.67 | 187.33 | 178.11 | .2849 | 217.58 | 225.32 | 212.60 | .2034 |
| SD | 34.71 | 36.46 | 26.48 | 31.95 | 29.59 | 38.90 | |||
| LDL cholesterol (mg/dl) | M | 112.13 | 101.71 | 95.56 | .2930 | 121.26 | 127.17 | 115.85 | .2112 |
| SD | 27.44 | 36.41 | 21.52 | 27.47 | 22.89 | 36.04 | |||
| HDL cholesterol (mg/dl) | M | 58.33 | 51.79 | 56.06 | .4481 | 71.41 | 67.80 | 66.15 | .2646 |
| SD | 13.01 | 18.43 | 15.63 | 16.36 | 17.74 | 14.31 | |||
| Triglycerides (mg/dl) | M | 145.60 | 166.96 | 126.72 | .4104 | 119.04 | 140.47 | 112.95 | .1889 |
| SD | 80.18 | 116.52 | 76.65 | 69.05 | 92.91 | 66.66 | |||
| Creatinine (mg/dl) | M | 0.85 | 0.94 | 0.98 | .1481 | 0.62 | 0.68 | 0.69 | .0011 |
| SD | 0.12 | 0.19 | 0.24 | 0.09 | 0.11 | 0.12 | |||
| Urea nitrogen (mg/dl) | M | 19.47 | 20.00 | 20.78 | .7530 | 17.25 | 19.58 | 19.95 | .0007 |
| SD | 4.64 | 4.22 | 6.25 | 4.10 | 3.75 | 4.84 | |||
| Uric acid (mg/dl) | M | 6.19 | 5.85 | 6.11 | .5818 | 4.29 | 4.89 | 4.64 | .0038 |
| SD | 0.94 | 1.20 | 0.99 | 1.10 | 1.01 | 1.21 | |||
| HbA1c (%) | M | 5.66 | 5.90 | 5.98 | .2333 | 5.61 | 5.77 | 5.74 | .0652 |
| SD | 0.37 | 0.67 | 0.50 | 0.40 | 0.50 | 0.42 | |||
Note. HDL = high-density lipoprotein; LDL = low-density lipoprotein; HbA1c = glycated hemoglobin. p values were derived using ANOVA.
The average BMI was within the normal range for men and women, although it was higher for women in Group Z (Table 3). The percentage of body fat was higher and basal metabolic rate (BMR) and muscle mass were lower for women in Group Z than for women in the other groups. There were no significant group differences in the percentage of body fat among the groups of men; however, compared with the other groups, BMR and muscle mass were significantly lower in Group Z.
Table 3.
Body Composition of Participants Stratified by Age and Sex.
| Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
X |
Y |
Z |
X |
Y |
Z |
|||
| Age (years) | 50-64 | 65-74 | 75+ | p | 50-64 | 65-74 | 75+ | p | |
| Height (cm) | M | 166.87 | 165.26 | 159.82 | .0003 | 154.84 | 151.57 | 149.57 | <.0001 |
| SD | 6.05 | 5.86 | 5.81 | 5.56 | 4.86 | 4.72 | |||
| Weight (kg) | M | 67.19 | 65.22 | 61.35 | .0913 | 54.07 | 51.87 | 54.64 | .1379 |
| SD | 9.00 | 10.24 | 6.64 | 8.94 | 7.63 | 9.45 | |||
| Body fat (%) | M | 21.96 | 21.88 | 22.11 | .9896 | 27.47 | 27.98 | 30.99 | .0471 |
| SD | 6.70 | 5.99 | 6.06 | 6.41 | 6.63 | 8.35 | |||
| Body water (kg) | M | 37.67 | 36.53 | 34.43 | .0261 | 27.99 | 26.71 | 27.00 | .0073 |
| SD | 4.35 | 4.29 | 3.10 | 2.87 | 2.46 | 4.22 | |||
| Muscle mass (kg) | M | 48.46 | 46.96 | 44.23 | .0238 | 35.81 | 34.18 | 34.46 | .0059 |
| SD | 5.64 | 5.46 | 4.08 | 3.58 | 3.10 | 5.51 | |||
| BMI (m/kg2) | M | 24.09 | 23.84 | 24.12 | .9315 | 22.57 | 22.62 | 24.46 | .0399 |
| SD | 2.80 | 3.19 | 3.17 | 3.67 | 3.34 | 4.34 | |||
| BMR (kcal) | M | 1,276.94 | 1,189.83 | 1,072.92 | .0009 | 1,061.34 | 996.21 | 975.96 | <.0001 |
| SD | 155.17 | 179.38 | 171.85 | 83.49 | 80.80 | 104.58 | |||
Note. BMI = body mass index; BMR = basal metabolic rate. p values were derived using ANOVA.
