ABSTRACT
Marburg virus (MARV) and Ebola virus (EBOV) have been a source of epidemics and outbreaks for several decades. We present here the generation and characterization of the first protective antibodies specific for wild-type MARV. Non-human primates (NHP), cynomolgus macaques, were immunized with viral-replicon particles expressing the glycoproteins (GP) of MARV (Ci67 isolate). An antibody fragment (single-chain variable fragment, scFv) phage display library was built after four immunogen injections, and screened against the GP1-649 of MARV. Sequencing of 192 selected clones identified 18 clones with distinct VH and VL sequences. Four of these recombinant antibodies (R4A1, R4B11, R4G2, and R3F6) were produced in the scFv-Fc format for in vivo studies. Mice that were challenged with wild-type Marburg virus (Ci67 isolate) receiving 100 µg of scFv-Fc on days −1, 1 and 3 demonstrated protective efficacies ranging from 75–100%. The amino-acid sequences of the scFv-Fcs are similar to those of their human germline counterparts, sharing an identity ranging between 68 and 100% to human germline immunoglobulin. These results demonstrate for the first time that recombinant antibodies offer protection against wild-type MARV, and suggest they may be promising candidates for further therapeutic development especially due to their human homology.
KEYWORDS: Antibody, biodefense, ebola, filovirus, hemorrhagic, Marburg, murine, protection, therapeutic
Introduction
Marburg virus (MARV), together with the five members of the Ebolavirus genus, constitutes the family Filoviridae of the order Mononegravirales. MARV causes severe and highly lethal viral hemorrhagic fevers (VHF) in both non-human primates (NHP) and humans.1 The primary transmission of MARV is through contact with infected bodily fluids from infected humans or animals.2 MARV was first identified in 1967 in Germany and Yugoslavia, and continues to cause sporadic outbreaks throughout equatorial Africa.3 In the absence of a licensed vaccine or therapeutic, there are limited options beyond supportive care.4 Although several vaccine and a few therapeutic options are currently in clinical trials for filoviruses, these are specific only to Ebola virus (EBOV). Additionally, issues with the logistics of a complete vaccination program present a strategic gap for this global threat and do not eliminate the need for a post-exposure therapeutic program.5
Filoviruses are nonsegmented, single-stranded negative sense RNA viruses that contain seven or more structural proteins.6 The transmembrane glycoprotein (GP) is expressed on the viral surface and is the primary facilitating protein of entry into the host cells. The location and abundance of this protein on the virion surface makes it an attractive candidate for the development of protective antibodies. Vaccine candidates have shown varying degrees of success in animal models and in clinical trials (for reviews, see references 7-9). Initial attempts focused on the use of inactivated whole virus with mixed success in NHP models, while later attempts utilized virus-like replicon particles (VRP), virus-like particles (VLP), viral vectors or plasmid DNA with greater levels of protection offered.10-12 The shared component of all these vaccine candidates was the concept of developing an immune response against GP, which would hopefully lead to the generation of protective antibodies and cellular responses.
Convalescent serum was used during the 1995 Kikwit Ebola outbreak, providing the first suggestion that an immunotherapeutic could be effective for the treatment of filovirus-infected individuals. In this small study (n = 8), with no control group, convalescent serum treatment reduced mortality from 80% seen in the broader outbreak to 12.5%, although the authors acknowledge the possibility of a standard-of-care effect.13 Since that time, there has been expanding, yet limited, success in developing protective antibody-based therapeutics against filoviruses. The recombinant anti-EBOV antibody KZ52, isolated from a human survivor, was shown to be protective in guinea pig models; however, it failed to protect in the NHP model.14,15 Dye et al. were the first to demonstrate the utility of antibody passive transfer therapies in NHP models of filovirus infections.16 EBOV- or MARV-infected NHPs were fully protected when treated with immunoglobulin G purified from species-matched convalescent serum, even when treatment was delayed 48 hours post-infection. The first utilization of a monoclonal antibody (mAb) therapy for MARV was recently reported by Fusco et al; they found two mAbs that bind to GP, which were able to provide protection, but to a mouse-adapted Ravn strain of Marburg virus (RAVV).17
In this study, we present the generation, isolation and characterization of a series of macaque, high-affinity single-chain variable fragments (scFvs) targeting MARV GP, as well as the protection in a mouse model obtained by these antibodies in the scFv-Fc format.
Results
Macaque immunization and antibody generation
A single cynomolgus macaque was intramuscularly (i.m.) immunized with four sequential injections of virus replicon particles (VRP) expressing the Marburg GP (isolate Ci67) at the surface of cells following viral replication of the complex. The macaque developed increasing anti-GP antibody titers as evaluated by ELISA with a titer of 1:316,000 after the second boost and 1:500,000 following the third (Table S1). The final boost was given three months after the third injection and eight days later bone marrow samples were harvested. Bone marrow samples were taken on days 3, 6, 8, 12, 18 and 21. The optimal DNA amplification was observed at the day eight time point (Fig. S1 and Fig. S1-3) before the quantity of the amplified variable gene products decreased. The amplified products of VH1 through VH9 and VL1 through VL7 were combined from day eight collections and cloned into pGemT for the respective construction of к light chains and Fd sub-libraries.
Library construction and isolation of scFvs specific to MARV-GP
For the construction of the immune library, the pGEM cloned V-Genes were amplified and cloned into pHAL35 in two subsequent steps. First the VL repertoire was clones and afterwards the VH repertoire, resulting in a library size of 6.04 × 10E8 independent clones.
