Abstract
Selection for yellow- and white-grain types has been central to postdomestication improvement of maize. While genetic control of carotenoid biosynthesis in endosperm is attributed primarily to the Yellow1 (Y1) phytoene synthase gene, less is known about the role of the dominant white endosperm factor White Cap (Wc). We show that the Wc locus contains multiple, tandem copies of a Carotenoid cleavage dioxygenase 1 (Ccd1) gene that encodes a carotenoid-degrading enzyme. A survey of 111 maize inbreds and landraces, together with 22 teosinte accessions, reveals that Wc is exclusive to maize, where it is prevalent in white-grain (y1) varieties. Moreover, Ccd1 copy number varies extensively among Wc alleles (from 1 to 23 copies), and confers a proportional range of Ccd1 expression in diverse organs. We propose that this dynamic source of quantitative variation in Ccd1 expression was created in maize shortly after domestication by a two-step, Tam3L transposon-mediated process. First, a chromosome segment containing Ccd1 and several nearby genes duplicated at a position 1.9 Mb proximal to the progenitor Ccd1r locus on chromosome 9. Second, a subsequent interaction of Tam3L transposons at the new locus created a 28-kb tandem duplication, setting up expansion of Ccd1 copy number by unequal crossing over. In this way, transposon-mediated variation in copy number at the Wc locus generated phenotypic variation that provided a foundation for breeding and selection of white-grain color in maize.
Keywords: copy number variation, macro-transposition, maize domestication, transposon rearrangement
STRUCTURAL rearrangements and gene copy number variation are important components of genetic diversity in plant genomes (Springer et al. 2009; DeBolt 2010; Hardigan et al. 2016). Although the sources of structural variation are not fully understood, transposons are a potent mechanism of genome remodeling in maize (Fu and Dooner 2002), including novel genotypes associated with domestication (Studer et al. 2011). In particular, Ac/Ds transposons belonging to the hAT (Hobo-Activator-Tam3) superfamily of DNA transposons (Kempken and Windhofer 2001) have been shown to mediate a rich repertoire of chromosome rearrangements in maize (Ralston et al. 1989; Zhang and Peterson 1999; Huang and Dooner 2008; Zhang et al. 2013, 2014). The potential of the Ac/Ds elements for generating gene duplications, transpositions, deletions, and inversions is attributable to three essential features of the Ac/Ds system: (1) transposition typically occurs during DNA replication; (2) Ac/Ds elements often transpose to sites that are near the donor site in the genome; and (3) compatible ends of nearby elements readily interact to form macro-transposons. Macro-transposition events can produce a range of structural outcomes depending on (i) relative orientation of the interacting elements, (ii) relative orientation of transposon ends at the insertion site, and (iii) position of the insertion site and interacting elements relative to nearby replication forks at the time of transposition (Ralston et al. 1989; Huang and Dooner 2008; Zhang et al. 2014). While, thus far, the Ac/Ds system has been studied extensively in maize, the broad distribution of hAT transposons in plant and other eukaryotic genomes (Kempken and Windhofer 2001) indicates that the mechanisms demonstrated may be a widespread source of structural variation.
Carotenoid content of the endosperm is a key trait that affects both the nutritional and aesthetic qualities of the maize grain (Brink 1930; Buckner et al. 1990, 1996). Both yellow- and white-endosperm varieties are agronomically important (Poneleit 2001). The white endosperm typical of teosinte grain is presumed to be the ancestral phenotype (Palaisa et al. 2004). In maize, carotenoid biosynthesis in endosperm requires dominant alleles of the Y1 gene that confer expression of phytoene synthase in both seed and plant tissues. In contrast, recessive y1 alleles have low phytoene synthase in endosperm, and produce a white-grain phenotype (Buckner et al. 1996). Association-genetic studies indicate that human selection for a dominant Y1 allele occurred during domestication of yellow-grain maize (Buckner et al. 1990; Palaisa et al. 2003, 2004; Zhu et al. 2008). However, at least two genes determine white vs. yellow endosperm. In addition to the recessive y1, a white endosperm can result from dominant alleles of White Cap (Wc). The dominant nature of Wc, which has been known for at least a century (White 1917; Brink 1930), implies a mechanism for negative regulation of carotenoid accumulation in the developing maize kernel. Wc occurs in commercially important white and sweet corn varieties (Hannah and McCarty 1991).
