ABSTRACT
Protein glycosylation is arguably the paramount post-translational modification on recombinant glycoproteins, and highly cited in the literature for affecting the physiochemical properties and the efficacy of recombinant glycoprotein therapeutics. Glycosylation of human immunoglobulins follows a reasonably well-understood metabolic pathway, which gives rise to a diverse range of asparagine-linked (N-linked), or serine/threonine-linked (O-linked) glycans. In N-linked glycans, fucose levels have been shown to have an inverse relationship with the degree of antibody-dependent cell-mediated cytotoxicity, and high mannose levels have been implicated in potentially increasing immunogenicity and contributing to less favorable pharmacokinetic profiles. Here, we demonstrate a novel approach to potentially reduce the presence of high-mannose species in recombinant human immunoglobulin preparations, as well as facilitate an approximate 100% replacement of fucosylation with arabinosylation in Chinese hamster ovary cell culture through media supplementation with D-arabinose, an uncommonly used mammalian cell culture sugar substrate. The replacement of fucose with arabinose was very effective and practical to implement, since no cell line engineering or cellular adaptation strategies were required. Arabinosylated recombinant IgGs and the accompanying reduction in high mannose glycans, facilitated a reduction in dendritic cell uptake, increased FcγRIIIa signaling, and significantly increased the levels of ADCC. These aforementioned effects were without any adverse changes to various structural or functional attributes of multiple recombinant human antibodies and a bispecific DVD-Ig. Protein arabinosylation represents an expansion of the N-glycan code in mammalian expressed glycoproteins.
KEYWORDS: ADCC, arabinose, afucosylation, Fc effector activity, protein glycosylation
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- CHO
Chinese hamster ovary cells
- DSC
Differential scanning calorimetry
- FcRn
Fc neonatal receptor
- FcγR
Fc gamma receptor
- Fuc
Fucose
- FucT
Glycoprotein 6-α-L-fucosyltransferase
- Gal
Galactose
- GalT
β-N-acetylglucosaminyl glycopeptide β-1,4 galactosyltransferase
- GlcNAc
N-acetylglucosamine
- GnT I
α-1,3-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase
- GnT II
α-1,6-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase
- HMW
High molecular weight species
- LC-MS
Liquid chromatography-mass spectrometry
- LMW
Low molecular weight species
- Man
Mannose
- Man I
Mannosyl-oligosaccharide 1,2-α-mannosidase
- Man II
Mannosyl-oligosaccharide 1,3–1,6-α-mannosidase
- N/A
Not applicable
- NK
Natural killer cells
- PK
Pharmacokinetics
- SEC
Size-exclusion chromatography
- VCD
Viable cell density
Introduction
N-linked glycosylation is commonly found in recombinant glycoprotein therapeutics, including antibodies, and follows a reasonably understood series of enzymatic events.1 The pathway begins in the endoplasmic reticulum (ER), where an initial precursor N-glycan comprised of Glc3Man9GlcNAc2 is transferred en bloc to a newly expressed protein during import into the lumen of the ER. Following a series of monosaccharide trimming reactions in the ER, a step-wise series of various sugar addition and trimming reactions occur in the Golgi apparatus (Fig. 1). The addition of these monosaccharides proceeds through a variable sequence of enzymatic reactions that are determined by a variety of factors, including enzymatic substrate specificity, the relative levels and localization of glycosylation enzymes, and the abundance of the sugar donor and protein acceptor co-substrates. The donor substrates for these monosaccharide addition reactions are nucleotide-sugars, which are recognized by the glycosyltransferase enzymes for addition onto the N-glycan. After traversing the secretory pathway, the protein is eventually transferred to its final destination. In the case of most recombinant glycoprotein therapeutics expressed from mammalian cells, this is essentially the constitutive secretion into the extracellular environment. There are only a few different glycosidase enzymes, glycosyltransferase enzymes, and nucleotide-sugars in the N-glycan biosynthetic pathway. However, there is a great deal of variability in this metabolic pathway, which gives rise to a diverse spectrum of N-glycans on an expressed protein (microheterogeneity), as well as variable occupancy of N-glycans at a particular site on the protein (macroheterogeneity).
Figure 1.
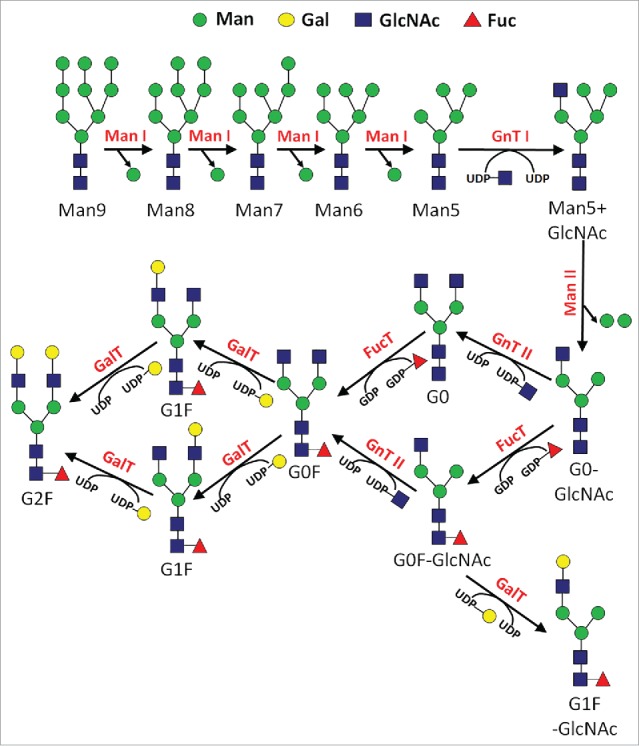
Simplified reaction pathway for N-glycan biosynthesis in mammalian cells.
Literature reports have highlighted the various sensitivities of the N-glycan biosynthetic pathway during the manufacturing of recombinant glycoprotein therapeutics. Some of these sources of variability include the source tissue of a particular cell, the cell line used to express the protein, the protein itself, the process used to culture the cells, as well as the various parameters used to culture the cells.2 Of particular note, many reports have highlighted the degree to which the final N-glycan profile is mediated through individual compounds in the culture media of the cells used to express the protein.2 It stands to reason that with the available information, one may conclude that the protein glycosylation pathway is very sensitive to a multitude of factors. This sensitivity is important for the commercial manufacturing of recombinant glycoprotein therapeutics for both the targeted optimization of efficacy, as well as ensuring consistency between different batches of the protein product, and directing glycoform profiles toward comparability to that of a desired reference material.
Mannose and fucose are 2 types of sugars attached to N-glycans on recombinant proteins. Oligomannose is the first sugar chain that is added onto the N-glycan before being serially removed. Frequently, mannose removal is not complete before the protein exits the ER and is eventually secreted outside the cell. Mannose is an important sugar that has been implicated in regulating cellular activities in a variety of studies.3 For example, it is known that high mannose N-glycans can resemble the glycosylation profile from proteins of adventitious organisms,4 and thus potentially induce both an immunogenic response, as well as a negative effect on the pharmacokinetic (PK) profile.,5,6 The mannose receptor is principally found on dendritic cells and macrophages, and has been found to be principally important toward the recognition of high mannose epitopes and the resulting clearance of the proteins that display them.4,7,8 Fucose is unique among common N-glycan sugars in the sense that it is added as the L- form of the sugar. Previous research by others has shown that lower levels of L-fucose attached to the N-glycan can actually increase antibody-dependent cell-mediated cytotoxicity (ADCC),9,10 and that this effect is dependent upon the activity of natural killer (NK) cells.11 This latter effect is of particular interest in many oncology protein therapeutics because the ADCC response could contribute to the molecule's principal mechanism of action.
The general mechanism for generating fucosylated glycoproteins through either the de novo pathway or the salvage pathway is shown in Fig. 2. Mannose and fucose are monosaccharides added or removed at various stages of the protein N-glycosylation pathway. Both sugars have been reported to affect either the efficacy or PK profile of therapeutically relevant proteins. Since the relative levels of mannose and fucose have been shown to play an important role toward the physiologic responses of the proteins that bear them, having a means with which to control their respective levels would be extremely beneficial for the targeted optimization of protein therapeutic safety and efficacy. Previous work by others has established the capability of producing afucosylated glycoproteins. Included in this are interventions at the enzyme level,12,13 as well as substrate level.14
Figure 2.
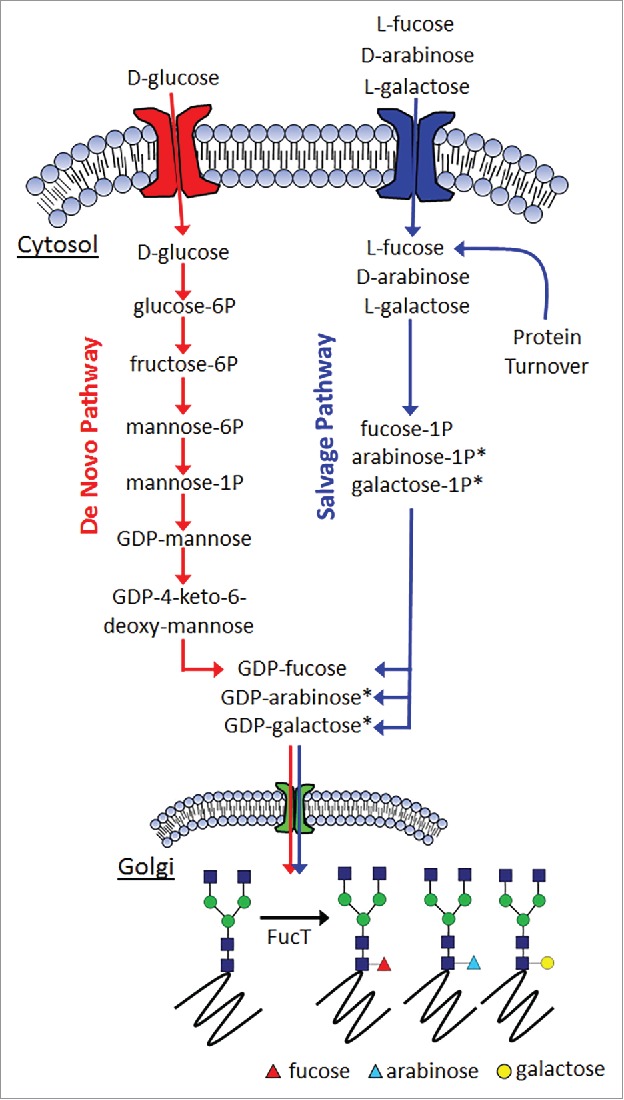
General mechanism for fucosylation in mammalian cells. (*denotes inferred metabolites).
Here, we highlight a novel means with which to reduce the levels of high mannose N-glycans while simultaneously reducing the levels of fucose through its replacement with arabinose. This was accomplished through the cell culture media supplementation of D-arabinose in cultured mammalian cell lines expressing therapeutically relevant glycoproteins. The chemical structure of D-arabinose resembles that of L-fucose (Fig. 3), facilitating the ability of arabinose to completely replace fucose on product N-glycans, and leading to approximately 100% arabinosylation of the N-glycans attached to multiple recombinant proteins. Protein arabinosylation case studies using both monoclonal antibodies (mAbs) and dual variable domain immunoglobulins (DVD-Igs) suggest that there is no adverse impact of arabinosylation on a diverse range of structural or functional attributes of these proteins. The ease with which fucose levels could be reduced through this method highlights its utility for GMP manufacturing of both new, legacy, and follow-on biologics.
Figure 3.
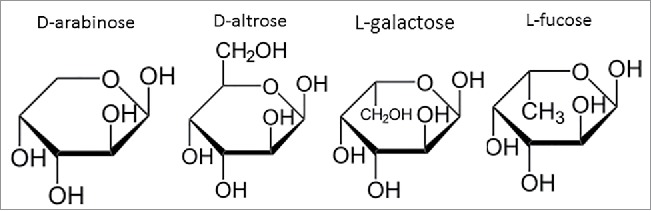
Chemical structure comparison between D-arabinose, D-altrose, L-galactose, and L-fucose, highlighting the similarity in structure and stereochemistry at select carbon positions.