The antibody selection using the generated anti-MARV immune library was performed on MARV Ci67 GP1-649 immobilized in microtiter plates. Four panning rounds were performed with increased stringency. Here, 5, 10, 20, 40 washing steps, respectively, were performed after each panning round. Finally, 3×10E7 antibody phage were eluted after the fourth panning round. Subsequently, 194 clones were sequenced and analyzed, resulting in 18 unique antibody sequences (Table 1). The overall identity of the macaque VH and VL sequences with their human germline counterparts averaged 76.7% for VH and 82.1% for VL
Table 1.
Germline sequence similarity of 18 distinct scFv antibody fragments isolated from panning. Macaque VH and VL similarity with their human germline counterparts were calculated.
| Heavy Chain (VH) |
Light Chain (VL) |
|||
|---|---|---|---|---|
| % | % | |||
| Antibody | V | Identity | V | Identity |
| R4A1 | IGHV3-11*04 | 88.8 | IGKV1-5*01 | 93.6 |
| R4B11 | IGHV1-69*09 | 80.6 | IGKV1D-12*01 | 88.4 |
| R4G2 | IGHV3-23*01 | 60.8 | IGKV1-5*01 | 78.9 |
| R4G7 | IGHV3-23*01 | 77.3 | IGKV1-5*01 | 78.9 |
| R4G8 | IGHV3-23*02 | 76.3 | IGKV1-5*01 | 81.1 |
| R4G9 | IGHV3-23*01 | 79.4 | IGKV1-5*01 | 81.1 |
| R4G10 | IGHV3-23*04 | 61.9 | IGKV1-5*01 | 78.9 |
| R4G11 | IGHV3-23*02 | 75.3 | IGKV1-5*01 | 81.8 |
| R4H11 | IGHV3-23*02 | 75.3 | IGKV1-5*01 | 81.1 |
| R4H12 | IGHV3-23*02 | 76.3 | IGKV1-5*01 | 81.1 |
| R3C4 | IGHV1-69*09 | 84.7 | IGKV1D-12*01 | 85.3 |
| R3D4 | IGHV3-23*02 | 75.3 | IGKV1-5*01 | 81.1 |
| R3F6 | IGHV3-23*01 | 78.4 | IGKV1-5*01 | 80.0 |
| R3G2 | IGHV3-23*02 | 71.1 | IGKV3-15*01 | 80.0 |
| R3G5 | IGHV4-b*02 | 75.8 | IGKV1-39*01 | 85.3 |
| R3H2 | IGHV3-23*02 | 77.3 | IGKV1-5*01 | 81.1 |
| R3H6 | IGHV3-23*02 | 77.3 | IGKV1-5*01 | 80.0 |
| R3H10 | IGHV3-23*01 | 89.7 | IGKV1-5*01 | 80.0 |
Antibody recovery and characterization
Each of the 18 distinct scFv recovered from the library was assessed for its binding capacity with MARV Ci67 GP1-649 by surface plasmon resonance (SPR; Table 2). The affinities of the anti-MARV scFv were evaluated under standard conditions, using 800 second elution steps in HBS-EP buffer (Fig. 1), and resulting values ranged from 155 nM for R3H2 to 0.14 nM for R3F6 (Table 2). Of note, R3G5 was unable to produce sufficient quantities of antibody to be further tested.
Table 2.
Antibody Affinities as measured by Bia-Core with Epitope groping
| kon | koff | KD | Epitope | |
| Antibody Fragment |
M−1s−1 |
s−1 |
(nM) |
Group |
| R4A1 | 6.42 × 104 | 2.85 × 10−4 | 4.4 | 2 |
| R4B11 | 8.76 × 104 | 4.07 × 10−4 | 4.6 | 1 |
| R4G2 | 1.19 × 104 | 4.54 × 10−4 | 38 | 2 |
| R4G7 | 8.19 × 104 | 3.79 × 10−4 | 4.6 | 1 |
| R4G8 | 9.23 × 104 | 6.3 × 10−3 | 68 | 3 |
| R4G9 | 3.1 × 104 | 1.75 × 10−4 | 5.6 | 3 |
| R4G10 | 1.39 × 104 | 7.46 × 10−4 | 54 | 2 |
| R4G11 | 7.5 × 104 | 4.75 × 10−4 | 6.3 | 2 |
| R4H11 | 3.75 × 104 | 4.17 × 10−4 | 11 | 1 |
| R4H12 | 1.46 × 105 | 5.65 × 10−4 | 3.9 | 2 |
| R3C4 | 1.16 × 104 | 3.86 × 10−4 | 33 | ND |
| R3D4 | 1.75 × 104 | 8.98 × 10−4 | 5.1 | 1 |
| R3F6 | 1.99 × 105 | 2.35 × 10−5 | 0.14 | 1 |
| R3G2 | 8.3 × 103 | 5.78 × 10−4 | 69.7 | 2 |
| R3H2 | 6.39 × 103 | 9.93 × 10−4 | 155.0 | 2 |
| R3H6 | 1.74 × 104 | 5.61 × 10−4 | 32.3 | 2 |
| R3H10 | 4.13 × 104 | 2.83 × 10−4 | 6.9 | 2 |
Figure 1.