The genetic map location of Wc on the long-arm of chromosome 9 (Stinard 1995) is near the Carotenoid cleavage dioxygenase 1 (Ccd1) gene (Vogel et al. 2008) in the B73 reference genome (Schnable et al. 2009). The CCD1 enzyme is a broad-specificity 9,10 (9′,10′) carotenoid dioxygenase that catalyzes cleavage of diverse carotenoids to their corresponding apo-carotenoid products (Tan et al. 1997, 2003; Sun et al. 2008; Vogel et al. 2008). In animals, apo-carotenoid signaling molecules such as retinoids and vitamin A are derived from specific cleavage of carotenoids (Giguere et al. 1987; Schwartz et al. 1997). In plants, apo-carotenoids are precursors for two important hormones, abscisic acid and strigolactone (Zeevaart et al. 1989; Schwartz et al. 1997; Tan et al. 1997; Gomez-Roldan et al. 2008; Umehara et al. 2008).
Here we show that the Wc locus, which confers a white-endosperm phenotype, contains multiple tandem copies of the Ccd1 gene. Alleles of Wc can have between 1 and 23 copies of a 28-kb repeat that contains Ccd1 and downstream glutamyl tRNA acyl transferase (Tglu) and cytochrome P450 (P450) genes. We find that Ccd1 mRNA levels in diverse tissues of Wc inbreds vary in direct proportion to Ccd1 copy number. Our analyses of Wc structure and distribution based on bac- and whole-genome-sequence (wgs) data indicate that the Wc locus was created by separate macro-transposition and gene amplification events – both mediated by interactions between closely spaced Tam3-like (Tam3L) transposons. First, a pair of Tam3L elements formed a macro-transposon that duplicated and transposed a chromosome segment containing Ccd1 and several nearby genes to a position 1.9 Mb proximal to the progenitor Ccd1 locus (Ccd1r). Next, a subsequent interaction of Tam3L transposons at the new locus formed a 28-kb tandem duplication. This in turn initiated further expansion of repeat copy number by unequal crossing over. Although the Wc phenotype is most dramatic in the endosperm of yellow-grained, Y1 genotypes, Wc occurs most often in y1 varieties lacking capacity for carotenoid biosynthesis in endosperm. We suggest that Wc intensifies the white-grain phenotype of recessive y1, thus providing a basis for human selection of the Wc y1 genotype. Our results indicate that variation in Ccd1 copy number and expression due to Wc enriched the genetic foundation for breeding and selection of grain color during postdomestication improvement of maize.
Materials and Methods
Genetic stocks
The Wc-ref Y1 stock (MGS 14545) was obtained from the Maize Cooperation Genetics Stock Center (Urbana, IL). The recessive wc line used as a reference in Figure 1 was extracted from the heterozygous Wc-ref stock (MGS 14545) provided by the Maize Coop Genetic Stock Center. The six diverse inbred stocks were a gift from J. Messing, Waksman Institute, Rutgers University. The Silver Queen hybrid sweet corn harboring a dominant white allele was obtained from L. C. Hannah at the University of Florida. Teosinte (Zea mays spp. parviglumis and Zea mays spp. Mexicana) accessions and the maize accessions listed in Table 2 were obtained from the United States Department of Agriculture North Central Regional Plant Introduction Station in Ames, IA. Maize lines were grown at the University of Florida field station in Citra, FL.