Results
D-Arabinose impact on culture performance
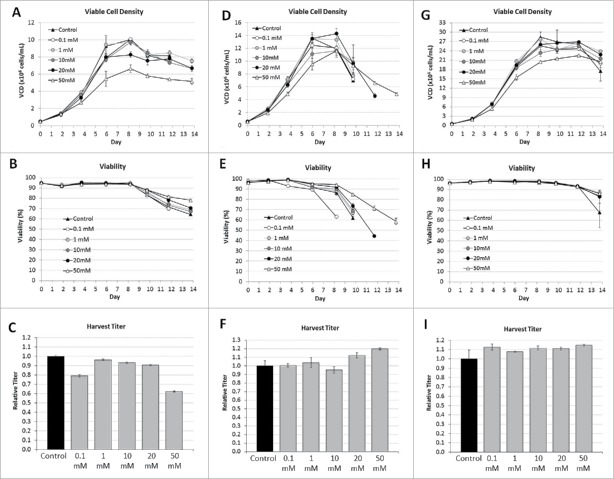
Chinese hamster ovary (CHO) cell lines 1, 2, and 3, expressing mAb-1, DVD-1, and mAb-2 were evaluated in shaker flasks in fedbatch operation mode. Variable amounts of D-arabinose were supplemented into both the chemically-defined basal and feed medias. The cell culture performance results are highlighted in Fig. 4. In cell line 1, the viable cell density (VCD) profiles suggest that 0.1, 1, and 10 mM concentrations introduce no adverse impact to either peak VCD, or culture longevity. At 20 and 50 mM concentrations, the cells also grow appreciably well, but with peak VCD values that were reduced compared with the unsupplemented control. Nevertheless, the cell viability results across the respective conditions were highly similar to each other, with all culture durations lasting either 13 or 14 days, when viability dropped below 70%. In cell line 2, the VCD profiles suggest that, across the range of tested concentrations, the majority of the cultures supported peak VCD's either on par, or only nominally lower, with that of the unsupplemented control. The cultures were also harvested when cell viability dropped below 70%. Only one of the D-arabinose supplemented cultures had a harvest date earlier than the unsupplemented control, with the higher supplemented conditions of 20 mM and 50 mM actually supporting a longer culture duration before the harvest criteria was triggered. In cell line 3, the supplement was also well-tolerated across the range of tested D-arabinose concentrations. Peak viable cell densities for each of the supplementation conditions were on par, on average, with that of the unsupplemented control. Only the 50 mM supplementation condition demonstrated average VCD results that dropped slightly below that of the control. Cell viability results were all reasonably the same across all of the evaluated conditions, with the unsupplemented control condition demonstrating a slight drop in cell viability toward the very end of the cultures. All cultures were harvested on Day 14.
Figure 4.
Cell culture performance of different CHO cell lines with different concentrations of D-arabinose supplementation (n = 3). Cell Line 1, (A) Viable cell density. (B) Cell viability. (C) Relative harvest titer. Cell Line 2, (D) Viable cell density. (E) Cell viability. (F) Relative harvest titer. Cell Line 3, (G) Viable cell density. (H) Cell viability. (I) Relative harvest titer.
Harvest titers from each of the respective cultures, from each of the evaluated cell lines, were measured. In cell line 1, across the range of D-arabinose concentrations the titers were only nominally lower compared with the unsupplemented control, which became more appreciable as the D-arabinose concentration approached 50 mM, where the titers dropped by ∼40%. For this particular cell line, one may make the conclusion that D-arabinose is well-tolerated as a media supplement; however, at appreciably high supplementation, cell culture process performance can potentially decrease. In cell line 2, regardless of the harvest timing, the harvest titers from each of the respective cultures were approximately the same, with the longer duration cultures actually increasing the total amount of expressed DVD-1. The cumulative results with CHO cell line 2 suggest that D-arabinose does not adversely affect cell culture performance across a wide range of concentrations. In cell line 3, the harvest titers from each of the D-arabinose supplemented cultures were on average slightly higher than the unsupplemented control condition. These results further highlight the benign nature of using D-arabinose as a cell culture media supplement with respect to both cell growth and productivity.
D-Arabinose impact on protein glycosylation
Cell culture harvests from cell line 1 were purified using Protein A, and the enriched antibody samples analyzed for their N-glycan profiles via liquid chromatography-mass spectrometry (LC-MS) on the reduced protein. A representative spectrograph for an arabinose-supplemented culture is shown in Fig. 5 compared with the unsupplemented control. There is a noticeable shift in mass for all the fucosylated N-glycan species by ∼14 Da in the cultures supplemented with arabinose. This decrease in mass is consistent with the difference in molecular weight between that of L-fucose (the native N-glycan sugar) and D-arabinose. The difference in molecular weight between that of arabinose and GlcNAc is ∼71 Da, and the difference between that of arabinose and galactose is ∼30 Da. The LC-MS results thus suggest that the N-glycans were potentially arabinosylated, with the fucose sugars replaced with arabinose.
Figure 5.
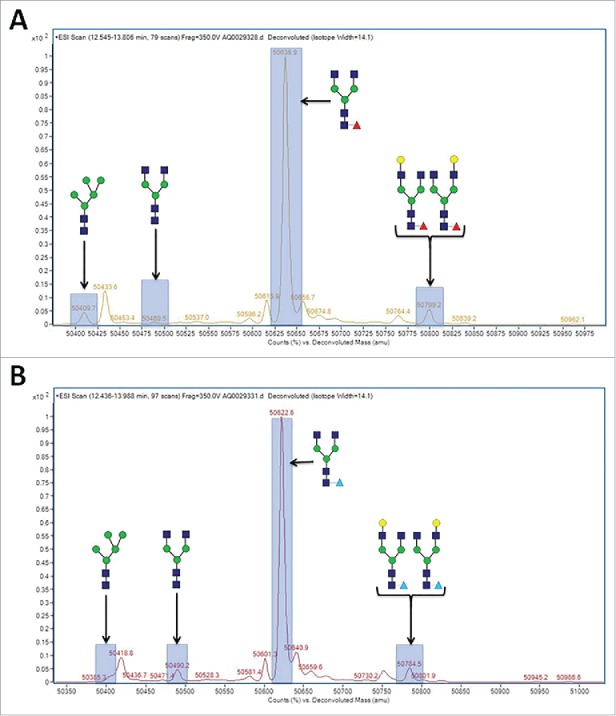
Reduced LC-MS spectragraph of purified mAb-1 expressed in CHO cell culture under different conditions. (A) Control culture, with glucose as the principal sugar. (B) Experimental culture, with glucose and D-arabinose as the principal sugars.
To confirm the replacement of core fucose with arabinose, 13C labeled D-arabinose (Sigma, St. Louis, MO) was supplemented into the cell culture media of cell line 1 at a concentration of 30 mM. Fig. 6 highlights the observed differences in reduced molecular weight of purified mAb-1 from these cultures as measured by LC-MS. It is readily apparent that there was a +1 Da shift in masses of all the N-glycans that typically contain fucose, suggesting that all fucosylated N-glycans had in fact been arabinosylated. Furthermore, Endo H (Prozyme, Hayward, CA) treatment of control and arabinosylated mAb-1 to cleave the N-glycan between the N,N’-diacetylchitobiose core GlcNAc sugars, followed by LC-MS analysis, confirmed the presence of a principal peak consistent with the theoretical mass of arabinose incorporation into the core N-glycan GlcNAc structure (Fig. 7). The cumulative results provide confidence that the incorporation of arabinose, a pentose sugar, for fucose, a larger hexose sugar, is a highly effective and specific process.
Figure 6.
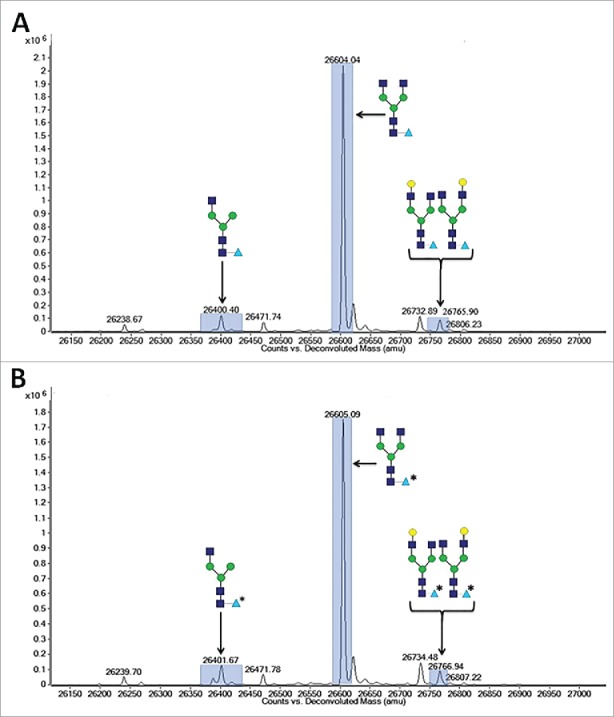
LC-MS spectragraph of Lys-C treated, and reduced mAb-1 expressed in CHO cell culture with different sugar supplements. (A) D-arabinose. (B) 13C labeled D-arabinose.
Figure 7.
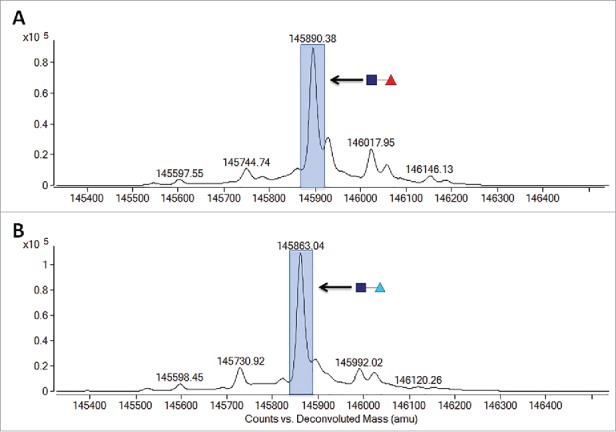
LC-MS spectragraph of Endo H treated mAb-1 expressed in CHO cell culture with different sugar supplements. (A) Glucose-only control. (B) D-arabinose.
The average oligosaccharide results for mAb-1 from all of the variably supplemented arabinose cultures of cell line 1 are shown in Table 1. The results indicate that D-arabinose supplementation also reduced the levels of high mannose N-glycans, especially at the 10 mM condition, with the most notable effect on the Man5 species. Arabinose was also able to increase the levels of G0 type N-glycans. The D-arabinose titration experiment also shows that the effective concentration at which protein arabinosylation begins to be observed is in the 0.1-1 mM range. At higher concentrations than this, the N-glycans were fully arabinosylated, with no appreciable presence of fucose on any of the N-glycans. These results are important because they highlight the ease with which complete removal of fucose can be achieved through this approach. Simple incubation of cells in culture with sufficient quantities of this particular sugar is enough for ∼100% incorporation of arabinose, thus facilitating an approximately equal percentage of fucose-free antibody. This is highly unexpected since most literature reports have shown that when cells in culture are exposed to a particular sugar, or compounds that facilitate an enhanced addition of that sugar, there is typically only a partial incorporation of that sugar on the N-glycans.15
Table 1.