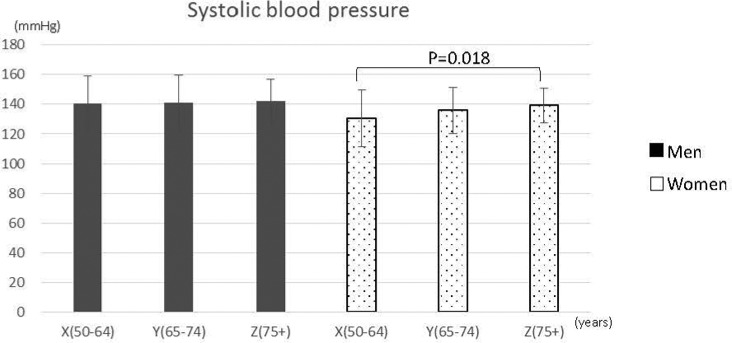
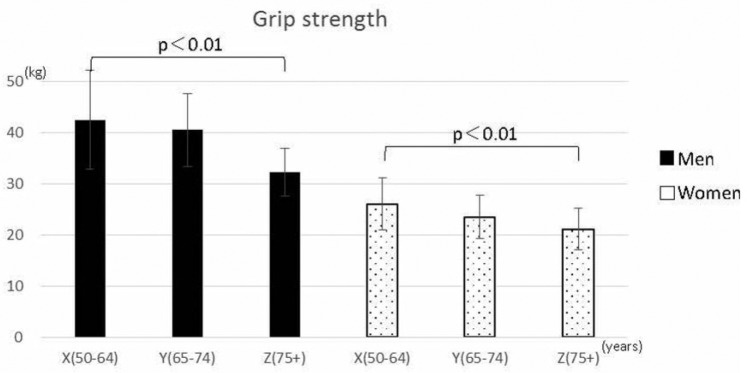
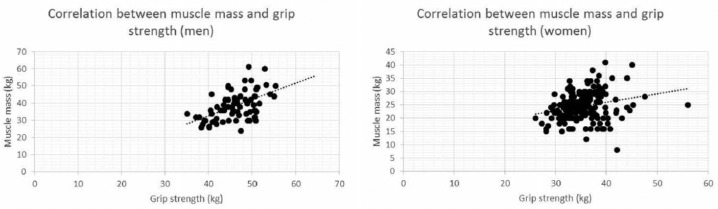
There were no significant differences in mean blood pressure among the three groups of men. However, there was a significant difference among the groups of women (Figure 1). In addition, grip strength was significantly lower (p < 0.01) in Group Z for both men and women (Figure 2). We found a positive correlation between grip strength and muscle mass for men (r = .55, p < .001) and a weak positive correlation for women (r = .26, p = .001) (Figure 3).
Figure 1.
Mean systolic blood pressure of participants stratified by age and sex (men, n = 79; women, n = 217).
Figure 2.
Mean grip strength of participants stratified by age and sex (men, n = 79; women, n = 217).
Figure 3.
Correlations between grip strength and muscle mass for men (top chart) and women (bottom chart).
In terms of dietary intake, there were no significant differences in any dietary intake category among the groups of men (Table 4). Among women, there were significant differences in all categories except carbohydrates and alcohol, with the dietary intake being highest in Group Z. No correlation was demonstrated between protein intake and muscle mass for men or women (men: r = .076, p = .25; women: r = .013, p = .20).
Table 4.
Dietary intake of participants derived from the BDHQ.
| Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
X |
Y |
Z |
X |
Y |
Z |
|||
| Age (years) | 50-64 | 65-74 | 75+ | p | 50-64 | 65-74 | 75+ | p | |
| Energy (kcal) | M | 1,835.35 | 1,935.84 | 1,910.59 | .0845 | 1,579.20 | 1,645.70 | 2,041.72 | .0004 |
| SD | 624.57 | 520.27 | 541.31 | 543.10 | 532.71 | 551.52 | |||
| Water (g) | M | 2,150.75 | 1,934.77 | 2,030.32 | .3690 | 1,647.32 | 1,736.17 | 2,080.96 | .0014 |
| SD | 631.11 | 461.96 | 523.31 | 572.25 | 514.32 | 581.29 | |||
| Protein (g) | M | 67.76 | 78.68 | 77.23 | .3779 | 65.64 | 72.67 | 101.57 | <.0001 |
| SD | 23.11 | 28.69 | 27.14 | 24.13 | 28.62 | 31.13 | |||
| Fat (g) | M | 50.31 | 51.33 | 48.15 | .7518 | 47.67 | 49.26 | 66.50 | <.0001 |
| SD | 15.50 | 16.05 | 16.27 | 18.51 | 20.36 | 23.15 | |||
| Carbohydrates (g) | M | 236.51 | 261.20 | 256.74 | .5875 | 212.46 | 219.11 | 250.42 | .0785 |
| SD | 82.19 | 83.12 | 80.75 | 81.25 | 73.92 | 80.38 | |||
| Saturated fatty acids (g) | M | 12.44 | 13.43 | 12.52 | .6624 | 13.21 | 13.30 | 16.71 | .0159 |
| SD | 4.59 | 4.52 | 4.57 | 5.84 | 5.69 | 6.08 | |||
| Cholesterol (g) | M | 389.70 | 381.37 | 417.60 | .7652 | 331.82 | 389.82 | 550.77 | <.0001 |
| SD | 212.12 | 166.76 | 206.43 | 150.25 | 207.22 | 211.66 | |||
| Soluble dietary fiber (g) | M | 3.15 | 3.54 | 3.89 | .2239 | 3.18 | 3.46 | 4.42 | .0003 |
| SD | 1.08 | 1.41 | 1.42 | 1.44 | 1.47 | 1.24 | |||
| Insoluble dietary fiber (g) | M | 8.30 | 9.77 | 10.76 | .0562 | 8.66 | 9.60 | 12.01 | <.0001 |
| SD | 2.82 | 3.09 | 3.49 | 3.67 | 3.64 | 3.27 | |||
| Total dietary fiber (g) | M | 11.90 | 13.81 | 15.24 | .0515 | 12.30 | 13.48 | 17.34 | <.0001 |
| SD | 3.82 | 4.65 | 5.09 | 5.30 | 5.26 | 4.71 | |||
| Salt equivalent (g) | M | 10.37 | 12.05 | 12.57 | .1773 | 9.78 | 10.66 | 14.46 | <.0001 |
| SD | 2.73 | 4.10 | 3.95 | 3.19 | 3.56 | 3.54 | |||
| Alcohol (g) | M | 20.35 | 12.91 | 17.88 | .5917 | 3.42 | 3.11 | 2.61 | .9052 |
| SD | 22.77 | 20.45 | 35.77 | 8.30 | 9.58 | 8.50 | |||
| Omega-3 fatty acids (mg) | M | 2.56 | 3.02 | 2.78 | .4622 | 2.43 | 2.76 | 4.37 | <.0001 |
| SD | 0.92 | 1.46 | 1.22 | 1.09 | 1.40 | 1.79 | |||
| Omega-6 fatty acids (mg) | M | 10.41 | 10.10 | 9.49 | .6228 | 8.93 | 9.13 | 12.28 | <.0001 |
| SD | 3.03 | 3.18 | 3.25 | 3.31 | 3.54 | 3.77 | |||
Note. BDHQ = Brief-Type Self-Administered Diet History Questionnaire. p values were derived using ANOVA.
Discussion
Other studies (Jingu, Egami, Kinukawa, Sano, & Takei, 2003; Nakamura et al., 2002) have reported that in Japan, 20% to 40% of self-rated health assessments tend toward the negative, that is, “not very healthy,” and the results of this study were similar. There is no reason to think that the participants in this study, as a group, had a bias that would affect their health assessments. Although this study’s results included participants with medical histories, all participants were living independently. All laboratory blood test results were within the Japanese reference ranges, with the exception of the triglyceride levels, which were high for women in Group Y.
Regarding bowel movements, it has been reported that older adults, especially those 80 years or older, have slower colonic transit (Madsen & Graff, 2004). The risk of constipation may increase with age, given that this study’s results were similar for women in Group Z. Despite copious intake of water and consumption of insoluble dietary fiber (Table 4), Group Z had a larger percentage of individuals with a low rate of bowel movements.