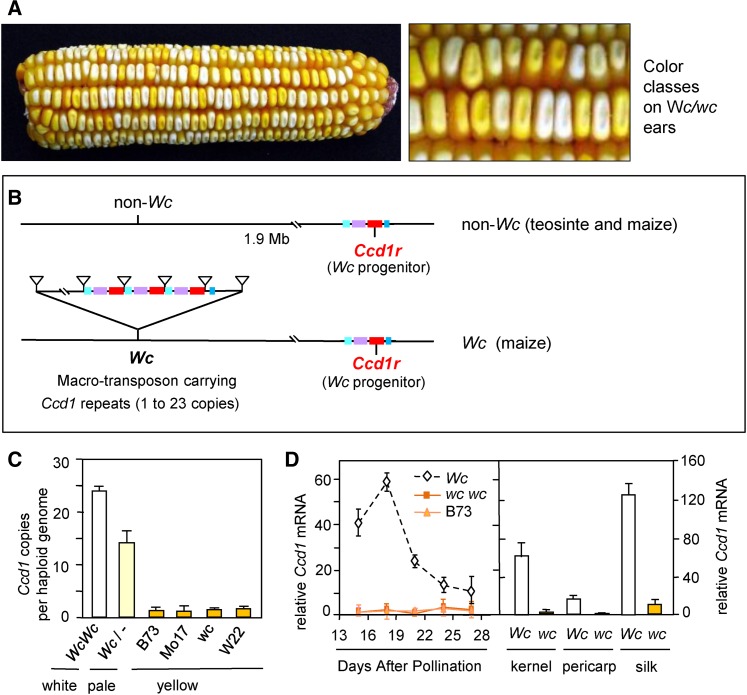
Figure 1.
White Cap (Wc), a dominant inhibitor of carotenoid accumulation in maize endosperm encodes a macro-transposon that contains 1–23 tandem copies of Ccd1 (Carotenoid cleavage dioxygenase-1) causing elevated expression in diverse tissues. (a). Kernels on a self-pollinated ear of a Wc wc heterozygote in a Y1 Y1 background segregate four classes of yellow pigmentation distinguished by Wc dosage in the triploid endosperm. Kernels with three doses of Wc (25% of kernels) have a fully white phenotype whereas zero doses (25% of kernels) conditions a normal yellow phenotype. Intermediate Wc dosage classes have pale yellow phenotypes. (b). Diagram comparing Wc (maize) and non-Wc (maize and teosinte) chromosomes. The Ccd1r reference locus is presumed to be present in all maize and teosinte haplotypes (see Table 3). In Wc maize, a macro-transposon insertion carrying from 1 to 23 tandem copies of the Ccd1 gene is located 1.9 Mb proximal to Ccd1r. The transposed chromosome segment includes Ccd1 (red), and neighboring genes Tglu (purple), P450 (light turquoise), and Rpl21 (blue). Supporting evidence is described herein (see also subsequent figures). (c). Quantification of Ccd1 copies per genome in the Wc reference allele (Wc Wc), a Wc heterozygote from a Silver Queen B73 F1 hybrid, several non-Wc inbreds, and wc, a control that is homozygous for the recessive allele segregating in the Wc-ref stock. (d) Expression of Ccd1 during seed development. mRNA abundance per ng total RNA determined by qPCR is expressed in relative units (see Materials and Methods).
Table 2. Distribution of Wc in white-maize inbreds and landraces.