Representative BiaCore sensorgram of anti-MARV antibody scFv-R4A1. R4A1 affinity was measured at 4.4 nM against MARV GP, utilizing an 800 second elution.
Classical epitope determination methods utilizing peptide arrays were not successful. To characterize these antibodies, we determined competition groups and isolated specific epitopic families within the 17 remaining antibodies using BiaCore analysis. SM5 chips were coated with GP1-649 and were pairwise added for sequential assessment by BiaCore. As an example, competitive antibodies had no change in signal (Fig. 2, blue line), while antibodies biding to a new epitope had an increased signal above the first set. (Fig. 2, red line). Using this analysis, we identified three distinct groupings of antibodies that were non-competitive (Table 2). The antibody R3C4 is not determined (ND) because its dissociation rate was too rapid to determine a competition grouping.
Figure 2.
BiaCore sensorgram of binding analysis between two competitive scFv antibodies (blue) and two non-competitive scFv antibodies (red).
Four antibodies from the groups above were chosen based on their high affinity to GP1-649, sequence homology and growth characteristics (data not shown). These antibodies were produced in the scFv-Fc format to assess for cross binding by western blot analysis, as well as for use in protection studies. The selected antibodies were assessed by western blot analysis, demonstrating cross reactivity to the MARV isolates Ci67, Musoke and Angola, but with no reactivity to Ravn virus (Fig. 3). Of the four antibodies assessed, all bound strongly to Ci67; R4A1, R4B11 and R3F6 bound strongly to Musoke; and only one of the antibodies, R3F6, demonstrated moderate binding to the Angola isolate.
Figure 3.
Western blots analysis of the reactivity of the scFv-Fc antibodies to whole irradiated virus. In each 4-12% gradient gel, each lane was loaded with 3uL of a 1:500 dilution of sucrose purified Marburg virus isolates corresponding to the labeled well.
In vitro antibody neutralization
The identification of neutralizing mAbs to MARV has been problematic, with neutralizers reported in the literature limited to vesicular stomatitis virus (VSV)-expressed Ravn17 or Uganda18 glycoprotein, although Kajihara et al. was able to demonstrate an inhibitory mechanism specific to viral budding.19 In this study, we utilized two separate assays to evaluate neutralization of MARV. ScFvs for 17 of the antibodies (R3C4 was not assessed due to low expression) were tested in a VSV pseudovirion assay expressing the Musoke variant of GP (Fig. S2), as well as the classical plaque reduction neutralization test (PRNT) assay utilizing Ci67 isolate of wild-type MARV. In both of these assays, no antibody reached a PRNT titer of 80% inhibitory concentration. In the classical neutralization assay, which measures the ability of a molecule to block viral entry to the cell, plaque sizes appeared as smaller “pinpoint” plaques and often took an extra day to detect, but failed to reach a PRNT titer of 80%. (Fig. 4a) The reduction of these plaque sizes were significant for four of the antibody fragments tested, R3F6, R4A1, R4B11, and R4G2 (Fig. 4).
Figure 4.
(A) Photographic representation of viral plaques and (B) corresponding sizes in Vero E6 cells. ***All plaque sizes were highly significant to a p-value <0.0001 by utilizing a two-tailed t-test for the four antibody fragments tested against control virus.
In vivo mouse protection study
To investigate the in vivo protection of the four selected candidates (R4A1, R4B11, R4G2 and R3F6), each of them was reformatted as scFv-Fc and tested in interferon (IFN) α/β receptor knockout mice (IFNAR-/-) challenged with wild-type MARV Ci67. Standard mouse models, using C57BL/6 or BALB/c, could only be utilized with a mouse-adapted Ravn17,20 or mouse-adapted Ci6718, as previously done by others. These antibodies bind to Ci67 with some cross reactivity to Angola or Musoke, but are not cross reactive to Ravn. All scFv-Fc antibodies were protective against a lethal MARV Ci67 challenge of 1000 PFU (Fig. 5). The antibody R3F6 demonstrated the best efficacy (100% protection) under the tested conditions, while R4A1, R4B11, and R4G2 had protective efficacies of 75%, 80%, and 85%, respectively. The protective efficacy at our dose range was similar to that reported in previous studies. Fusco et al. demonstrated post-exposure prophylactic protection using 500 µg of antibody administered 1 hr after 1000 PFU challenge with mouse-adapted Ravn.21 Flyak et al. used a similar approach to our experimental design, but with i.m. administration of a 100 µg of antibody, with multi-dose regimen beginning 24-hr pre-exposure followed by 24 hr post-exposure against 1000 PFU mouse-adapted Ci67 strain.18
Figure 5.
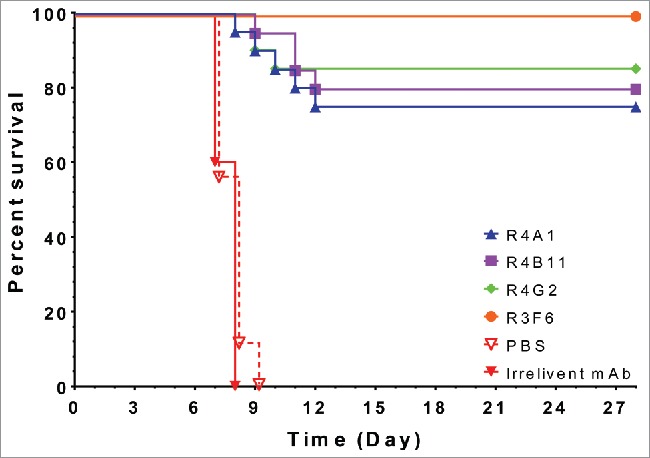
Percent survival of IFNAR-/- mice administered MARV GP specific scFv-Fc antibodies. Each mouse was administered 100ug of scFv-Fc antibody treatment, irrelevant antibody (n=10), or PBS (n=10) on Days −1, 1, and 3. Mice were challenged with 1000 pfu MARV Ci67 on D0.