N-glycan Profiles from Arabinose Supplemented Cultures.
| D-Arabinose Conc. (mM) | Man5 (%) | Man6 (%) | G0A- GlcNAc (%) | G0F- GlcNAc (%) | G0(%) | G0A(%) | G0F (%) | G1A (%) | G1F (%) | Fucose Reduction (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line 1 (n = 3) | ||||||||||
| 0 | 2.1+/−0.1 | 1.0+/−0.1 | 0 | 8.6+/−0.1 | 0 | 0 | 83.3+/−0.6 | 0 | 3.6+/−0.1 | 3.2+/−0.1 |
| 0.1 | 1.9+/−0.1 | 1.0+/−0.1 | 0 | 8.5+/−0.1 | 0 | 0 | 82.6+/−0.1 | 0 | 3.4+/−0.0 | 2.8+/−0.1 |
| 1 | 2.7+/−0.1 | 0.3+/−0.5 | 0 | 7.1+/−0.1 | 0.5+/−0.1 | 13.6+/−1.2 | 70.1+/−0.9 | 0.7+/−0.1 | 3.1+/−0.1 | 17.9 +/− 1.0 |
| 10 | 0 | 1.0+/−0.0 | 10.8+/−0.3 | 0 | 3.5+/−0.1 | 79.6+/−0.5 | 0 | 3.2+/−0.1 | 0 | 98.2+/−0.2 |
| 20 | 0 | 1.5+/−0.1 | 13.4+/−0.4 | 0 | 3.5+/−0.1 | 76.3+/−0.4 | 0 | 3.4+/−0.1 | 0 | 98.1+/−0.1 |
| 50 | 0 | 3.6+/−0.1 | 11.7+/−0.3 | 0 | 2.7+/−0.0 | 73.7+/−0.5 | 0 | 6.4+/−0.0 | 0 | 98.1+/−0.2 |
| Cell Line 2 (n = 3) | ||||||||||
| 0 | 2.1+/−0.0 | 0 | 0 | 6.6+/−0.2 | 0 | 0 | 85.0+/−0.3 | 0 | 4.4+/−0.2 | 2.1+/−0.0 |
| 0.1 | 2.1+/−0.0 | 0 | 0 | 6.5+/−0.0 | 0 | 0 | 85.3+/−0.1 | 0 | 4.2+/−0.1 | 2.1+/−0.0 |
| 1 | 2.4+/−0.1 | 0 | 0 | 5.9+/−0.1 | 0 | 0 | 85.1+/−0.3 | 0 | 4.8+/−0.2 | 2.4+/−0.1 |
| 10 | 2.1+/−0.1 | 0.5+/−0.4 | 2.8+/−0.0 | 3.6+/−0.0 | 3.8+/−0.0 | 34.5+/−0.4 | 48.3+/−0.3 | 1.2+/−0.0 | 2.6+/−0.2 | 44.9+/−0.6 |
| 20 | 2.5+/−0.0 | 2.1+/−0.2 | 3.5+/−0.0 | 3.1+/−0.0 | 4.2+/−0.1 | 38.8+/−0.2 | 40.9+/−0.2 | 1.6+/−0.0 | 2.4+/−0.1 | 52.7+/−0.3 |
| 50 | 5.3+/−0.3 | 5.0+/−0.1 | 3.8+/−0.2 | 4.0+/−0.1 | 4.4+/−0.0 | 33.5+/−0.3 | 37.8+/−0.3 | 2.1+/−0.0 | 3.3+/−0.0 | 54.1+/−0.1 |
| Cell Line 3 (n = 3) | ||||||||||
| 0 | 1.8+/−0.1 | 0 | 0 | 6.6+/−0.1 | 0.2+/−0.1 | 0 | 85.0+/−0.6 | 0 | 4.2+/−0.4 | 1.9+/−0.1 |
| 0.1 | 1.6+/−0.1 | 0 | 0 | 6.2+/−0.2 | 0.2+/−0.3 | 0 | 86.7+/−0.9 | 0 | 3.6+/−0.1 | 1.6+/−0.2 |
| 1 | 0 | 0 | 2.8+/−0.5 | 3.6+/−0.0 | 1.2+/−0.0 | 34.2+/−0.2 | 53.7+/−0.6 | 0.6+/−0.0 | 3.2+/−0.1 | 38.8+/−0.7 |
| 10 | 0 | 0 | 7.5+/−0.2 | 0 | 2.8+/−0.0 | 84.8+/−0.6 | 0 | 2.9+/−0.4 | 0 | 98.0+/−0.1 |
| 20 | 0 | 0 | 7.8+/−0.2 | 0 | 2.9+/−0.0 | 84.0+/−0.2 | 0 | 3.3+/−0.2 | 0 | 98.0+/−0.1 |
| 50 | 0 | 0 | 10.4+/−0.3 | 0 | 2.8+/−0.0 | 81.0+/−0.5 | 0 | 3.6+/−0.5 | 0 | 98.7+/−0.0 |
Note: Glycan %'s represent mean +/− 1 SD.
Cell culture harvests from cell line 2 were purified using Protein A, and the DVD-1 samples were analyzed for their N-glycan profiles. The average results (Table 1) indicate D-arabinose supplementation was able to elicit a similar effect of increasing the levels of G0 type N-glycans, as well as significantly replacing fucose with arabinose in a DVD-Ig. The D-arabinose titration experiment also highlights the effective concentration to be slightly higher to that for IgGs, i.e., at between 1 and 10 mM, protein arabinosylation begins to be observed. At higher concentrations than this, the N-glycans reached peak arabinosylation levels, supporting an overall 54% decrease in fucose. Interestingly, DVD-1 reached a limit of arabinosylation that was not complete. This may be due to the unique protein structure of the DVD-Ig molecule.16
To evaluate broader applicability of this method, another antibody (mAb-2) was cultured and purified under similar conditions to mAb-1. Similar to mAb-1, arabinose was able to facilitate increased levels of G0 type N-glycans, as well as completely replace fucose with arabinose. The D-arabinose titration experiment highlights that the effective concentration at which protein arabinosylation takes place is somewhere between 0.1 mM and 1 mM. At concentrations higher than this, the molecules were fully arabinosylated, with no appreciable presence of fucose on any of the N-glycans.
D-arabinose's ability to serve as a replacement for L-fucose is likely attributable to its structural similarity to L-fucose (Fig. 3). The stereochemistry at the C-2 to C-4 position is exactly the same. Due to the chemical similarity of these 2 sugars, it is apparent that the 5 carbon containing arabinose can be used as an alternative substrate for the addition reaction onto the N-glycan as that of the 6 carbon containing fucose. The use of D-arabinose in this regard has not, to our knowledge, been historically exploited. This could be due to the fact that arabinose is more commonly found in nature in its L-form. L-arabinose supplementation into cell culture did not facilitate any detectable levels of protein arabinosylation (results not shown). The structural and functional consequences of protein arabinosylation on the mAbs and DVD-Ig studied in this work are highlighted below.
Cell culture performance with D-Altrose and L-Galactose
Other sugars in nature resemble D-arabinose, including D-altrose (Fig. 3). To investigate whether D-altrose could also be used to replace L-fucose on N-glycans, CHO cell line 1 was cultured in chemically-defined media supplemented with 10 mM of D-altrose. Upon purifying the expressed protein from the culture milieu, LC-MS-MS glycopeptide mapping was used to analyze the oligosaccharide diversity on the principal N-glycosylation site of the molecule (Fig. 8). Upon inspection of the results, and comparison of the relative peak intensities between both arabinosylated mAb-1, and the predominantly fucosylated glycovariant, we found that D-altrose was actually leading to arabinosylation. In this particular case, it is likely that the D-altrose was being broken down (perhaps due to the metabolism of the CHO cell line) into D-arabinose, which was then subsequently added onto the molecule. This experiment hints at a novel means with which to conduct native N-glycan sugar replacement. That is, one may target the use of novel precursor sugars, which are then processed by cells in culture toward a different sugar, which can then be used to elicit the desired native N-glycan sugar replacement effect. Additional studies are needed to identify more sugars that pose this potential.
Figure 8.
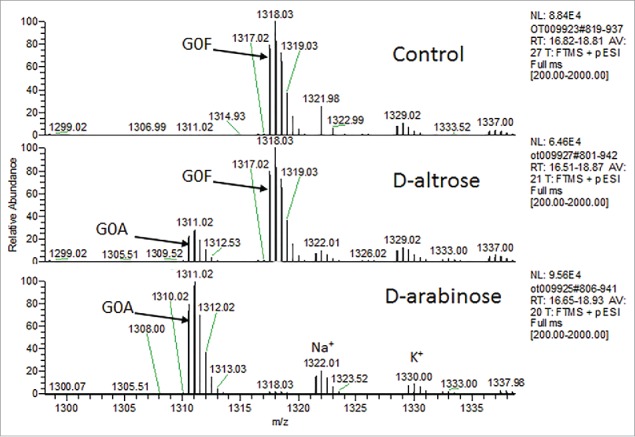
N-glycan glycopeptide mapping of purified mAb-1 expressed from CHO cells supplemented with D-altrose or D-arabinose.
L-galactose also resembles D-arabinose (Fig. 3). To investigate whether L-galactose could also be used to replace L-fucose on N-glycans, variable amounts of L-galactose were supplemented into both the chemically-defined basal and feed medias of CHO cell line 1. Cell culture harvests from these cultures were Protein A purified, and the protein samples were analyzed for their N-glycan profiles via LC-MS of the Lys-C-treated and reduced protein. In all instances, a noticeable shift in mass for all the fucosylated N-glycan species by ∼ +16 Da in the cultures supplemented with L-galactose was observed. This increase in mass was consistent with the difference in molecular weight between that of L-fucose with L-galactose. These results suggest that the N-glycans had fucose replaced with L-galactose. The N-glycan oligosaccharide results from these particular cultures are shown in Table 2.
Table 2.
Average N-glycan Profiles from L-Galactose Supplemented Cultures.
| L-Gal Conc. (mM) | Man5 (%) | Man6 (%) | G0G*- GlcNAc (%) | G0F- GlcNAc (%) | G0(%) | G0G*(%) | G0F (%) | G1G* (%) | G1F (%) | Fucose Reduction (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.3 | 0 | 0 | 5.6 | 0.4 | 0 | 83.3 | 0 | 6.7 | 1.7 |
| 0.1 | 1.9 | 0 | 0 | 6.0 | 0.5 | 13.5 | 70.6 | 1.3 | 5.1 | 17.2 |
| 1 | 2.0 | 0.9 | 4.1 | 4.0 | 0.5 | 37.0 | 43.6 | 2.5 | 3.6 | 47.4 |
| 5 | 2.4 | 0 | 7.8 | 0.7 | 0 | 72.9 | 6.5 | 5.1 | 1.2 | 91 |
| 10 | 2.5 | 0.4 | 8.5 | 0 | 0 | 80.5 | 0 | 5.7 | 0 | 100 |
Note: G* represents galactose in the L-fucose position on the N-glycan.
At a 1 mM concentration of L-galactose, there was almost an equal mixture of L-fucose and L-galactose observed on the product N-glycans. At 10 mM, all of the fucose was completely swapped for L-galactose. This suggests that L-galactose is quite potent for facilitating fucose reduction via enabling the targeted addition of this non-native N-glycan sugar onto recombinant glycoproteins.
Structural & functional characterization of arabinosylated glycoproteins
Different mAb-1, mAb-2, and mAb-3 samples with different spectra of oligosaccharides were subjected to extended structure/function testing to evaluate the effect of the levels of arabinose, fucose, and mannose on key protein attributes. From CHO cell line 1, mAb-1 samples were generated through cell culture media supplementation of D-arabinose, sucrose, as well as a glucose-only experimental control condition. Sucrose (70 mM) was supplemented into both the basal and feed medias to facilitate production of large quantities of high mannose N-glycans,17 to contrast with the reduced levels of high mannose N-glycans facilitated through the use of D-arabinose. A summary of the N-glycoform profile for mAb-1 generated for the purposes of this testing is shown in Table 3. For CHO cell line 3 and CHO cell line 4, testing was performed on samples generated through supplementation of just D-arabinose, and the corresponding glucose-only control.