| Variety | Wca present | Grain colorb | Accession |
|---|---|---|---|
| Puebla 32, Mexico | Wc | Pale yellow | PI484595 |
| Puebla 27, Puebla, Mexico | Wc | seg white, yellow | PI628480 |
| Puebla 42, Mexico | Wc | seg white, yellow | PI388974 |
| Lima 19, Peru | Wc | seg white, yellow | PI485353 |
| Jalisco 43, Mexico | Wc | seg white, yellow | PI483560 |
| Country Gentleman | Wc | White | NSL5613 |
| MO24W | Wc | White | PI587144 |
| KY228 | Wc | White | PI587136 |
| Tzi8, Nigeria | Wc | White | PI506246 |
| Chile 301, Santiago Chile | Wc | White | PI485410 |
| Santander S 356, Columbia | Wc | White | PI445401 |
| Boyaca 462, Columbia | Wc | White | PI444165 |
| NC336 | Wc | White | Ames 27164 |
| CML10 | Wc | White | Ames 27072 |
| K55, Kansas | Wc | White | Ames 22754 |
| Mexico 37 | Wc | White | Ames 19558 |
| White Dent OP | Wc | White | Ames 04836 |
| Hays White, WI | Wc | White | Ames 01829 |
| Cuzco 9, Lima Peru | Wc-f | Pale yellow | PI503671 |
| Huancavelica 147, Peru | Wc-f | White | PI571793 |
| CML218 | Wc-f | White | Ames 27086 |
| CML91 | Wc-f | White | Ames 27079 |
| CML247 | — | White | PI595541 |
| H105W | — | White | PI587127 |
| MO15W | — | White | PI558518 |
| Aguascalientes 8, Mexico | — | White | PI484401 |
| White midget | — | White | NSL5631 |
| NC33 | — | White | Ames 27139 |
| I29 | — | White | Ames 27115 |
| Guanajuato 36, Mexico | — | White | Ames 19481 |
| Guerrero 3, Mexico | — | White | Ames 19467 |
Wc genotype was determined by Southern blots probed with Ccd1 for presence of an intense 6.1-kb Bam HI fragment. Wc-f indicates presence of a faint band consistent with a low-copy number Wc allele.
Phenotypes were recorded for seed grown for this experiment; seg, segregating.
Nucleic acid methods
DNA extraction, Southern analysis, sequence determination, and other routine molecular biology methods were conducted as previously described (Tan et al. 1997, 2003). The Ccd1 genomic DNA region was cloned via construction and screening of a lambda phage genomic library prepared from a Wc wc heterozygote as previously described (Tan et al. 1997). Briefly, genomic DNA was digested with BamHI and resolved through 10–35% sucrose gradient centrifugation at 26,000 × g for 24 hr at 4°. The fraction enriched in 6.5-kb fragments was purified and ligated to lambda-ZAPII vector (Stratagene, La Jolla, CA). The library was screened using a 1.1-kb Ccd1 partial cDNA (EST CSU453) as a probe. The genomic sequence was extended by PCR amplification of a 2-kb fragment from Wc genomic DNA that included the presumptive translation start.
Cloning of Ccd1 cDNA and partial genomic clones from maize and teosinte
A near full-length cDNA clone containing the complete coding sequences of Ccd1 was obtained by RT-PCR with forward primer (5′-CCCTTCGCTACAAGCCTACA-3′) and reverse primer (5′-TTCGAATACACGTCCTGCAA-3′). RNA extracted from developing Wc kernels at 18 days after pollination was used to synthesize the cDNA. The PCR products were cloned in pCR4-TOPO vector and completely sequenced. Several genomic fragments identified from Southern analysis were cloned by construction and screening of λ-phage libraries. Briefly, genomic DNA was digested with appropriate DNA restriction enzymes and fractionated via a 15–40% sucrose gradient centrifuged at 25,000 × g at 4° for 24 hr in a swing bucket rotor. Selected DNA fractions were ligated into phage cloning vectors (λ-ZAP II or λ-ZAP Express, Stratagene) and packaged according to the manufacturer’s instructions. The library was screened and positive clones were isolated and converted into phagemid by in vivo excision.
BAC library construction and sequencing
A custom bac library was constructed from genomic DNA of the homozygous Wc reference stock and screened with a Ccd1 cDNA probe by the Bio S&T (Montreal, Canada). Two positive clones (19F and H10) were identified. The BAC clones were characterized by restriction mapping and partial sequencing of selected subcloned restriction fragments. Of the two clones, H10 extended farthest into the Wc locus, and was selected for complete sequencing and assembly using a combination of circular-consensus and linear long format reads from the PacBiosystems instrument. Trimmed circular-consensus sequence reads (>5000 bp) were assembled into contigs using CAP3 (Huang and Madan 1999) and linear long reads were assembled using CANU (Koren et al. 2016). The contigs were further evaluated and assembled manually to obtain an assembly (Genbank accession KX760165) that was consistent with the bac restriction map, bac end sequences, wgs analysis, and subclone sequences.