The mAbs described in our studies were assessed in both male and female groups of mice with no efficacy differences observed between sexes. In addition to the baseline protection study, we re-challenged our mice thirty-five days after the initial exposure. Surviving mice were challenged with a second injection of 1000 PFU by i.p. with no antibody treatment given. All mice survived the second challenge with no loss in weight, demonstrating that these mice were able to develop a protective memory immune response. (Supplementary Figure)
Discussion
Previous studies have demonstrated that post-exposure polyclonal antibodies, as well as recombinant mAbs, provide protection against filoviruses in NHP models. Although there is no clear path for the down selection of antibodies, we chose an approach that identified high binding affinity to the antigen, sequence homology, production capacity and finally protection. Previous work has identified that neutralization may not be the result of higher affinity.22 This gap in understanding initial in vitro characteristics to protection could be one reason that few mAbs have advanced to protection studies. To our knowledge, the observation of reduced plaque size in non-neutralizing filovirus mAbs has not been reported in the literature. Reduced plaque size has also been shown with therapeutics and vaccines to Dengue virus, and could indicate that these antibodies are able to inhibit spread of this virus in vitro.23 The decreased plaque size in our studies suggests a blockage of viral spread, but further studies are needed. To date, there are no reports of mAbs that protect against wild-type MARV infection in the literature. Previous studies have proposed that the protection of MARV may proceed by a mechanism other than the classical mechanism that blocks virus entry.16 It has recently been shown that antibodies can inhibit the virus by a separate mechanism, viral budding.19 In this study, we present the first mAbs developed from NHP immune libraries utilizing the scFv and scFv-Fc format, providing protection in an animal model for MARV. Although these mAbs were produced and characterized in the scFv and scFv-Fc formats to allow rapid down selection and identification of lead candidates, future studies will address the effector functionality and protection of these candidates in an IgG format.
Although availability of both vaccines and pre-exposure therapeutics against viral hemorrhagic fevers would be optimal, the reality is that viral diseases can occur in areas that were not previously known to have a history of that disease or strain, so a vaccination campaign would not be easy to implement. This occurred in 2014 with the EBOV outbreak in western Africa, demonstrating that the emergence of a virus could present itself in a population unvaccinated or prepared for such an epidemic. Given the long half-life of human antibodies (∼20 days),24 a pre-treatment comprising a protective antibody or cocktail of antibodies could be administered in a prophylactic regimen. This prophylactic treatment could provide sufficient protection for a significant duration based on the antibody pharmacokinetics, with titers possibly similar to or higher than those provided by a rapid vaccine programs. Furthermore, providing these antibodies would afford immediate protection to an individual while an immunological response is still developing in a vaccinee. Antibodies could thus represent effective pre- and post-exposure treatments.
In these studies, we report the first in vivo protective recombinant antibodies against wild type MARV. We believe these antibodies are promising candidates for use in the development of an antibody cocktail for therapeutic applications.
Materials and methods
Macaque immunization
VRPs on a Venezuelan equine encephalitis virus platform were first developed by Pushko et al.25 Filovirus-specific VRPs expressing MARV GP at their surface have previously shown protection in rodents and NHPs.26 VRPs expressing MARV GP were injected i.m. into a cynomolgus macaque (Macaca fascicularis). The first injection consisted of MARV VRP at a concentration of 9.0 ×108 VRP/mL. Two additional injections were completed at 30 day intervals followed by a final booster (fourth) injection 88 days after the third injection, all at 9.0 × 108 VRP/mL.
The macaque immunizations were approved by the Institut de Recherche Biomédicale des Armées Ethics committee (Comité d’éthique de l'Institut de Recherche Biomédicale du Service de Santé des Armées) under authorization no. 2008/03.0 and were performed in accordance with all relevant French laws and ethical guidelines, including, in particular (1) “partie règlementaire du livre II du code rural (Titer I, chapitre IV, section 5, sous-section 3: expérimentation sur l'animal),” (2) “décret 87–848 du 19-10/1987 relatif aux expériences pratiquées sur les animaux vertébrés modifié par le décret 2001/464 du 29/05/2001,” (3) “arrêté du 29 octobre 1990 relatif aux conditions de l'expérimentation animale pour le Ministère de la Défense,” and (4) “instruction 844/DEF/DCSSA/AST/VET du 9 avril 1991 relative aux conditions de réalisation de l'expérimentation animale.” Animal care procedures complied with the regulations detailed under the Animal Welfare Act and in the Guide for the Care and Use of Laboratory Animals. Animals were kept at a constant temperature (22 °C ± 2 °C) and relative humidity (50%), with 12 h of artificial light per day. Animals were anesthetized before the collection of blood or bone marrow by an i.m. injection of 10 mg/kg ketamine (Imalgene®, Merial). If the animal technicians suspected that the animal was in pain, on the basis of their observations of animal behavior, analgesics were subsequently administered, through a single i.m. injection of 5 mg/kg flunixine (Finadyne®, Schering Plough) in the days after interventions.