It has been reported that loss of muscle mass is associated with aging (Keys, Taylor, & Grande, 1973); our findings supported this (Table 1). The older adults living in a region prone to heavy snowfall studied here lost muscle mass with age even though they were healthy and exercised habitually. BMR also decreases with age. Longitudinal studies have reported that BMR decreases by approximately 1% to 3% over the course of 10 years and that the rate of decrease is particularly rapid for men (Henry, 2000; Pannemans & Westerterp, 1995). It is assumed that this phenomenon is a result of the decline in lean mass that accompanies aging (Poehlman et al., 1993). However, the decrease in BMR associated with aging is not necessarily a linear change. Some studies have shown that there is a significant decline in BMR in men in their 40s and in BMR for women in their 50s (Poehlman, 1992; Volpi, Mittendorfer, Rasmussen, & Wolfe, 2000); however, the causes of these decreases have not been fully explained. In this study, the decrease in BMR stratified by age was significant for both men and women. Therefore, this study provides data obtained from healthy elderly people whose activity was restricted by snow. Activity during the winter can be a criterion for health management indices for those who cannot leave their living space because of weather conditions.
In terms of grip strength and muscle mass, grip strength for both men and women was significantly lower in Group Z (p < 0.01). Many studies have reported that the measurement of grip strength in older adults correlates with factors such as muscle strength and walking ability (Houston et al., 2008); the results of the present study may support those findings. Measuring grip strength is a convenient and useful way to assess the overall physical strength of healthy individuals aged above 75 years and who are living independently.
The results of body composition measurements showed a decline in muscle mass. It has been reported that in older adults, there is a decline in the responsiveness of skeletal muscles to synthesize protein when stimulated by the intake of dietary protein (Volpi et al., 2000). In a 3-year observational study of older community-dwelling Americans in their 70s, the 3-year decline in lean mass was 40% less for the group with the highest baseline dietary protein intake per total energy intake (average 91.0 g/day, 1.2 g/kg weight/day) than for the group with the lowest dietary protein intake per total energy intake (average 56.0 g/day, 0.8 g/kg weight/day), even after adjusting for confounding factors (Houston et al., 2008). Low dietary intake of protein has also been related to a 3-year decline in muscle strength (Bartali et al., 2012). Furthermore, for older women, it has been confirmed that a low intake of dietary protein increases the risk of incident frailty after 3 years (Beasley et al., 2010). In addition, cross-sectional research of elderly Japanese women has shown that there is a relationship between the incidence of frailty and dietary protein intake (Kobayashi, Asakura, Suga, Sasaki, & Three-Generation Study of Women on Diets Health Study Group, 2013). In this study, both men and women had a dietary protein intake of ≥60 g/day. Even though intake was adequate, both men and women in Group Z lost muscle mass (Table 3); however, intake was not correlated with the loss of muscle mass. This suggests that older adults may develop anabolic resistance; therefore, even when amino acids are supplied to muscles, protein anabolism is weaker in older individuals than it is in younger adults (Paddon-Jones & Rasmussen, 2009). In general, protein anabolism in the muscle cells of older adults can be adequately activated by increasing the supply of amino acids. Therefore, to stimulate protein synthesis in the skeletal muscles, the concentration of amino acids in the blood needs to be higher for older adults than for younger adults, which requires sufficient intake of protein or administration of amino acids (Drummond et al., 2008; Pennings et al., 2011; Symons et al., 2007). The results of the present study demonstrated age-related changes in the sample, thereby supporting the finding that, unless the intake of high-quality protein at every meal is at least approximately 25 to 30 g, effective protein synthesis in skeletal muscles may not be maintained throughout the day (Pennings et al., 2011).
Although protein intake is important, some studies have reported misgivings about the safety of high-protein diets for older adults with renal insufficiency (Knight, Stampfer, Hankinson, Spiegelman, & Curhan, 2003; Walrand et al., 2008). For example, an increased risk of renal insufficiency in healthy older adults has been reported with high protein intake (2.0 g/kg weight/day). An 11-year observational study reported that, for older women with mild renal insufficiency (estimated glomerular filtration rate: 55-88 ml/min/1.73 m2), high protein intake (>1.3 g/kg weight/day) during the 11-year observation period caused a decline in renal function (Knight et al., 2003). In our study, although the results of renal function tests for women were in the normal range, we observed a decline. Further study is needed to verify the nature of the relationship between renal function and diet.
According to many epidemiological studies, if a person’s salt intake can be reduced by 1 g per day, a reduction in systolic blood pressure of 1 to 2 mmHg can be expected. According to the 2013 National Health and Nutrition Examination Survey, the average salt intake for men is 11.1 g and that for women is 9.4 g. Our study verified that there was a significant age group difference in salt intake for women; this difference was highest in the oldest group. However, we were not able to confirm any relationship between salt intake and blood pressure. In Japan, although salt intake is one of the causes of hypertension, understanding that relationship in independently living older adults would require a multifaceted study, including not only salt intake but also factors such as smoking and psychological stress, which will require further investigation in future.