Quantitative real-time RT-PCR
Total RNA was extracted using RNeasy (QIAGEN, Germany) and treated with RNase-free DNase. The complete removal of DNA was verified by a quantitative real-time RT-PCR analysis without reverse transcription. The conditions used are as described in detail previously (Tan et al. 2003). For TacMan qPCR primers used for Ccd1 were forward (5′-GGGAAGAGGGTGATGAAGTTGT-3′) and reverse (5′-TGATATCCATTCACCTTGTCCAAA-3′), and the probe was 5′-CTCATTACCTGCCGCCTTGAGAATCCA-3′. The probe was labeled with fluorescent reporter dye 6-carboxyfluorescein (FAM) at 5′ and 6-carboxy-N,N,N′,N′-tetramethylrhodamine (TAMRA) at 3′. The standard curve was derived with a plasmid containing Ccd1 cDNA. Reactions were carried out in the GeneAmp 5700 Sequence Detection (Perkin-Elmer, Norwalk, CT). The transcript abundance was normalized as copy number per nanogram of total RNA. qPCR of pericarp tissue was performed as described by Sun et al. (2008) using 5′-CTGCTGTGGATTTTCCTCGTG-3′ and 5′-TATGATGCCAGTCACCTTCGC-3′ as forward and reverse primers, respectively. Relative expression levels were calculated from E−ΔCt values setting the mean of the B73 wc control to one.
Identification of the Tam3Ld left junction sequence
To identify the left border sequence of the Wc macro-transposon, we employed a modified TAIL-PCR protocol that used four AD3 primers (AD3-1 to 4), each of which contains an AD3 primer fused with an arbitrary degenerate primer (Liu and Chen 2007). Three nested primers based on the predicted Tam3Ld flanking sequence (Wc3P-R3, Wc3P-R4, and Wc3P-R5) were used with the AD3 primers (Table 1). Following three rounds of TAIL-PCR fragments were sequenced and analyzed to identify products that contained Tam3L-3′ termini. The sequences were in turn used to design a Tam3L-specific primer (Wc3P-R5), which was then used with Wc3P-R3 to amplify the left border of the Tam3Ld candidate sequence. The resulting Tam3Ld-3′ flanking sequence contained an 8-bp target site duplication (GTTCTAGT) that matched the right junction of Tam3La confirming the macro-transposon hypothesis.
Table 1. Primers used to identify the Tam3Ld-3′ junction.