Construction and screening of the anti-MARV antibody gene library
RNA from lymphocytes of the macaque bone marrow was prepared with Tri Reagent (Molecular Research Center Inc., Cincinnati, USA). The isolated RNA was reverse transcribed to cDNA using Superscript II and oligo (dT) (Invitrogen, USA). Combinations of forward and reverse primers were used to amplify the regions coding for the variable regions VLK and VH as previously described.27 PCR products were cloned in the pGemT vector (Promega, Madison, Wisconsin) according to the manufacturer's instructions, yielding two sub-libraries encoding the heavy chains (Fd fragment) or the kappa light chains.
The pGemT cloned PCR products were reamplified using two macaque oligonucleotide primer sets to introduce restriction sites for library cloning as described before.22,28,29 In brief, the secondary PCRs were carried out for each forward oligonucleotide primers separately to keep the diversity. Each PCR was performed in a volume of 100 µl using 100 ng purified PCR reaction product of the pGemT cloned cDNA, 2.5 U Go Taq polymerase (Promega, Mannheim, Germany), 200 µM dNTPs each and 200 nM of each oligonucleotide primer for 20 cycles (30 s 94°C, 30 s 57°C, 30 s 72°C), followed by 10 min 72°C. The PCR products were separated by 1.5% (w/v) agarose gel, cut out and purified using Nucleospin Extract II Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions.
The construction of the library was completed in two subsequent steps. First, the PCR products encoding VL were cloned into pHAL35. pHAL35 was derived from pHAL14 with an additional SfiI site for VH cloning and Myc-His tag orientation instead of His-Myc.30 Second, the VH PCR fragments were cloned. A total of 5 µg pHAL35 and 2 µg VL were digested using 50 U MluI and 50 U NotI (NEB, Frankfurt, Germany) in a 100 µl reaction volume for 2 h at 37°C. Afterwards, 0.5 U calf intestinal phosphatase (MBI Fermentas) was added and incubated for further 30 min. This dephosphorylation step was repeated once. The vector was purified using the Nucleospin Extract II Kit. 270 ng VL were cloned into 1 µg of the dephosporylated pHAL35 using 1 U ligase (Promega, Mannheim, Germany) overnight at 16°C. The ligation reactions were precipitated with ethanol and sodium acetate and the pellet was washed twice with 70% ethanol. These reactions were electroporated (1.7 kV) in 25 µl XL1-Blue MRF' (Agilent, Böblingen, Germany). The transformed bacteria were plated onto 2xYT agar plates (Sambrook and Russell, 2001) (25 cm petri dishes) supplemented with 100 µg/mL ampicillin, 20 µg/mL tetracycline and 100 mM glucose. The colonies were harvested by suspending in 40 mL 2xYT media with a Drigalsky spatula. Plasmids were isolated using the Nucleobond Plasmid Midi Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. Afterwards, 5 µg of each VL chain library as well as 2 µg of the VH fragments were digested using 50 U HindIII (NEB) in a 100 µl reaction volume overnight at 37°C followed by 50 U SfiI (NEB) for 2.5 h at 50°C. In total, 4 transformations were performed and pooled. The harvested bacteria representing the final antibody gene libraries were aliquoted and stored at −80°C.
Library packaging
400 mL 2xYT medium supplemented with 100 µg/mL ampicillin and 100 mM glucose were inoculated with the library glycerin stock of the pooled library.31 The bacteria were grown to O.D.600 = 0.4 - 0.5 at 37°C and 250 rpm. 25 mL bacteria (∼1.25×1010 bacteria) were infected with 2.5×1011 Hyperphage, incubated at 37°C for 30 min without shaking, followed by 30 min at 250 rpm.32,33 The infected cells were harvested by centrifugation for 10 min at 3220 xg and the pellet was resuspended in 30 mL 2xYT supplemented with 100 µg/mL ampicillin and 50 µg/mL kanamycin, and cultivated over night at 30°C and 250 rpm. Bacteria cells were pelleted for 10 min at 10000 xg. Phage particles in the supernatant were precipitated with 1/5 volume of 20% PEG/2.5 M NaCl solution for 1 h on ice with gentle shaking and pelleted 1 h at 10000 xg at 4°C. The precipitated phage were re-suspended in 10 mL phage dilution buffer (10 mM TrisHCl pH7.5, 20 mM NaCl, 2 mM EDTA), sterile filtered using a 0.45 µm filter and precipitated again with 1/5 volume of PEG solution for 20 min on ice, and pelleted 30 min at 10000 xg at 4°C. The precipitated phage were re-suspended in 300 µL phosphate-buffered saline (PBS) and cell debris was pelleted by additional centrifugation for 5 min at 15400 xg at 20°C. The supernatant containing the scFv phage were stored at 4°C. The library packaging was analyzed by SDS-PAGE, Western blot and anti-pIII immunostaining as described before.22
Screening of the library was performed as described elsewhere,34 except that 5, 10, 20, and 40 washes were performed for each successive round of panning. MARV GP1-649 was utilized as the antigen and TBS-Tween 20 0.1% as the washing buffer.