Table 3.
N-glycan Profiles from Arabinose & Sucrose Supplemented Cultures (n = 3).
| Condition | Man5 (%) | G0A- GlcNAc (%) | G0F- GlcNAc(%) | G0(%) | Man6(%) | G0A(%) | G0F(%) | Man7(%) | G1A(%) | G1F(%) | Man8(%) | G2A(%) | G2F(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.7+/− 0.03 | 0 | 10.2+/− 0.03 | 0 | 0.6+/−0.15 | 0 | 80.6+/−0.27 | 0 | 0 | 4.7+/−0.12 | 0 | 0 | 0.2+/−0.03 |
| +Sucrose | 22.3+/−0.05 | 0 | 16.3+/−0.13 | 0 | 10.0+/−0.27 | 0 | 40.2+/−0.20 | 5.6+/−0.08 | 0 | 3.3+/−0.14 | 2.0 | 0 | 0.4+/−0.02 |
| +D-Arabinose | 2.5+/−0.06 | 5.2+/−0.21 | 0 | 4.0+/−0.08 | 0 | 82.7+/−0.50 | 0 | 0 | 5.2+/−0.15 | 0 | 0 | 0.1+/−0.13 | 0 |
Note: Glycan %'s represent mean +/− 1 SD.
As expected, both D-arabinose and sucrose were very effective at eliciting changes in the protein oligosaccharide profile compared with the unsupplemented control. Arabinose decreased high mannose N-glycan species, increased G0, and facilitated arabinose replacement of fucose. Sucrose had a very significant effect on high mannose type N-glycans, and was evaluated as a control condition for high mannose to be tested alongside the lower mannose samples elicited through the use of arabinose.
Size-exclusion chromatography
The presence of high molecular weight (HMW) species has been shown to affect protein immunogenicity,18 and thus the respective levels are monitored and controlled during production of recombinant glycoprotein therapeutics. Protein glycosylation has been shown to protect against aggregation.19 Purified samples of mAb-1 were subjected to size-exclusion chromatography (SEC) analysis to evaluate if the oligosaccharide impact mediated through either arabinose or sucrose supplementation affected the presence of either HMW or low molecular weight (LMW) species. The results are shown in Table 4.
Table 4.
SEC Results from Arabinose & Sucrose Supplemented Cultures of Cell Line 1.
| Condition | HMW (%) | Monomer (%) | LMW (%) |
|---|---|---|---|
| Control | 1.7 | 98.2 | 0.2 |
| +Sucrose | 1.7 | 98.0 | 0.3 |
| +D-Arabinose | 1.6 | 98.2 | 0.2 |
These results suggest that neither HMW nor LMW species are affected by the cell culture supplementation of either sucrose or arabinose.
Viscosity measurements
Suitable viscosity levels are important for high concentration formulations of proteins, which must deliver sufficient dosages of soluble proteins in small volumes. Purified samples of mAb-1, mAb-2, and DVD-1, were subjected to viscosity measurements after concentrating all to 50 mg/mL in the same formulation buffer (15 mM histidine, pH 6.0). The results are shown in Table 5.
Table 5.
Measured Viscosities From Arabinosylated Glycoproteins.
| Protein | Condition | Absolute Viscosity (cP) |
|---|---|---|
| mAb-1 | Control | 1.15 |
| +D-arabinose | 1.24 | |
| DVD-1 | Control | 1.48 |
| +D-arabinose | 1.48 | |
| mAb-2 | Control | 1.30 |
| +D-arabinose | 1.32 |
There was relatively little change in viscosity observed for mAb samples, and no change in the DVD-1 sample, in comparison to their arabinosylated counterparts. The cumulative results suggest that arabinosylation of glycoproteins does not adversely affect the viscosity of protein formulations.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a common laboratory technique for investigating higher order structure of macromolecules such as proteins. Understanding the role of N-glycans in higher order structure is important because oligosaccharides have been shown to affect the higher order structure20 and thermal stability of the proteins to which they are attached.21 Fig. 9 highlights the DSC thermal scans of the 4 CHO expressed glycoproteins, both with and without D-arabinose supplementation to the cell culture media. The results indicate that there is no difference in the thermal stability of the glycoprotein samples that were arabinosylated compared with the control samples. ΔHTotal was in agreement by 96.1% for mAb-1, 95.9% for mAb-2, and 97.0% for mAb-3, with all deconvolution Tm's less than 1°C different. Deconvolution of the scan for the arabinose processed DVD-1 showed only a nominal 9% difference in ΔHTotal between the control and arabinosylated samples, and one transition difference greater than Δ1°C. All data analyzed demonstrate arabinose supplementation does not affect the overall thermal stability of the proteins studied.
Figure 9.
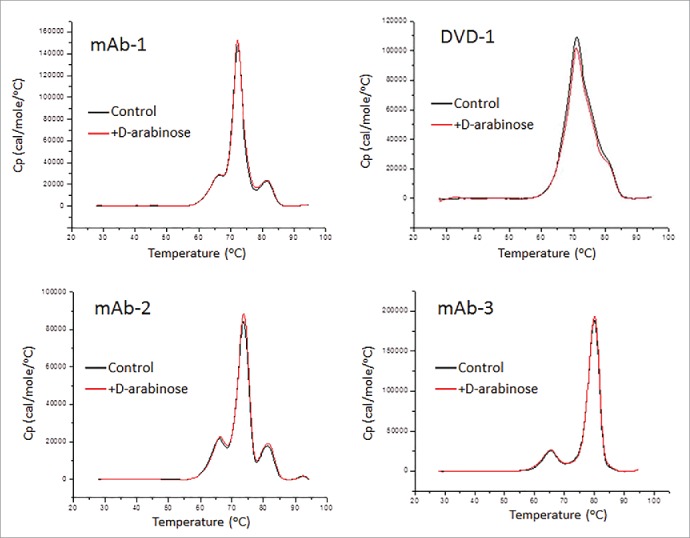
DSC thermal scans of 4 CHO expressed glycoproteins both with and without D-arabinose supplementation to the cell culture media.
Dendritic cell uptake
Dendritic cells are potent, professional antigen-presenting cells that play a critical role toward both the innate and adaptive immune responses. Uptake of antigens by dendritic cells is important for the activation and initiation of immunogenic responses. A specific type of dendritic cells, inflammatory dendritic cells, are present during inflammation.22 Thus, any antigen capable of enhancing or reducing dendritic cell uptake in vitro may potentially be correlated with the activation or attenuation of their immune response toward the antigen in vivo. In previous reports, high levels of mannose receptors have been shown on the surface of dendritic cells.23,24 To evaluate whether the levels of high mannose N-glycans could be correlated with the uptake of recombinant glycoproteins, purified mAb-1 was evaluated in a dendritic cell uptake assay (Fig. 10).
Figure 10.
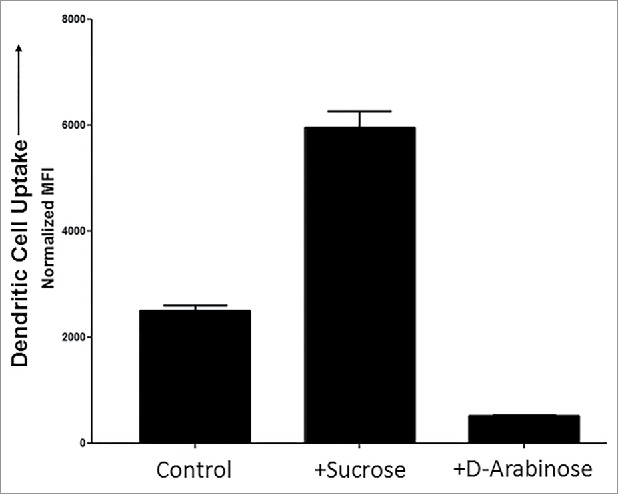
Dendritic cell uptake of purified mAb-1 expressed in CHO cell culture with different sugar supplements.
The results suggest that the highly mannosylated sample (generated by sucrose in the culture media) is correlated with an elevated dendritic cell uptake. Thus, this material would be anticipated to elevate dendritic cell antigen display activity, including any associated immunogenic and inflammatory responses. The arabinosylated sample (generated by arabinose in the culture media) facilitated a lower level of high mannose type N-glycans species and exhibited significantly lower uptake by dendritic cells compared with the control. This material thus would be anticipated to reduce dendritic cell antigen display activity, including any associated attenuation of immunogenic and inflammatory responses. The results indicate that the control of protein mannosylation levels may provide a potential means to improve the therapeutic profile, thus decreasing the mannose levels, and may enable the manufacturing of a potentially more efficacious therapeutic immunoglobulin, with perhaps less off-target cellular uptake.
FcγR/FcRn binding
Numerous therapeutic proteins are designed to bind to antigens on cell surfaces and then kill the targeted cells through ADCC, complement-dependent cytotoxicity (CDC), and direct apoptosis. Fcγ receptors serve a variety of in vivo immune functions and are needed for therapeutic proteins to elicit their desired effect. FcγRIIIa (CD16) is the receptor on NK effector cells that antibodies bind to through their Fc region. Upon binding antigen, the same antibodies are then able to bind NK cells and elicit ADCC activity. Studies have shown that both target binding and the effector interactions of antibodies are important for ADCC activity,25 and that the docking between an antibody and NK effector cells is mediated through the carbohydrate interactions associated with both.26 Thus, changing the protein glycosylation profile of recombinant protein therapeutics, especially fucose levels, is anticipated to affect the resulting ADCC activity, as has been reported in the literature.9,10 Purified mAb-1 was evaluated via Biacore for FcγR binding, and the results are shown in Table 6.
Table 6.
FcγR Binding of mAb-1 from Arabinose and Sucrose Supplemented Cell Cultures.
| Sample | huFcγRIIb KD(x10−6 M) | huFcγRIIa131H KD(x10−6 M) | huFcγRIIa131R KD(x10−6 M) | huFcγRIIIa158F KD(x10−7 M) | huFcγRIIIa158V KD(x10−7 M) |
|---|---|---|---|---|---|
| Control | 5.1 | 2.2 | 5.5 | 8.9 | 1.3 |
| 6.7 | 2.5 | N/A | 8.8 | 1.3 | |
| +Sucrose | 9.9 | 3.9 | 5.5 | 1.3 | 0.6 |
| 10.5 | 3.8 | 6.3 | 1.4 | 0.6 | |
| +D-Arabinose | 6.2 | 2.5 | 5.5 | 5.9 | 0.6 |
| 6.4 | 2.6 | 5.7 | 6.2 | 0.6 |
Compared to the glucose-only control culture, the results suggest that mAb-1 purified from the sucrose supplemented culture demonstrated a nominal decrease in binding to FcγRIIb as well as FcγRIIa131H, with no substantial effect on FcγRIIa131R. The binding to both FcγRIIIa158F and FcγRIIIa158V increased nominally. Compared to the glucose-only control culture, the material purified from the arabinose supplemented cell culture demonstrated negligible impacts toward FcγRIIb, FcγRIIa131H, and FcγRIIa131R binding. The binding to FcγRIIIa158V increased, albeit by a nominal amount. Fucose levels have been shown to have an inverse relationship toward both FcγRIIIa binding and ADCC activity.9 Although in vitro binding results can be predictive of in vivo activity, how ADCC activity might be affected cannot be determined from this data set. The potential capability of arabinosylated N-glycans to provide beneficial improvements to ADCC activity is highlighted in a later section.
The Fc neonatal receptor (FcRn) facilitates transport of IgG from mother to fetus, and it has also been implicated in IgG turnover.27 Purified mAb-1 was evaluated via Biacore for FcRn binding, and the results shown in Table 7.
Table 7.