Conclusion
In this study, we were able to assess body composition and living habits in a sample of independently living older adults in a region of Japan subject to heavy snowfall. We found that the BMI values of these older adults, who had good health expectancy, were within the normal range, but BMR and muscle mass declined with age. In addition, although these older adults had sufficient caloric intake and ate a variety of foods, changes in body composition across age groups suggested that, with advancing physical age, food is used less effectively.
Acknowledgments
The authors sincerely thank H. Moriyama of Hamanasu Information Company Limited who has supported for this research project. They also thank all the participants who participated in this study.
Footnotes
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Ethics Committee for the Graduate School of Health Sciences at Hokkaido University (15-96) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Statement of Informed Consent: The authors explained that anonymity of data would be maintained strictly, and obtained written consent regarding study participation.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by COI (Center of Innovation) under the “Radical Innovation and Entrepreneurship Program” (COI STREAM) by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan and by Japan Science and Technology Agency (JST).
References
- Annual Health, Labour and Welfare Report. (2012-2013). Retrieved from http://www.mhlw.go.jp/wp/hakusyo/kousei/13-2/dl/02.pdf
- Bartali B., Frongillo E. A., Stipanuk M. H., Bandinelli S., Salvini S., Palli D., . . . Ferrucci L. (2012). Protein intake and muscle strength in older persons: Does inflammation matter? Journal of the American Geriatric Society, 60, 480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley J. M., LaCroix A. Z., Neuhouser M. L., Huang Y., Tinker L., Woods N., . . . Prentice R. L. (2010). Protein intake and incident frailty in the women’s health initiative observational study. Journal of the American Geriatric Society, 58, 1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaum C. S., Xue Q. L., Michelon E., Semba R. D., Fried L. P. (2005). The association between obesity and the frailty syndrome in older women: The women’s health and aging studies. Journal of the American Geriatric Society, 53, 927-934. [DOI] [PubMed] [Google Scholar]
- Chan C. B., Ryan D. A., Tudor-Locke C. (2006). Relationship between objective measures of physical activity and weather: A longitudinal study. International Journal of Behavioral Nutrition and Physical Activity, 3, Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. R., Safford M. M. (2012). Management of osteoporosis among the elderly with other chronic medical conditions. Drugs and Aging, 29, 549-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. J., Dreyer H. C., Pennings B., Fry C. S., Dhanani S., Dillon E. L., . . . Rasmussen B. B. (2008). Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. Journal of Applied Physiology, 104, 1452-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C. J. (2000). Mechanisms of changes in basal metabolism during ageing. European Journal of Clinical Nutrition, 54(Suppl. 3), S77-S91. [DOI] [PubMed] [Google Scholar]
- Hildrum B., Mykletun A., Dahl A. A., Midthjell K. (2009). Metabolic syndrome and risk of mortality in middle-aged versus elderly individuals: The Nord-Trøndelag Health Study (HUNT). Diabetologia, 52, 583-590. [DOI] [PubMed] [Google Scholar]
- Houston D. K., Nicklas B. J., Ding J., Harris T. B., Tylavsky F. A., Newman A. B., . . . Health, Aging, and Body Composition Study. (2008). Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. American Journal of Clinical Nutrition, 87, 150-155. [DOI] [PubMed] [Google Scholar]
- Hozawa A., Okamura T., Oki I., Murakami Y., Kadowaki T., Nakamura K., . . . NIPPON DATA80 Study Group. (2008). Relationship between BMI and all-cause mortality in Japan: NIPPON DATA80. Obesity (Silver Spring), 16, 1714-1717. [DOI] [PubMed] [Google Scholar]
- Jingu S., Egami Y., Kinukawa N., Sano S., Takei H. (2003). Factors related to functional capacity in community dwelling elderly people. Nihon Koshu Eisei Zasshi, 50, 92-105. [PubMed] [Google Scholar]
- Keys A., Taylor H. L., Grande F. (1973). Basal metabolism and age of adult man. Metabolism, 22, 579-587. [DOI] [PubMed] [Google Scholar]