| Primer name | Sequence |
|---|---|
| AD3-1 | 5′-AGTTTTTGGGTGGTGG(G/C/A)N(G/C/A)NNNGGAA-3′ |
| AD3-2 | 5′-AGTTTTTGGGTGGTGG(G/C/T)N(G/C/T)NNNGGTT-3′ |
| AD3-3 | 5′-AGTTTTTGGGTGGTGG(G/C/A)(G/C/A)N(G/C/A)NNNCCAA-3′ |
| AD3-4 | 5′-AGTTTTTGGGTGGTGG(G/C/T)(G/A/T)N(G/C/T)NNNCGGT-3′ |
| AD3 | 5′-AGTTTTTGGGTGGTGG-3′ |
| Wc3P-R3 | 5′-CCTCGTCAAAACATTGTCATCTTTCAAACCC-3′ |
| Wc3P-R4 | 5′-TTAGAACCGGTTGATGGTAAAAGCTGGCAG-3′ |
| Wc3P-R5 | 5′-GGCAGTAAATTAAGGGGGTG-3′ |
Quantification of Ccd1 copy number in the Wc reference allele
Real-time quantitative PCR was used to determine the gene copy number of Ccd1 in each DNA sample. A single-copy gene Vp14 (Tan et al. 1997) was used as an internal standard. In addition, the inbred line B73, which was confirmed to contain a single copy of Ccd1 by hybridization and sequencing, was used as a standard to normalize the Ccd1 probe. To increase the accuracy of real-time quantitative PCR, genomic DNA samples were digested with EcoRI to completion, generating Vp14 and Ccd1 fragments in a 6- to 7-kb size range. The DNAs were further purified by a Turbo genomic DNA purification kit (Qbiogene, Carlsbad, CA). The concentration of the DNAs was determined spectrophotometrically. Equal amounts of DNA were analyzed by real-time quantitative PCR. The analysis conditions were the same as the real-time quantitative RT-PCR described above except without reverse transcription. Ccd1 primers and probe were the same as above. The Vp14 primers are forward (5′-GCTGGCTTGGCTTGTATACTCTG-T-3′) and reverse (5′-CCATCAGTCATATACTGTGAACAAATGT-3′), and the gene-specific probe is (5′-CACGCACCGATAGCCACAGGGAA-3′) labeled with FAM and TAMRA at 5′ and 3′, respectively.
Copy number estimation in maize genomes by analysis of k-mer frequencies
Frequencies of 22-mers in the B73 reference genome were profiled using JELLYFISH (Marçais and Kingsford 2011). The resulting database was then queried with 22-mers from 39,424 genes in the maize filtered gene set (gramene.org) to identify a subset of genic 22-mers that were single-copy in the B73 genome. Frequencies of the resulting set of 124 million single-copy, genic 22-mers were in turn profiled in wgs sequence data obtained from the Sequence Read Archive (ncbi.nlm.nih.gov) for each of 102 maize and teosinte accessions in the HapMap2 collection (Chia et al. 2012). Gene copy numbers in each genome were then estimated by normalizing the average frequency of single-copy 22-mers from Ccd1r to the average frequency of 124 M genic single-copy 22-mers in wgs data for each inbred. The estimated effective sequence coverage of each genome is listed in Supplemental Material, Table S1.
Analysis of Wc and Ccd1r allele-specific features in maize genomes
The wgs data from HapMap2 genomes was searched for sequence reads that contained diagnostic features of the Wc locus and Ccd1r alleles using the Global search Regular Expression Print (GREP) utility. Simple text searches were made in both orientations using 18–22 base sequences that were unique to transposon insertion sites and other characteristic features of Wc or Ccd1r alleles. Sequence reads identified by text searches were then validated by full-length blastn alignment to the Wc bac assembly and B73 reference genome (Schnable et al. 2009) sequences.
Data availability
The Wc Ccd1 genomic and cDNA sequences are deposited in Genbank (accessions: DQ100348, DQ100347, and cDNA DQ100346). The Wc bac sequence is Genbank accession KX760165. Genetic strains used in this study are available by request.
Results
Wc contains a Ccd1 gene cluster that confers high Ccd1 expression
In a Y1 genetic background, which leads to biosynthesis of yellow carotenoids in the endosperm, the dominant Wc allele confers a dosage-dependent white-endosperm phenotype (Figure 1a). In the triploid endosperm, kernels that have a single dose of Wc have a pale yellow or white crown, reflecting a partial inhibition of carotenoid accumulation. In contrast, kernels with three doses of Wc have a nearly white endosperm. A gradation of yellow to white-kernel phenotypes can thus be discerned on a self-pollinated ear of a Wc wc Y1 Y1 heterozygote consistent with the four expected gene dosage classes (Figure 1a).