Sequence analysis
A sequence analysis of similarities between macaque VH and VL and the closest human germline genes encoding VH and VL was performed utilizing the IMGT database (http://www.imgt.org/IMGTlect/) (Table 1). Additionally, the degrees of identities between the macaque V regions with their most similar human germline counterparts were calculated with DomainGapAlign (http://www.imgt.org/3Dstructure-DB/cgi/DomainDisplay.cgi).34
Affinity measurements
Affinities were measured by SPR utilizing a BIAcore-3000 instrument (Biacore, Uppsala, Sweden). The MARV GP1-649 was immobilized at a maximum of 1000 RU on a CM5 chip (BiaCore) via amine coupling according to the manufacturer's instructions. A 30 µL/min flow rate was maintained for the measurement. For each scFv, eight dilutions were prepared in HBS-EP buffer (Biacore) with elution times greater than 1000 seconds. Following each dilution, the chip was regenerated with 1.5 M glycine buffer (Biacore) run at 10 µL/min for 50 seconds. For competition BiaCore epitope binding, MARV GP1-649 was immobilized at a maximum of 400 RU on a CM5 chip (BiaCore) as above. Sets of two antibodies were injected in tandem with the second antibody injection just after the maximal saturation of the epitope. Following the second antibody injection, the chip was regenerated with 1.5 M glycine buffer (Biacore) run at 10 µL/min for 50 seconds. This process was completed until all antibodies could be assessed with one another.
ScFv-Fc production and purification
ScFv fragments isolated by antibody-phage display were subcloned into pCSE2.5-mIgG2c-Fc-XP and produced as scFv-Fc in HEK293-6E cells (National Research Council (NRC), Biotechnological Research Institute (BRI), Montreal, Canada) cultured in chemically defined medium F17 (Invitrogen, Life Technologies, Darmstadt, Germany) supplemented with 1 g/L pluronic F68 (Applichem, Darmstadt, Germany), 4 mM L-glutamine (GE Healthcare, Freiburg, Germany) and 25 mg/L G418 (GE Healthcare, Freiburg, Germany), as previously described.35 The Fc was of murine origin and the scFv-Fc format is similar to IgG in that it contains two antigen binding sites and an Fc domain. For the scFv-Fc production, DNA was used for the transient transfection of 25 mL cultures of HEK293-6E cells in 125 mL Erlenmeyer shake flasks. After 48 hours of culture with shaking at 110 rpm in a Minitron orbital shaker (Infors, Bottmingen, Switzerland) at 37 °C, under an atmosphere containing 5% CO2, one volume of culture medium, with a final concentration of 0.5% (w/v) tryptone N1 (TN1, Organotechnie S.A.S., La Courneuve, France) was used for the purification of a scFv, whereas scFv-Fc were purified on a UNOsphere SUPrA column (Biorad, Munich, Germany) with a Profinia apparatus (Biorad, Hercules, California, USA), according to the manufacturer's instructions.
Cell based neutralization assay
Antibody samples, in the scFv format, were titrated in complete MEM supplemented with 10% fetal bovine serum (FBS). Antibody dilutions were added, in decreasing dilutions, to a constant viral titer for 65 PFU per well for a 1 hr incubation at 37°C. Dilutions were plated in triplicate on 6-well plates containing 95–98% confluent Vero E6 cells. After a 1 hr incubation at 37°C, wells were overlaid with 1% agarose in Eagle's Basal medium (EBME) with 10% FBS and 0.1% gentamicin and returned to the incubator for 7 days. On day 7, a 1% agarose secondary overlay containing 4% neutral red was added and after 1 more day at 37 °C, plaques were counted.36
Western blot analysis
Irradiated MARV antigen (Ci67, Ravn, Angola, and Musoke) was mixed with 4X loading buffer (Life Technologies) and 2-betamercaptoethanol (BioRad). The samples were heated at 70 °C for 10 minutes and 10 ul was loaded on 4–12% Bis-Tris precast gels (Life Technologies). 10 ul of precision plus protein dual color standard (BioRad) was also added to the gels. The gels were run at 150 V for 90 minutes in 1X MOPS running buffer (Life Technologies). The gels were transferred to nitrocellulose membranes (Life Technologies) via the IBLOT. The membranes were blocked with 5% milk (Microbiology) in PBS (Sigma) plus 0.02% Tween20 (Sigma Aldrich) (PBST) for 2 hrs at RT on a shaker. The primary antibodies were added at 1 ug/ml in 10 ml of blocking buffer and incubated for 1hr at RT on a shaker. The membranes were washed 3X with PBST at 10 minutes each. Secondary antibody horseradish peroxidase goat anti-mouse gamma (Kirkegaard & Perry Labs, cat. #074-1802, lot #101088) was added at 1:5000 in blocking buffer for 1hr at RT on shaker. The membranes were washed 3X with 10ml of PBST for 10 minutes. Gels were imaged on BioRad imager after staining with TMB (Life Technologies).
Murine protection study
Specific pathogen-free 6- to 8-week-old male and female IFN α/β receptor knockout (IFNAR -/-) mice were utilized (Jackson Laboratory, Bar Harbor, ME) as a model for filovirus infection. Research was conducted under an Institutional Animal Care and Use Committee-approved protocol in compliance with the Animal Welfare Act, Public Health Service policy, and other US Federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the 2011 Guide for the Care and Use of Laboratory Animals from the National Research Council. One hundred micrograms of each antibody was administered intraperitoneally (i.p.) to groups of mice (n = 10/sex with n = 20/treatment group) as a scFv-Fc fusion, on Days −1, 1, and 3. On Day 0, mice were transferred to a Biosafety Level 4 containment area and challenged by i.p. inoculation utilizing 1000 plaque-forming units (PFU) MARV Ci67. Mice were weighed and monitored once or twice daily upon onset of symptoms for 28 days post infection.