FcRn Binding of mAb-1 from Arabinose and Sucrose Supplemented Cell Cultures.
| Sample | huFcRn KD(x10−6 M) |
|---|---|
| Control | 7.1 |
| 7.2 | |
| + Sucrose | 7.4 |
| 7.6 | |
| + D-Arabinose | 7.3 |
| 7.3 |
The results suggest that the material purified from the sucrose and arabinose supplemented cell cultures were not grossly different with respect to FcRn binding at pH 6.
Antigen binding
MAb-1 is a neutralizing antibody that binds to its target antigen with strong affinity. To further characterize the functional effect of the oligosaccharide changes toward the antibody, antigen binding assays were performed via Biacore. The results from this study are shown in Table 8.
Table 8.
Antigen Binding of mAb-1 from Arabinose and Sucrose Supplemented Cell Cultures (n = 3).
| Sample | Antigen BindingKD (x10−11 M)a |
|---|---|
| Control | 8.14 +/− 0.34 |
| + Sucrose | 8.15 +/− 0.41 |
| + D-Arabinose | 7.96 +/− 0.29 |
Mean +/− 1 SD
The results suggest that the various oligosaccharide changes incurred through cell culture supplementation of sucrose or arabinose do not affect antigen binding. These glycomodulated antibodies thus can be considered similar to each other with respect to antigen binding.
Cytokine release
Cytokines are a broad class of proteins produced by immune cells, as well as non-immune cells such as fibroblasts. These proteins serve numerous functions, including humoral and cell-based immune response, and frequently modulate other target cells, or the cells that secrete the cytokines. Many cytokines are categorized as pro-inflammatory cytokines for their role associated with inflammation. Thus, monitoring the levels of these particular cytokines upon exposure to a specific compound provides an indication of the resulting levels of inflammatory responses associated with this exposure. In this study, a series of inflammatory cytokines were monitored upon administration of various glycomodulated mAb-1 samples to human peripheral blood mononuclear cells (PBMC). The results using PBMC from 3 different donors are shown in Table 9. Results were considered positive when the measured cytokine levels exceeded 2 times the levels elicited with the negative control.
Table 9.
Cytokine Release Elicited with mAb-1 Purified from Arabinose and Sucrose Supplemented Cell Cultures.
| Cytokines Measured (pg/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| Sample | IL-1β | IFNγ | IL-2 | IL-6 | IL-8 | TNFα | |
| PBMC Source: Donor1 | |||||||
| Glucose-only Control | 8 | 2 | 16 | 41 | 12056 | 36 | |
| 70 mM Sucrose | 10 | 4 | 14 | 47 | 23594 | 60 | |
| 15 mM D-Arabinose | 15 | 3 | N/A | 40 | 19198 | 65 | |
| Negative Control | 12 | 6 | 12 | 48 | 23172 | 223 | |
| PBMC Source: Donor 2 | |||||||
| Glucose-only Control | 19 | 3 | 19 | 37 | 15886 | 68 | |
| 70 mM Sucrose | 29 | 4 | 17 | 44 | 20315 | 96 | |
| 15 mM D-Arabinose | 23 | 4 | 15 | 27 | 16726 | 104 | |
| Negative Control | 24 | 9 | 69 | 38 | 22405 | 232 | |
| PBMC Source: Donor 3 | |||||||
| Glucose-only Control | 10 | 12 | N/A | 25 | 6726 | 57 | |
| 70 mM Sucrose | 15 | 27 | 23 | 70 | 14554 | 89 | |
| 15 mM D-Arabinose | 15 | 17 | N/A | 37 | 6949 | 93 | |
| Negative Control | 15 | 26 | 13 | 39 | 20334 | 305 | |
Note: Negative control, trastuzumab.
With the purified mAb-1 protein from the sucrose and D-arabinose supplemented cultures, there were no overtly negative effects on cytokines (i.e., increase in cytokine release) compared with a control antibody (trastuzumab). Additionally, the cytokine release induced by the test article antibody samples remained similar to the glucose-only control condition.
Complement activation
The complement system, which is part of the innate immune system, comprises a series of proteins that are activated by a variety of triggers, including the classical, alternative, and lectin complement pathways. Upon activation, proteases in these pathways degrade pathway-specific proteins, which leads to further molecular events that cascade and amplify, ultimately leading to cell lysis or opsonization of foreign cells, as well as other immune defensive responses.28,29
We investigated whether glycomodulated mAb-1 generated through arabinose or sucrose cell culture media supplementation facilitated any change toward human complement activation. The results from experiments using serum from 2 different donors (Fig. 11) show that complement activation elicited through either sucrose or arabinose cell culture supplementation was not higher than the unsupplemented, glucose-only, experimental control. Thus, it can be concluded that there was no adverse impact to complement activation as a result of either of these glycomodulation techniques. This is a notable result since previous studies using highly afucosylated antibodies elicited a reduced degree of complement activation.30,31
Figure 11.
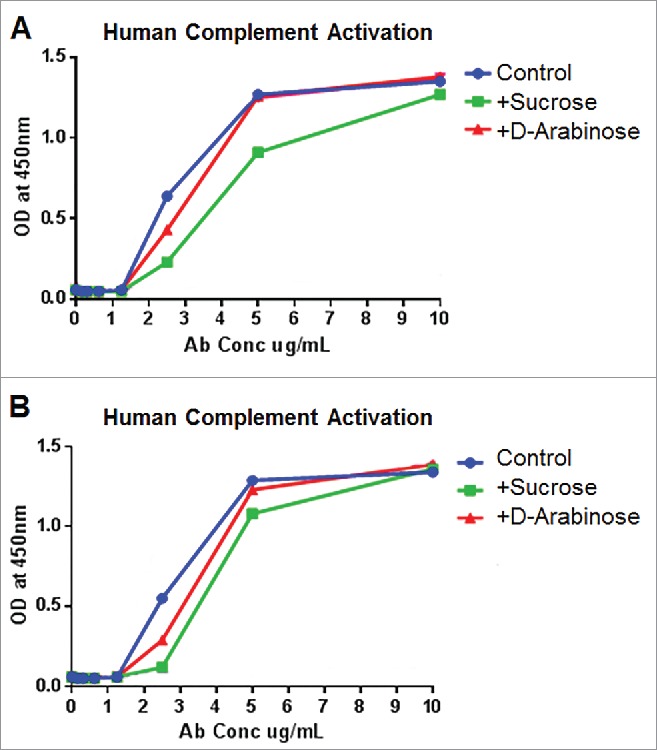
Complement activation elicited by purified mAb-1 expressed in CHO cell culture with different sugar supplements. (A) Response from cells from human donor 1. (B) Response from cells from human donor 2.
Rodent pharmacokinetics
Studies to evaluate the effect of arabinosylation on the PK of mAb-1 were conducted in Sprague-Dawley rats. Results were compared with mAb-1 purified from unsupplemented control culture conditions, and are shown in Fig. 12 and Table 10.
Figure 12.
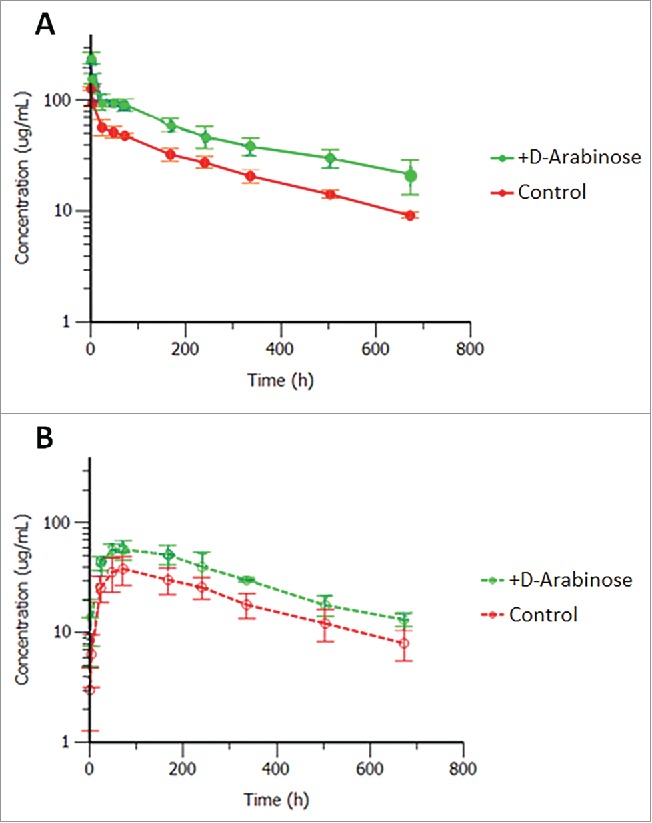
Mean ( ± SD) rat serum concentrations versus time after a single 5 mg/kg (A) intravenous or (B) subcutaneous administration of control and arabinosylated mAb-1 in Sprague Dawley rats; n = 5 rats per group (3 rats for arabinosylated mAb-1 in SC group).
Table 10.
Mean PK parameters after a single 5 mg/kg intravenous and subcutaneous administration of control and arabinosylated mAb-1 in Sprague Dawley rats.
| Intravenousa |
Subcutaneousa |
|||||
|---|---|---|---|---|---|---|
| Sample | T1/2b (day) | CL (mL/h/kg) | Vss(mL/kg) | T1/2b (day) | Cmax (μg/mL) | F (%) |
| Control (n = 5) | 11.8 +/− 2.0 | 0.23 +/− 0.01 | 88.8 +/− 11.6 | 11.7 +/− 1.4 | 41.1 +/− 8.7 | 78.6 +/− 21.4 |
| + D-Arabinose (n = 5 IV, n = 3 SC) | 14.7 +/− 1.5 | 0.11 +/− 0.02 | 55.1 +/− 10.6 | 11.1 | 58.9 +/− 9.7 | 58.3 +/− 6.6 |
All numbers represent mean +/− 1 SD
Harmonic mean
Both the control and arabinosylated mAb-1 upon intravenous (IV) administration displayed low clearances, small volumes of distributions and long half-lives. Interestingly the arabinosylated form exhibited a somewhat reduced volume of distribution and clearance, but not a significantly different half-life, compared with the control. Upon subcutaneous (SC) administration the arabinosylated antibody had higher Cmax and average serum concentrations than the control antibody indicating good absorption, but lower overall bioavailability due to the still higher exposure upon IV dosing. The reason or the effect of this potential difference in tissue penetration between the 2 forms is unknown at this time and may warrant further investigation. Two of the 5 animals that were administered the arabinosylated antibody SC displayed rapid antibody clearance starting around 14 days, and were therefore eliminated from the PK parameter calculations due to the apparent onset of an anti-drug antibody response (not evaluated). There was no apparent onset of an anti-drug antibody response in the IV administered control or arabinosylated mAb-1 in Sprague Dawley rats. The appearance of an immunogenic response to a human antibody dosed in rodents is not considered predictive of immunogenicity in humans.
ADCC and FcγRIIIa signaling
The cumulative effect on N-glycosylation through media supplementation with D-arabinose could be very significant due to modulation of the particular sugars, which have been shown in the literature to be important for immunogenicity, PK, and ADCC. Decreasing the levels of mannose is potentially beneficial because the high mannose forms exhibit potentially increased immunogenicity and shorter systemic half-life.5,6 Lowering fucose levels could be beneficial because fucose removal is positively correlated with an increase in ADCC activity.9,10 Collectively, changes in the glycosylation profile caused by arabinose might impact multiple attributes that are associated with improvements in the therapeutic profile of antibodies.
mAb-2, an IgG1 antibody with potential relevance in oncology, has ADCC as a principal component of its mechanism of action. Antibody expressed by CHO cells with a high degree of arabinosylation was purified and analyzed in a cell-based ADCC assay (Fig. 13). The results suggest a very similar behavior between both the higher affinity and lower affinity allele of FcγRIIIa evaluated as part of the assay. mAb-2 generated from the cultures supplemented with more than 0.1 mM arabinose exhibited significantly increased ADCC activity under the conditions tested, with the material containing the higher proportion of arabinosylation showing the largest increases in ADCC activity. These results are consistent with what one would expect through the enhancement of FcγRIIIa binding. In previous reports, the removal of fucose has been correlated with an increase in ADCC activity, but, in the present case, fucose was replaced with a different, and smaller, sugar. Our results suggest that the complete removal of fucose may not be necessary to elicit an increase in ADCC activity. Simply reducing the size by removing a methyl group from fucose (i.e., converting to arabinose) is sufficient to elicit higher ADCC. This result is surprising and unprecedented in the established literature. Detailed crystallography studies by other researchers comparing fucosylated and afucosylated antibody samples has demonstrated the existence of a direct, steric inhibition caused by fucose in the carbohydrate-mediated interaction between the Fc glycan and FcγRIIIa.26 It was further shown how the introduction of an increased number of newly formed hydrogen bonds and van der Waal's contacts facilitated the higher binding affinity introduced as a result of afucosylation. In our study, it is likely that some of these same molecular events are also occurring as a result of protein arabinosylation, and further experiments are required to determine the specific interactions.