- Knight E. L., Stampfer M. J., Hankinson S. E., Spiegelman D., Curhan G. C. (2003). The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Annals of Internal Medicine, 138, 460-467. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Asakura K., Suga H., Sasaki S., Three-Generation Study of Women on Diets Health Study Group. (2013). High protein intake is associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutrition Journal, 12, Article 164. [Google Scholar]
- Kobayashi S., Honda S., Murakami K., Sasaki S., Okubo H., Hirota N., . . . Date C. (2012). Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. Journal of Epidemiology, 22, 151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya M. (2012). Process of physical disability among older adults—Contribution of frailty in the super-aged society. Nagoya Journal of Medical Science, 74, 31-37. [PMC free article] [PubMed] [Google Scholar]
- Madsen J. L., Graff J. (2004). Effects of ageing on gastrointestinal motor function. Age and Ageing, 33, 154-159. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Labour, and Welfare. (2010). Summary report of comprehensive survey of living conditions 2010. Retrieved from http://www.mhlw.go.jp/english/database/db-hss/dl/report_gaikyo_2010.pdf
- Ministry of Health, Labour, and Welfare. (2015). Abridged life table. Retrieved from http://www.mhlw.go.jp/toukei/saikin/hw/life/life15/dl/life15-02.pdf
- Ministry of Internal Affairs and Communications. (2015). National census. Retrieved from http://www.stat.go.jp/data/kokusei/2015/kekka/pdf/c_youyaku.pdf
- Nakamura Y., Kaneko I., Kawamura Y., Sakano T., Naito K., Maeda K., . . . Hashimoto S. (2002). Factors associated with self-rated health for non-institutionalized aged persons. Nihon Koshu Eisei Zasshi, 49, 409-416. [PubMed] [Google Scholar]
- Paddon-Jones D., Rasmussen B. B. (2009). Dietary protein recommendations and the prevention of sarcopenia. Current Opinion on Clinical Nutrition and Metabolism Care, 12, 86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannemans D. L., Westerterp K. R. (1995). Energy expenditure, physical activity and basal metabolic rate of elderly subjects. British Journal of Nutrition, 73, 571-581. [DOI] [PubMed] [Google Scholar]
- Pennings B., Koopman R., Beelen M., Senden J. M., Saris W. H., van Loon L. J. (2011). Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. American Journal of Clinical Nutrition, 93, 322-331. [DOI] [PubMed] [Google Scholar]
- Poehlman E. T. (1992). Energy expenditure and requirements in aging humans. Journal of Nutrition, 122, 2057-2065. [DOI] [PubMed] [Google Scholar]
- Poehlman E. T., Goran M. I., Gardner A. W., Ades P. A., Arciero P. J., Katzman-Rooks S. M., . . . Sutherland P. T. (1993). Determinants of decline in resting metabolic rate in aging females. American Journal of Physiology, 264(3, Pt. 1), E450-E455. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration, Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., . . . Collins R. (2007). Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. The Lancet, 370, 1829-1839. [DOI] [PubMed] [Google Scholar]
- Shimoda T., Yoshimura S., Yoshida Y., Okazaki M., Gotou T., Tamura S., . . . Ogasawara K. (2015). Development and current status of an advanced telehealth consultation system in Japan. Journal of Telemedicine and Telecare, 21, 176-178. [DOI] [PubMed] [Google Scholar]
- Symons T. B., Schutzler S. E., Cocke T. L., Chinkes D. L., Wolfe R. R., Paddon-Jones D. (2007). Aging does not impair the anabolic response to a protein-rich meal. American Journal of Clinical Nutrition, 86, 451-456. [DOI] [PubMed] [Google Scholar]
- Tamakoshi A., Yatsuya H., Lin Y., Tamakoshi K., Kondo T., Suzuki S., . . . JACC Study Group. (2010). BMI and all-cause mortality among Japanese older adults: Findings from the Japan collaborative cohort study. Obesity (Silver Spring), 18, 362-369. [DOI] [PubMed] [Google Scholar]
- United Nations. (2015). World Population Prospects, the 2015 Revision. Retrieved from https://esa.un.org/unpd/wpp/Download/Standard/Population/
- Volpi E., Mittendorfer B., Rasmussen B. B., Wolfe R. R. (2000). The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. Journal of Clinical Endocrinology & Metabolism, 85, 4481-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrand S., Short K. R., Bigelow M. L., Sweatt A. J., Hutson S. M., Nair K. S. (2008). Functional impact of high protein intake on healthy elderly people. American Journal of Physiology, Endocrinology, and Metabolism, 295, E921-E928. [DOI] [PMC free article] [PubMed] [Google Scholar]