Pseudovirion neutralization assay
Viral pseudotypes bearing MARV Musoke GP were generated by infecting 293T cells expressing MARV Musoke GP with VSVΔG, as described previously.37 Vero cells were maintained at 37 °C and 5% CO2 in high-glucose Dulbecco's modified Eagle medium (DMEM) (Invitrogen Corp., Carlsbad, CA) supplemented with 10% FBS. For antibody neutralization experiments, pre-titrated amounts of pseudotype VSV-MARV particles (MOI ≈1 IU per cell) were incubated with increasing concentrations of test antibody or scFv molecule, starting at 350 nM concentration, at room temp for 1 h, prior to addition to cell monolayers in 96-well plates. Viral infectivities were measured by automated enumeration of eGFP+ cells (infectious units; IU) using a CellInsight CX5 imager (Thermo Fisher) at 12–14 h post-infection. Viral neutralization data were subjected to nonlinear regression analysis to extract EC50 values (4-parameter, variable slope sigmoidal dose-response equation; GraphPad Prism)
Supplementary Material
Disclosure of potential conflicts of interest
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
Research was conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
Funding
We kindly acknowledge the support of Joint Science Technology Office - Defense Threat Reduction Agency (JSTO-DTRA) project CB0477.
References
- 1.Kuhn RJ. Togaviridae: The Viruses and Their Replication In: Knipe DM, Howley PM, eds. Fields Virology. 5 ed Philadelphia: Lippincott Williams & Wilkins; 2007: 1001–22 [Google Scholar]
- 2.MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Neglected Trop Dis 2012; 6(6): e1546; PMID:22761967; http://dx.doi.org/ 10.1371/journal.pntd.0001546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martini G, Siegert R. Marburg Virus Disease In: Martini GA, GSiegert R, editors. Marburg virus disease – Congresses Springer-Verlag; 1971. p. 250; http://dx.doi.org/ 10.1007/978-3-662-01593-3 [DOI] [Google Scholar]
- 4.Edwards T, Semple MG, De Weggheleire A, Claeys Y, De Crop M, Menten J, Ravinetto R, Temmerman S, Lynen L, Bah EI, et al.. Design and analysis considerations in the Ebola_Tx trial evaluating convalescent plasma in the treatment of Ebola virus disease in Guinea during the 2014-2015 outbreak. Clin Trials 2016; 13(1): 13–21; PMID:26768570; http://dx.doi.org/ 10.1177/1740774515621056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froude JW, Stiles B, Pelat T, Thullier P. Antibodies for biodefense. Mabs 2011; 3(6): 517–27; PMID:22123065; http://dx.doi.org/ 10.4161/mabs.3.6.17621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradfute SB, Dye JM Jr, Bavari S. Filovirus vaccines. Hum Vaccin 2011; 7(6): 701–11; PMID:21519188; http://dx.doi.org/ 10.4161/hv.7.6.15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Exp Rev Vaccin 2014; 13(4): 521–31; PMID:24575870; http://dx.doi.org/ 10.1586/14760584.2014.885841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al.. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017; 389(10068): 505–18; PMID:28017403; http://dx.doi.org/ 10.1016/S0140-6736(16)32621-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis 2002; 8(5): 503–7; PMID:11996686; http://dx.doi.org/ 10.3201/eid0805.010284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet 1980; 2(8207): 1294–5; PMID:6108462; http://dx.doi.org/ 10.1016/S0140-6736(80)92352-1 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature 2000; 408(6812): 605–9; PMID:11117750; http://dx.doi.org/ 10.1038/35046108 [DOI] [PubMed] [Google Scholar]
- 12.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 2005; 23(23): 3033–42; PMID:15811650; http://dx.doi.org/ 10.1016/j.vaccine.2004.11.070 [DOI] [PubMed] [Google Scholar]
- 13.Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, Colebunders R, Muyembe-Tamfum JJ. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis 1999; 179 Suppl 1: S18–23; PMID:9988160; http://dx.doi.org/ 10.1086/514298 [DOI] [PubMed] [Google Scholar]
- 14.Oswald WB, Geisbert TW, Davis KJ, Geisbert JB, Sullivan NJ, Jahrling PB, Parren PW, Burton DR. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog 2007; 3(1): e9; PMID:17238286; http://dx.doi.org/ 10.1371/journal.ppat.0030009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol 2002; 76(12): 6408–12; PMID:12021376; http://dx.doi.org/ 10.1128/JVI.76.12.6408-6412.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, Ortiz RA, Prugar LI, Pratt WD. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A 2012; 109(13): 5034–9; PMID:22411795; http://dx.doi.org/ 10.1073/pnas.1200409109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fusco ML, Hashiguchi T, Cassan R, Biggins JE, Murin CD, Warfield KL, Li S, Holtsberg FW, Shulenin S, Vu H, et al.. Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs. PLoS Pathog 2015; 11(6): e1005016; PMID:26115029; http://dx.doi.org/ 10.1371/journal.ppat.1005016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flyak AI, Ilinykh PA, Murin CD, Garron T, Shen X, Fusco ML, Hashiguchi T, Bornholdt ZA, Slaughter JC, Sapparapu G, et al.. Mechanism of human antibody-mediated neutralization of Marburg virus. Cell 2015; 160(5): 893–903; PMID:25723164; http://dx.doi.org/ 10.1016/j.cell.2015.