Figure 13.
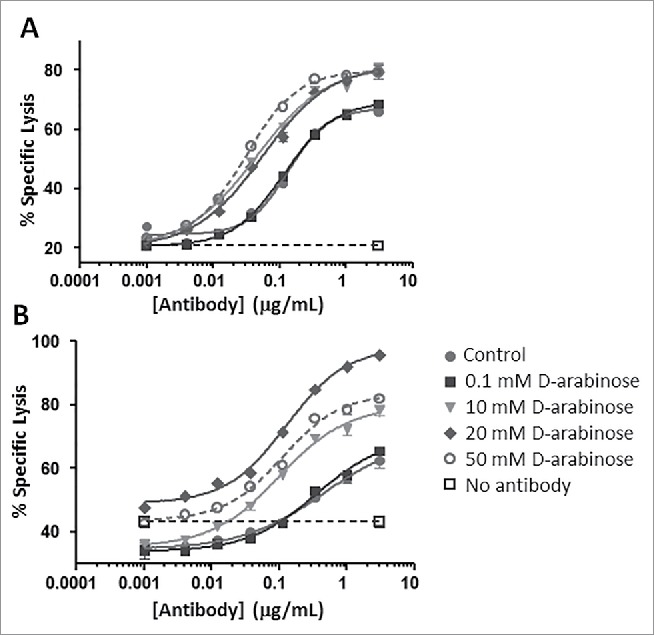
ADCC measurements of arabinosylated mAb-2 vs. predominantly fucosylated control mAb-2. (A) V158 higher affinity allele of FcγRIIIa (B) F158 lower affinity allele of FcγRIIIa.
mAb-3, a human IgG1 generated for an immunology indication, was also purified from arabinose supplemented and control cultures and analyzed for FcγRIIIa (CD16) signaling in a reporter assay (Fig. 14). An elevated signal from the assay would suggest stronger binding of FcγRIIIa, and would reinforce the previous results that the functionality of glycoproteins whose mechanism of action relies on FcγRIIIa binding (e.g., ADCC) could be enhanced through arabinosylation. Arabinosylated mAb-3 and predominantly fucosylated mAb-3 (control) were purified and compared with reference mAb-1 samples that were predominantly fucosylated, as well as afucosylated through the addition of kifunensine, a small molecule inhibitor of Man I enzyme that arrests glycan maturation at the high mannose, afucosylated N-glycan stage.32 The reporter assay results from both the high and low affinity allele of FcγRIIIa suggest that the arabinosylated mAb-3 significantly enhanced binding of FcγRIIIa. The results also suggest that signaling through FcγRIIIa from the arabinosylated mAb3 approximates that of the afucosylated mAb-1. One can reasonably make the argument that protein arabinosylation can yield an enhancement in signaling through FcγRIIIa that is comparable to the response seen with the complete absence of fucose.
Figure 14.
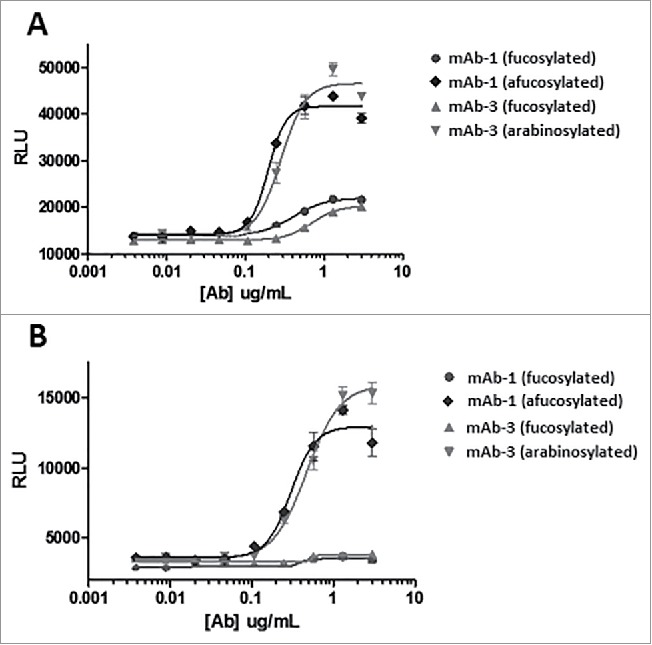
CD16 reporter assay of predominantly fucosylated control mAb-3 vs. predominantly arabinosylated mAb-3. (A) V158 higher affinity allele of FcγRIIIa (B) F158 lower affinity allele of FcγRIIIa.
Discussion
The use of pentose sugars, especially arabinose, as a supplement for cells in culture is not without precedent. Indeed, L-arabinose has been supplemented into bacterial cell cultures for the ara operon for decades,33 and in Saccharomyces cerevisiae cultures.34 The addition of L-arabinose into protein oligosaccharides such as arabinogalactan structures, which coat the surface of mycobacteria, is also well known.35 Our work is novel because it shows that supplementation of D-arabinose into the culture media was effective in re-distributing the protein glycosylation profiles in mammalian cells. The shifting of protein glycosylation profiles was demonstrated to provide numerous beneficial changes in the function of human immunoglobulin molecules. D-arabinose was shown to lead to a reduction in high mannose type N-glycans for some glycoproteins, an increase in G0 type N-glycans, and a substantial incorporation of arabinose, resulting in a predominant reduction, or the complete abrogation of N-glycan fucosylation. Through the use of multiple, recombinant CHO cell lines, we demonstrated that purified, arabinosylated recombinant glycoproteins provided particular functional benefits for both immunology- and oncology-relevant proteins, including a significant reduction in dendritic cell uptake, an increase in FcγRIIIa binding and signaling, as well as a significant increase in ADCC activity. In rodent PK studies, there was no significant difference in half-life; however, there appeared to be a difference in clearance and volumes of distribution between the control and arabinosylated forms of mAb-1, which requires further study. Among the structural, functional, and in vivo tests performed on these glycoproteins, there was no adverse impact identified as a result of replacing fucose with arabinose on product N-glycans. Protein arabinosylation was also confirmed to be possible for both mAbs, as well as bispecific DVD-Ig molecules, suggesting its utility across multiple types of protein modalities.
The capability of mammalian cells to utilize D-arabinose as a replacement sugar for L-fucose is an important observation and provides an additional tool for glycomodulation. It also highlights exactly which carbon positions on a sugar are principally important for the ability of the FucT enzyme, as well as the associated nucleotide-sugar biosynthetic enzymes, to recognize D-arabinose as a substrate. In this regard, it is apparent that carbon positions 1, 2, 3, and 4 are principally important for these corresponding enzymatic reactions to proceed. It is reasonable to presume that other sugars that preserve the stereochemistry at these positions will behave similarly to the D-arabinose, L-altrose, L-galactose examples presented in this work.
Although not specifically measured, it is likely that arabinose is forming a guanosine diphosphate (GDP)-arabinose nucleotide-sugar inside the CHO cells evaluated, since the default substrate for FucT is a sugar conjugated with GDP. The presence of GDP-arabinose is not without precedent, and, in fact, it has been detected previously in Leishmania major,36 as well as additional cell types. Fucose is unique among the sugars that comprise a typical N-glycan in the sense that it is an L-sugar, whereas the others are D-sugars. Arabinose is also more typically found in nature in its L-form. Here, we demonstrated that the D form of arabinose was effective at eliciting changes in the protein glycosylation profile, whereas the L form was not (results not shown).
The use of D-arabinose as a novel substrate for the FucT enzyme for addition onto N-glycans is not obvious since protein glycosylation enzymes in mammalian cells generally exhibit strong specificity for both the nucleotide-sugar donor and the protein acceptor substrates.37 We have highlighted here how this is not necessarily always true when it comes to the enzymatic activity of the FucT enzyme, which is capable of recognizing both its native 6-carbon, GDP-fucose substrate and the smaller 5-carbon, arabinose substrate. Fig. 2 highlights the efficiency of the salvage pathway (i.e., fewer enzymatic steps) for the incorporation of fucose (and arabinose), compared with the de novo pathway starting with glucose metabolism. In all instances of D-arabinose supplementation, there were still g/L levels of glucose in the culture media, and it can be inferred that the de novo pathway for GDP-fucose formation should still be active. It is not clear if the predicted GDP-arabinose formation happens to be a more preferred substrate for the FucT enzyme compared with GDP-fucose, or the local concentration of GDP-arabinose is simply higher than that of GDP-fucose. However, what is clear is that in all instances when observing protein arabinosylation in CHO cells, the FucT enzyme is much busier contending with arabinose addition as compared with fucose addition. This competitive inhibition of FucT activity by arabinose through the salvage pathway is novel, specific, and quite powerful for glycomodulation.
The use of D-arabinose is also suitable for manufacturing under ‘good manufacturing practice’ (GMP) conditions. Since this sugar is commercially available from multiple sources with transparent supply chains, the ability to incorporate this sugar as a raw material is relatively straight-forward. Thus, it might be possible through one simple raw material introduction to manufacture drug substance at the multi-thousand liter scale, with a significantly altered glycan profile and anticipated improvement in biologic activity. No cell line engineering or cell line adaptation toward this particular sugar was required, and no expensive sugar variants were needed; thus, this approach can be incorporated into the development of any protein biologic in a relatively straight-forward manner. In other studies performed in the presence of hydrolysates in the culture media, these less-defined media formulations still enabled protein arabinosylation to proceed at significantly high levels (results not shown).
Five principal sugars comprise the native N-glycan code in mammalian cells. Researchers have highlighted how the pattern of native N-glycan sugars can be changed or abrogated through various culture perturbations,2 and highlighted how variants of native N-glycan sugars can be functionalized with reactive groups for subsequent chemistry purposes.38 In our work, the assortment of native N-glycan sugars wasn't just changed, but new, non-native sugars were added onto product N-glycans. The preclinical and clinical validation of therapeutic glycoproteins that display these sugar-swapped N-glycan moieties will be of ongoing interest and will need to be investigated further. Similar to other reports where there was an expansion of the amino acid code for proteins,39 our work highlights how adding arabinose onto N-glycans truly represents an expansion of the N-glycan code in mammalian cells in culture. The work also represents an expansion of the cell culture toolbox for the production of glycoproteins with not just a well-defined and well-controlled target product profile, but potentially an optimal one for particular glycoproteins.
Materials and methods
Cell culture
Various CHO cell lines expressing recombinant glycoproteins were evaluated in shaker flask cultures. All cultures used the same chemically defined basal media, and chemically defined feed media, both of which contained D-glucose as the primary sugar. Each of the experimental conditions were supplemented with a variety of different sugars, including D-arabinose, D-altrose, L-galactose, or sucrose (Sigma Aldrich, St. Louis, MO) to evaluate their potential effect on the resulting N-glycan oligosaccharide profile. In preparation of the cultures, the cell lines were expanded through separate seed train inoculums to generate enough cell biomass for inoculation of multiple cultures. Process conditions used during the cultures were similar between each experimental condition and the respective non-novel sugar supplemented control condition (Table 11).