Figure 1b summarizes the structure of the Wc locus and its relationship to the Ccd1 reference (Ccd1r) locus in Wc and non-Wc (wc) haplotypes. Evidence presented below indicates that Wc originated as a macro-transposon. The macro-transposon duplicated a region including Ccd1 and several nearby genes and inserted it at a position 1.9 Mb proximal to the Ccd1r locus on the long-arm of chromosome 9. Southern blot analysis (Figure S1a in File S1) showed that Wc cosegregated with a gene cluster that includes multiple copies of the Ccd1 coding sequence. Multiple restriction digests probed with a Ccd1r cDNA yield single, intense bands of up to 16 kb indicating that the multiple copies are highly homogeneous (Figure S1a in File S1). In line with these results, our Ccd1 cDNA probe detects an intense FISH signal on the long arm of chromosome 9 in Wc plants (Han et al. 2007).
To quantify Ccd1 copy number in Wc and non-Wc (wc) genotypes (Figure 1c), we developed a gene-specific, real-time PCR assay. As a single-copy, internal control, we used the well-characterized Vp14 gene (Tan et al. 1997). Consistent with the Southern blot results, PCR data indicate that genomes of B73 and other yellow inbreds carry a single copy of Ccd1. We attribute this single Ccd1 to Ccd1r. In contrast, plants homozygous for the Wc reference allele are estimated to have 23.9 (±2.0) copies per genome. In agreement with this estimate, heterozygotes carrying a Wc allele extracted from “Silver Queen” sweet corn (Hannah and McCarty 1991) have an estimated 13.5 (±2.4) copies. This is an expected value for a heterozygote carrying 24 copies on one chromosome and one copy on the other [(24 + 1)/2 = 12.5 copies per genome].
Based on these results, we reasoned that amplification of Ccd1 copy number at the Wc locus could account for the dominant white phenotype by elevating expression of the CCD1 enzyme in endosperm. Enhanced carotenoid cleavage would result from activity of the broad-specificity CCD1 9,10 carotenoid dioxygenase (Vogel et al. 2008). As shown in Figure 1d, relative expression of Ccd1 mRNA is indeed markedly higher throughout development of Wc kernels compared to isogenic non-Wc and B73 inbreds. In Wc kernels, Ccd1 expression peaks during midgrain fill at 18 days after pollination (DAP) then declines gradually toward maturity. In addition, Wc causes elevated Ccd1 expression in diverse tissues including silks and pericarp.
Structure of the Wc locus
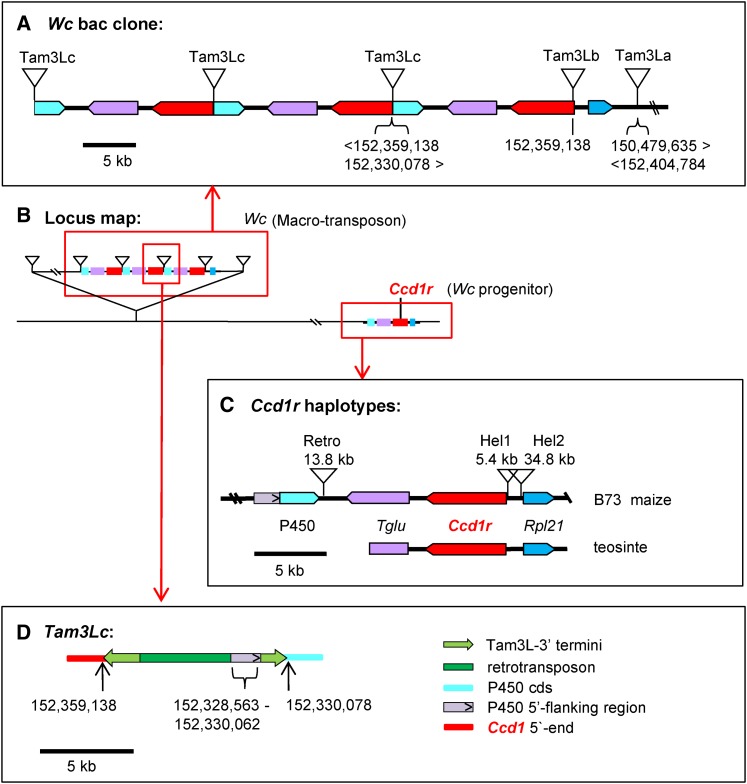
To determine the structure of the Wc locus, we constructed a bac library from the Wc reference stock and isolated a 106-kb bac clone that spanned one boundary of the Ccd1 gene cluster. The bac sequence assembly diagrammed in Figure 2a was confirmed by (1) restriction mapping, (2) sequencing of bac-ends and selected subclones, (3) genomic Southern blot data (Table 2, Figure S2, a and b in File S1), (4) consistency with whole-genome-sequence (wgs) data from diverse Wc inbreds (Table 3), and (5) by mapping and partial sequencing of a second overlapping bac clone (data not