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajihara M, Marzi A, Nakayama E, Noda T, Kuroda M, Manzoor R, Matsuno K, Feldmann H, Yoshida R, Kawaoka Y, et al.. Inhibition of Marburg virus budding by nonneutralizing antibodies to the envelope glycoprotein. J Virol 2012; 86(24): 13467–74; PMID:23035224; http://dx.doi.org/ 10.1128/JVI.01896-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warfield KL, Bradfute SB, Wells J, Lofts L, Cooper MT, Alves DA, Reed DK, VanTongeren SA, Mech CA, Bavari S. Development and characterization of a mouse model for Marburg hemorrhagic fever. J Virol 2009; 83(13): 6404–15; PMID:19369350; http://dx.doi.org/ 10.1128/JVI.00126-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fusco ML, Hashiguchi T, Cassan R, Biggins JE, Murin CD, Warfield KL, Li S, Holtsberg FW, Shulenin S, Vu H, et al.. Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs. PLoS Pathog 2015; 11(6): e1005016; PMID:26115029; http://dx.doi.org/ 10.1371/journal.ppat.1005016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenzel A, Kugler J, Wilke S, Schirrmann T, Hust M. Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol Biol 2014; 1060: 215–43; PMID:24037844; http://dx.doi.org/ 10.1007/978-1-62703-586-6_12 [DOI] [PubMed] [Google Scholar]
- 23.Goh KC, Tang CK, Norton DC, Gan ES, Tan HC, Sun B, Syenina A, Yousuf A, Ong XM, Kamaraj US, et al.. Molecular determinants of plaque size as an indicator of dengue virus attenuation. Sci Rep 2016; 6: 26100; PMID:27185466; http://dx.doi.org/ 10.1038/srep26100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov 2003; 2(1): 52–62; PMID:12509759; http://dx.doi.org/ 10.1038/nrd984 [DOI] [PubMed] [Google Scholar]
- 25.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, Jahrling PB, Smith JF. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 2000; 19(1): 142–53; PMID:10924796; http://dx.doi.org/ 10.1016/S0264-410X(00)00113-4 [DOI] [PubMed] [Google Scholar]
- 26.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 1998; 251(1): 28–37; PMID:9813200; http://dx.doi.org/ 10.1006/viro.1998.9367 [DOI] [PubMed] [Google Scholar]
- 27.Pelat T, Hust M, Hale M, Lefranc MP, Dubel S, Thullier P. Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol 2009; 9: 60; PMID:19563687; http://dx.doi.org/ 10.1186/1472-6750-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rulker T, Voss L, Thullier P, Isolatio O' Brien LM, Pelat T, Perkins SD, Langermann C, Schirrmann T, Dübel S, Marschall HJ, et al.. Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PloS One 2012; 7(5): e37242; PMID:22666347; http://dx.doi.org/ 10.1371/journal.pone.0037242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutte M, Thullier P, Pelat T, Wezler X, Rosenstock P, Hinz D, Kirsch MI, Hasenberg M, Frank R, Schirrmann T, et al.. Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus. PloS One 2009; 4(8): e6625; PMID:19675673; http://dx.doi.org/ 10.1371/journal.pone.0006625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hust M, Meyer T, Voedisch B, Rülker T, Thie H, El-Ghezal A, Kirsch MI, Schütte M, Helmsing S, Meier D, et al.. A human scFv antibody generation pipeline for proteome research. J Biotechnol 2011; 152(4): 159–70; PMID:20883731; http://dx.doi.org/ 10.1016/j.jbiotec.2010.09.945 [DOI] [PubMed] [Google Scholar]
- 31.Sambrook JDR. Molecular Cloning: A Laboratory Manual, Third Edition Joe Sambrook: Publisher: Cold Spring Harbor Laboratory Press, 2001. ISBN 10: 0879695773 ISBN 13: 9780879695774 [Google Scholar]
- 32.Rondot S, Koch J, Breitling F, Dubel S. A helper phage to improve single-chain antibody presentation in phage display. Nat Biotechnol 2001; 19(1): 75–8; PMID:11135557; http://dx.doi.org/ 10.1038/83567 [DOI] [PubMed] [Google Scholar]
- 33.Soltes G, Hust M, Ng KK, Bansal A, Field J, Stewart DI, Dübel S, Cha S, Wiersma EJ. On the influence of vector design on antibody phage display. J Biotechnol 2007; 127(4): 626–37; PMID:16996161; http://dx.doi.org/ 10.1016/j.jbiotec.2006.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelat T, Thullier P. Non-human primate immune libraries combined with germline humanization: an (almost) new, and powerful approach for the isolation of therapeutic antibodies. Mabs 2009; 1(4): 377–81; PMID:20068407; http://dx.doi.org/ 10.4161/mabs.1.4.8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jager V, Bussow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 2013; 13: 52; PMID:23802841; http://dx.doi.org/ 10.1186/1472-6750-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe JB, Lambert RD, Lupton HW. Plaque assay for Ebola virus. J Clin Microbiol 1981; 13(4): 791–3; PMID:7014628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci U S A 1997; 94(26): 14764–9; PMID:9405687; http://dx.doi.org/ 10.1073/pnas.94.26.14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