Table 11.
Summary of cell culture process conditions and sugar supplementation details.
| Variable | Cell Line 1 | Cell Line 2 | Cell Line 3 | Cell Line 4 |
|---|---|---|---|---|
| Cell Line Type | CHO | CHO | CHO | CHO |
| Expressed Protein | mAb-1 | DVD-1 | mAb-2 | mAb-3 |
| Isotype | IgG1 | IgG1 | IgG1 | IgG1 |
| Culture Vessel | Shaker Flasks | Shaker Flasks | Shaker Flasks | Shaker Flasks |
| Culture Mode | Fedbatch | Fedbatch | Fedbatch | Fedbatch |
| Initial Culture Temperature (°C) | 36 | 36 | 36 | 36 |
| Supplemented Sugars | D-arabinose | D-arabinose | D-arabinose | D-arabinose |
| L-galactose | ||||
| D-altrose | ||||
| Sucrose | ||||
| Sugar Concentrations (mM)a | 0.1,1,10,15,20,50 | 0.1,1,10,20,50 | 0.1,1,10,20,50 | 30 |
| 0.1,1,5,10 | ||||
| Ten | ||||
| 70 |
VCD and cell viability values were measured through trypan blue exclusion via Cedex automated cell counters (Roche Applied Science, Indianapolis, IN), glucose and lactate values were measured with a ABL-805 (Radiometer Medical, Denmark) blood gas analyzer. Media pH measurements were also performed with an ABL-805 (Radiometer Medical, Denmark) blood gas analyzer. Media osmolality was measured on a Multi-Osmette 2430 osmometer (Precision Systems, Natick, MA).
Protein A affinity chromatography
Antibody titers were measured from clarified cell culture harvests on a Poros A™ (Life Technologies, Carlsbad, CA) affinity column using an HPLC system operating with a low pH, step elution gradient with detection at 280 nm. Absolute concentrations were assigned with respect to reference standard calibration curves. Purified antibodies subjected to additional analytical characterization were purified using MabSelect™ Protein A (GE Healthcare Lifesciences, Pittsburgh, PA) using a low pH, step elution gradient, followed by buffer exchange using Spin Concentrator X UF columns (Corning Lifesciences, Tewksbury, MA), or equivalent, according to the manufacturer's recommended procedure.
N-glycan oligosaccharide profiling-LC/MS
The heavy chain of protein samples were analyzed using an HPLC (Agilent 1260, Santa Clara, CA) with a C4 reversed phase column (W.R. Grace, Columbia, MD) coupled to a Q-TOF mass spectrometer (Agilent 6510, Santa Clara, CA). Protein A purified samples from cell culture harvests were first reduced using dithiothreitol (DTT) at 37°C for 30 minutes. The samples were then loaded using 95% mobile phase A (0.08% formic acid and 0.02% TFA in water) and 5% mobile phase B (0.08% formic acid and 0.02% TFA in acetonitrile), and then eluted using a gradient of increasing levels of mobile phase B. The flow rate was 50 μL/min and the column temperature was set to 60°C. The mass spectrometer was operated in positive ion mode with an ion spray voltage of 4500 V and a source temperature of 350°C. Resolved peaks centered on the known masses of different N-glycans were subsequently used to identify the different types of N-glycans, and the relative peak heights were used to quantitate their respective levels.
N-glycan glycopeptide mapping-LC/MS/MS
Protein samples were denatured with 6 M guanidine-HCl at pH 8.0. The proteins were reduced with 10 mM DTT for 30 minutes at 37°C. The proteins were alkylated with 25 mM iodoacetic acid for 30 minutes at 37°C in the dark. The proteins were buffer exchanged to 10 mM tris pH 8.0 using NAP-5 columns. The proteins were digested with trypsin (1:20 trypsin:antibody) for 4 hours at 37°C. The digest was quenched by adding 5 µL of formic acid. Peptide mapping was performed by LC/MS/MS using a Waters (Milford, MA) Acuity UPLC coupled to a Thermo (ThermoFisher Scientific, Grand Island, NY) Orbitrap Velos. The summed spectrum was extracted for the chromatographic region where all the glycopeptides eluted. The relative peak intensities of all the glycovariants were determined as a percent of the total.
Rodent PK study
Studies were conducted in accordance with the AbbVie Institutional Animal Care and Use Committee guidelines. Sprague Dawley male rats (n = 5 per group/dose route) were purchased from Charles River Laboratories (Kingston, NY) and were acclimated for at least 2 d before receiving antibody by single SC injection or slow IV bolus dose injection (via a jugular vein catheter). Blood samples were collected from the lateral tail vein at 0.25, 4, and 24 hours, and then 2, 3, 7, 10, 14, 21, and 28 d post dose, and processed for serum. All samples were stored at −80°C until analysis. Dosed antibody serum concentrations were measured using a GYROS ligand binding method using biotinylated human antigen as the capture reagent (prepared in house), and Alexa Fluor 647 goat, anti-human IgG for detection (Catalog# R32AJ-1, Meso Scale Discovery, Rockville, MD). Serum concentrations were calculated relative to calibration curves using XLfit4 software with a 4-parameter logistic model for curve fitting. PK parameters were calculated with Phoenix™ WinNonlin®, Version 6.3 (Pharsight Corporation, St. Louis, MO) by non-compartmental analysis.
Dendritic cell uptake
Peripheral blood mononuclear cells (PBMC) were isolated from leukopacks of healthy donors by density gradient centrifugation over Ficoll-Paque (GE Healthcare Lifesciences, Pittsburgh, PA). Monocytes were isolated by magnetic sorting using CD14 microbeads (Miltenyi Biotec, San Diego, CA). Monocytes were cultured in RPMI1640 medium supplemented with 2 mM L-glutamine, 100 ng/mL of recombinant human GM-CSF (in-house prepared), 5 ng/mL of human IL-4 (Peprotech, Rocky Hill, NJ), 100 μg/mL penicillin and streptomycin, and 10% fetal bovine serum (Catalog# SH30070.03, GE Healthcare Lifesciences, Pittsburgh, PA) at a density of 1 × 106 cells/mL at 37°C with 5% CO2 for 5 d. Dendritic cells were generated by culturing monocytes in RPMI1640 medium supplemented with 100ng/mL of recombinant human GM-CSF and 5 ng/mL of human IL-4 (Peprotech, Rocky Hill, NJ) for 4 d. Dendritic cells were blocked with human IgG (Catalog# 14506, Sigma Aldrich, St. Louis, MO), stained with pHrodo red (Thermo Fisher, Grand Island, NY) labeled mAb-1 on ice, and incubated at 37°C. A mAb-1control purified from a glucose-only cell culture was used as a basis of comparison. All experimental samples were conjugated with pHrodo red using an antibody labeling kit (Thermo Fisher, Grand Island, NY) according to the manufacturer's protocol. Samples were analyzed on a Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and analysis was performed using Flowjo software (TreeStar Inc., Ashland, OR).
Antigen binding
A Biacore T100 (GE Healthcare Bio-Sciences, Pittsburgh, PA) was used to evaluate differences in antigen binding between control and experimental samples. Approximately 5000 RU of polyclonal, goat anti-human Fc IgG (Catalog#31125, ThermoFisher Scientific, Grand Island, NY) was immobilized on the flowcells of the biosensor chip. Purified samples from cell culture harvest were diluted as needed in HBS-EP buffer (GE Healthcare Lifesciences, Pittsburgh, PA) to approximately the same concentrations. The samples were then captured on flow cells at a flowrate of 10 μL/min for 60s. Dissociation was then monitored for 900s. Regeneration was performed after each run. Purified cell culture samples were compared with mAb-1 reference standard. All samples were assayed in triplicate.
Size exclusion chromatography
Protein A-purified antibody samples from cell line 1 were diluted when necessary to 0.5–5 mg/mL in 1X phosphate-buffered saline (PBS), and measured on a TSKgel G3000SWXL column (Tosoh Bioscience, South San Francisco, CA) using an isocratic gradient on an HPLC with detection at 280 nm. HMW, monomer, and LMW species were assigned and subsequently quantitated.
Viscosity measurement
Taylor dispersion analysis (TDA) was performed with the Viscosizer200 (Malvern, Westborough, MA) to determine sample viscosities. Ten µL of samples were injected into a cellulose-coated capillary under a continuous flow of buffer (15 mM histidine pH 6.0) at 2000 mBar of pressure. The same formulation buffer was used as the running buffer. Two detector heads were positioned in the capillary system and temperature was controlled at 25°C. The capillary was washed between experiments with buffer at 2000 mBar of pressure as per the manufacturer's protocol. The UV detection wavelength used was 280 nm. The specific viscosity for the sample solution was calculated using Poiseuille's Law and relied upon the accurate timing of UV absorbance migrating past 2 separate points within the capillary tube. Both the total length of the capillary and distance between the 2 observation points were known. The specific viscosities of protein formulations were determined by comparing the migration of a known sample against that of a reference buffer spiked with caffeine.
Differential scanning calorimetry
DSC was performed using an automated cap DSC instrument from Microcal (Northampton, MA). The thermal scans were obtained from 25°C to 95°C at a scan rate of 60°C/hr. A corresponding buffer scan was taken immediately after for deconvolution of the various melting profiles. All samples were analyzed at a concentration of 1 mg/mL in 15 mM histidine, pH 6.0. The difference in onset Tm's observed was less than 2°C between repeat scans.
Cytokine release assay
96-well propylene plates were coated with 1 μg of purified protein from experimental samples in 60 μL of DPBS per well for 1.5 hrs at room temperature. After rinsing, 1 × 105 human peripheral blood mononuclear cells (PBMC) were added and incubated for ∼2 d at 37°C. Well plate supernatants were then assayed for IL-1β, IL-6, IL-8, IL-2, TNFα, and IFNγ using MSD (Meso Scale Discovery, Rockville, MD) technology. Values were measured using a Sector Imager 6000 reader (Meso Scale Discovery, Rockville, MD). Experimental samples at 1 μg were compared with both experimental and negative controls. A cytokine response was considered positive when the levels were greater than twice the level released from the negative control (clinical grade trastuzumab).
Complement activation
96-well EIA (Corning, Corning, NY) ELISA plates were coated with various concentrations of purified antibody protein from the experimental samples. Plate wells were then incubated with human serum from healthy donors as a source of complement for 1 hr at 37°C. Complement activation was determined by measuring the levels of human iC3 bound to the plate using mouse, anti-human iC3b antibody, (Catalog# A209, Quidel, San Diego, CA) followed by incubation with anti-mouse IgG-HRP. Absorbance at 450 nm was read on a Spectramax 340PC plate reader (Molecular Devices, Sunnyvale, CA).
FcγR, FcRn binding
Biacore was used to evaluate for differences in binding of purified mAb-1 toward both human FcγRs and human FcRn between control and experimental samples. For the FcγR binding assays, the following FcγRs were immobilized on a CM5 chip using immobilized goat anti-histidine antibody: huFcγRIIa138H-His, huFcγRIIa131R-His, huFcγRIIb-His, huFcγRIIIa158F-His, huFcγRIIIa158V-His. Experimental purified samples were then added to the chip over a range of concentrations, and the binding results monitored using a Biacore T200 instrument (GE, Healthcare Lifesciences, Pittsburgh, PA). For the FcRn binding assays, the experimental purified samples were directly immobilized to a CM5 chip. huFcRn was then added over a range of protein concentrations at pH 6.0, and the binding results monitored by the Biacore T200 instrument. Association rates were monitored for 1–2 minutes followed by 1–5 minutes dissociation phase. Raw results were analyzed with the use of the Biacore T200 Evaluation software version 2.0.
Cell lysis assay for ADCC measurement
ADCC activity was assessed using a calceinAM based assay. Briefly, DoHH2 target cells were incubated for 30 minutes with 10–15 µM calceinAM in DMSO at a concentration of 4 × 106 cell/mL at 37°C, washed 2 times with culture medium, and then incubated with antibodies for 15 minutes on ice. The cells were then plated at 10000/well in a 96-well v-bottom plate. NK-92 V158 or NK-92 F158 effector cells (E) were added at a 9:1 E:T ratio. After a 2.5 hour incubation at 37°C, 100 µL of the supernatant was collected from each well after spinning the plate at 1300 RPM for 5 minutes, and released calcein was counted with an Envision multilabel reader (Perkin-Elmer, Hopkinton, MA). Triplicate wells were set up for each experimental condition. Background fluorescence due to medium or triton was subtracted from all data values. The results were then expressed as the percentage of lysis, calculated using following formula:
where experimental release represents the mean relative fluorescence units (RFU) for the target cells in the presence of effector cells; spontaneous release represents the mean RFU for target cells incubated without effector cells; and maximal release represents the mean RFU for target cells incubated with 1% Triton X-100.
FcγRIIIa (CD16) reporter assay
FcγRIIIa signaling was assessed using FcγRIIIa transfected reporter cells (Promega, Madison, WI). Reporter cells and human, transmembrane-antigen expressing HEK293 cells were incubated in a 96-well plate with increasing concentrations of antibody for 18 hours at a ratio of 1 reporter to 2 targets. After incubation, FcγRIIIa-mediated luciferase induction was measured by adding Bio-Glo luciferase substrate (Promega, Madison, WI). Relative luciferase units were measured on an Envision 2103 Multilabel Reader (Perkin Elmer, Hopkinton, MA). Data was graphed using GraphPad Prism software (GraphPad Software, La Jolla, CA).
Statistics
Experimental results are expressed as the mean for those results generated from 3 or more independent values. Experimental results are expressed directly (not averaged) for those results generated with less than 3 independent values.
Disclosure of potential conflicts of interest
All highlighted work was contributed to by authors that were used at AbbVie, Inc. at the point of data generation. PH, CC, CR, DO, AI, DS, AM, SM, BL, SS, CG, GP, SB, and RC were AbbVie employees at the date of submission of this work. Financial support for performing this work was provided by AbbVie, Inc. The authors were responsible for experimental design, data collection, analysis and interpretation, as well as the writing and review of this work. AbbVie was responsible for this work's approval. All authors have or had a financial interest in AbbVie, and have no conflict of interest to declare.
Acknowledgments
We thank Benjamin Shepard for cell culture support, Karen Hurkmans and Georgeen Gaza-Bulseco for antigen binding results, Subramanya Hegde and Pratibha Mishra for dendritic cell uptake method development and assay support, respectively.
References
- 1.Hossler P, Goh LT, Lee MM, Hu WS. GlycoVis: Visualizing glycan distribution in the protein N-glycosylation pathway in mammalian cells. Biotechnology and Bioengineering 2006; 95(5):946-960; PMID:16807922; http://dx.doi.org/ 10.1002/bit.21062 [DOI] [PubMed] [Google Scholar]
- 2.Hossler P, Khattak S, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009; 19(9):936-949; PMID:19494347; http://dx.doi.org/ 10.1093/glycob/cwp079 [DOI] [PubMed] [Google Scholar]
- 3.Sathyamoorthy N, Decker JM, Sherblom AP, Muchmore A. Evidence that specific high mannose structures directly regulate multiple cellular activities. Mol Cell Biochem 1991; 102(2):139-147; PMID:1881387; http://dx.doi.org/ 10.1007/BF00234571 [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: The double-faced macrophage mannose receptor. Crit Rev Immunol 2004; 24(3): 179-192; PMID:15482253; http://dx.doi.org/ 10.1615/CritRevImmunol.v24.i3.20 [DOI] [PubMed] [Google Scholar]
- 5.Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, Wong A, Stephan JP, Bayer R. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. mAbs 2012; 4(4): 475-487; PMID:22699308; http://dx.doi.org/ 10.4161/mabs.20737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetze A, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21(7): 949-959; PMID:21421994; http://dx.doi.org/ 10.1093/glycob/cwr027 [DOI] [PubMed] [Google Scholar]
- 7.Stahl PD. The mannose receptor and other macrophage lectins. Curr Opin Immunol 1992; 4(1): 449-52; PMID:1382452; http://dx.doi.org/ 10.1016/0952-7915(92)90123-V [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Lee YC, Feizi T, Langen H, Nussenzweig MC. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2002; 295(5561):1898-1901; PMID:11884756; http://dx.doi.org/ 10.1126/science.1069540 [DOI] [PubMed] [Google Scholar]
- 9.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcGRIII and antibody-dependent cellular toxicity. Journal of Biological Chemistry 2002; 277(30): 26733; PMID:11986321; http://dx.doi.org/ 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 10.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki H, et al.. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. Journal of Biological Chemistry 2003; 278(5):3466-73; PMID:12427744; http://dx.doi.org/ 10.1074/jbc.M210665200 [DOI] [PubMed] [Google Scholar]
- 11.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WWK, Dechant M, Beyer T, Repp R, van Berkel PHC, Vink T, van de Winkel JGJ, et al.. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 2008; 112(6): 2390-99; PMID:18566325; http://dx.doi.org/ 10.1182/blood-2008-03-144600 [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, et al.. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng 2004; 88(7): 901-8; PMID:15515168; http://dx.doi.org/ 10.1002/bit.20326 [DOI] [PubMed] [Google Scholar]
- 13.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, et al.. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 2004; 87(5): 614-22; PMID:15352059; http://dx.doi.org/ 10.1002/bit.20151 [DOI] [PubMed] [Google Scholar]
- 14.Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, Jeffrey SC, Klussman K, Law CL, Sussman D, et al.. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A 2013; 110(14): 5404-9; PMID:23493549; http://dx.doi.org/ 10.1073/pnas.1222263110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gramer MJ, Eckblad JJ, Donahue R, Brown J, Shultz C, Vickerman K, Priem P, van den Bremer ET, Gerritsen J, van Berkel PH. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol Bioeng 2011; 108(7):1591-602; PMID:21328321; http://dx.doi.org/ 10.1002/bit.23075 [DOI] [PubMed] [Google Scholar]
- 16.Correia I, Sung J, Burton R, Jakob CG, Carragher B, Ghayur T, Radziejewski C. The structure of dual-variable-domain immunoglobulin molecules alone and bound to antigen. MAbs 2013; 5(3):364-72; PMID:23572180; http://dx.doi.org/ 10.4161/mabs.24258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossler P, McDermott S, Racicot C, Chumsae C, Raharimampionona H, Zhou Y, Ouellette D, Matuck J, Correia I, Fann J. Cell culture media supplementation of uncommonly used sugars sucrose and tagatose for the targeted shifting of protein glycosylation profiles of recombinant protein therapeutics. Biotechnology Progress 2014; 30(6): 1419-31; PMID:25132658; http://dx.doi.org/ 10.1002/btpr.1968 [DOI] [PubMed] [Google Scholar]
- 18.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: Influence of aggregation. J Immunotoxicol 2014; 11(2): 99-109; PMID:23919460; http://dx.doi.org/ 10.3109/1547691X.2013.821564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannou YA, Zeidner KM, Grace ME, Desnick RJ. Human alpha-galactosidase A: Glycosylation site 3 is essential for enzyme solubility. Biochem J 1998; 332( Pt 3): 789-97; PMID:9620884; http://dx.doi.org/ 10.1042/bj3320789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houde D, Arndt J, Domeier W, Berkowitz S, Engen JR. Characterization of IgG1 conformation and conformational dynamics by hydrogen/deuterium exchange mass spectrometry. Anal Chem 2009; 81(7): 2644-51; PMID:19265386; http://dx.doi.org/ 10.1021/ac802575y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, Brickelmaier M, Muldowney C, Jones W, Goelz SE. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res 1998; 15(4): 641-9; PMID:9587963; http://dx.doi.org/ 10.1023/A:1011974512425 [DOI] [PubMed] [Google Scholar]
- 22.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends in Immunology 2013; 34(9): 440-5; PMID:23831267; http://dx.doi.org/ 10.1016/j.it.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 23.Linehan SA. The mannose receptor is expressed by subsets of APC in non-lymphoid organs. BMC Immunol 2005; 6: 4; PMID:15701168; http://dx.doi.org/ 10.1186/1471-2172-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie EJ, Taylor PR, Stillion RJ, Lucas AD, Harris J, Gordon S, Martinez-Pomares L. Mannose receptor expression and function define a new population of murine dendritic cells. J Immunol 2007; 178(8): 4975-83; PMID:17404279; http://dx.doi.org/ 10.4049/jimmunol.178.8.4975 [DOI] [PubMed] [Google Scholar]
- 25.Iida S, Kuni-Kamochi R, Mori K, Hirofumi M, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer 2009; 9: 58; PMID:19226457; http://dx.doi.org/ 10.1186/1471-2407-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al.. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences, USA 2011; 108(31): 12669-74; PMID:21768335; http://dx.doi.org/17703228 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nature Reviews Immunology 2007; 7: 715-725; PMID:17703228; http://dx.doi.org/ 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 28.Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol 2013; 33(6): 479-92; PMID:24161035; http://dx.doi.org/ 10.1016/j.semnephrol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricklin D, Reis ES, Lambris JD. Complement in disease: A defence system turning offensive. Nat Rev Nephrol 2016; 12(7): 383-401; PMID:27211870; http://dx.doi.org/ 10.1038/nrneph.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalle S, Reslan L, Besseyre de Horts T, Herveau S, Herting F, Plesa A, Friess T, Umana P, Klein C, Dumontet C. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 2011; 10(1): 178-85; PMID:21220500; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0385 [DOI] [PubMed] [Google Scholar]
- 31.Illidge T, Klein C, Sehn LH, Davies A, Salles G, Cartron G. Obinutuzumab in hematologic malignancies: Lessons learned to date. Cancer Treat Rev 2015; 41(9): 784-92; PMID:26190254; http://dx.doi.org/ 10.1016/j.ctrv.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem 1990; 265(26): 15599-605; PMID:2144287 [PubMed] [Google Scholar]
- 33.Schleif R. Regulation of the L-arabinose operon of Escherichia coli. Trends Genet 2000; 16(12): 559-65; PMID:11102706; http://dx.doi.org/ 10.1016/S0168-9525(00)02153-3 [DOI] [PubMed] [Google Scholar]
- 34.Subtil T, Boles E. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels 2011; 4: 38; PMID:21992610; http://dx.doi.org/ 10.1186/1754-6834-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderwick LJ, Birch HL, Mishra AK, Eggeling L, Besra GS. Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: Arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochem Soc Trans 2007; 35(Pt 5): 1325-8; PMID:17956343; http://dx.doi.org/ 10.1042/BST0351325 [DOI] [PubMed] [Google Scholar]
- 36.Novozhilova NM, Bovin NV. D-arabinose methabolism: Characterization of bifunctional arabinokinase/pyrophosphorylase of leishmania major. Acta Naturae 2009; 1(3): 81-3; PMID:22649617 [PMC free article] [PubMed] [Google Scholar]
- 37.Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006; 16(2): 29R-37R; PMID:16037492; http://dx.doi.org/ 10.1093/glycob/cwj016 [DOI] [PubMed] [Google Scholar]
- 38.Okeley NM, Toki BE, Zhang X, Jeffrey SC, Burke PJ, Alley SC, Senter PD. Metabolic engineering of monoclonal antibody carbohydrates for antibody-drug conjugation. Bioconjug Chem 2013; 24(10): 1650-5; PMID:24050213; http://dx.doi.org/ 10.1021/bc4002695 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct 2006; 35: 225-49; PMID:16689635; http://dx.doi.org/ 10.1146/annurev.biophys.35.101105.121507 [DOI] [PubMed] [Google Scholar]