shown). To aid interpretation of the Wc assembly, the bac sequence was then aligned to the region surrounding the single-copy Ccd1r gene in the B73 reference genome (Figure 2, b and c). In addition, we cloned and sequenced a 12-kb region containing the Ccd1r gene from the teosinte (Z. mays spp. parviglumis) genome (Figure 2c and Figure S2b in File S1). The 106-kb Wc bac sequence includes three tandem copies of Ccd1 arranged in direct orientation. Each Ccd1 copy is embedded in a 28-kb direct repeat that also includes a glu-tRNA acyltransferase (Tglu, GRMZM2G057491) and a cytochrome P450 (GRMZM2G057514) gene, P450. The Tglu and P450 genes are located downstream of Ccd1r in the B73 reference genome (Figure 2, b and c). The rightmost Ccd1 copy in the bac sequence contains a 4460-bp, Tam3-like transposon insertion (Tam3Lb) near its 5′-prime end. As expected, Tam3Lb is flanked by an 8-bp, host site duplication typical of the hAT transposon family. Although multiple Tam3L copies are detected in the B73 genome (data not shown), the Tam3L transposon family has not previously been characterized in maize.
Figure 2.
Structure of the Wc locus and Ccd1r haplotypes of maize and teosinte. (a) Sequencing and assembly of a bac clone containing one border of the Wc locus revealed two complete and one partial copy of a 28-kb repeat that included copies of Ccd1 (red) and nearby Tglu (purple) and P450 (turquoise) genes. The repeats are punctuated by a composite Tam3L-like sequence (Tam3Lc), which is shown in greater detail in part d. The rightmost copy of Ccd1 is bordered by an intact Tam3L transposon (Tam3Lb) with a flanking 8-bp host site duplication. The 10-kb segment between Tam3Lb and a second Tam3L transposon (Tam3La) is colinear with the region upstream of Ccd1r in the B73 reference and teosinte genomes that includes Rpl21 (blue, see part c). The 13.5-kb sequence that extends from Tam3La to the right end of the bac clone (abridged for clarity) aligns to sequence located 1.9 Mb proximal to Ccd1r in the B73 reference. Consequently, Tam3La is not flanked by an 8-bp host site duplication. (b) Location of Wc relative to the Ccd1r progenitor locus on the long arm of chromosome 9 (see Figure 1b). (c) Comparison of Ccd1r haplotypes found in maize (B73 reference) and teosinte (data on distributions of Ccd1r haplotypes in maize and teosinte are summarized in Table 3). The teosinte structure was determined by sequencing a 12-kb region containing Ccd1r in Z. mays spp. parviglumis. Gray box with arrow inset, position and orientation of P450 5′-flanking sequence included in Tam3Lc. (d). Structure of Tam3Lc, a composite transposon-like element that punctuates the Wc 28-kb repeats. Numbers in parts a and d indicate coordinates of sequence alignments to the B73 genome (version 3).
Table 3. Sequence features detected in genomes of maize and teosinte accessions.
| Inbred/accession | Endosperm colora | PZE0680879922b | Ccd1 copy numberc | Macro-transposon right junction (Tam3La-5') | Tam3La-3' junction | Macro-transposon left junction (Tam3Ld-3') | Tam3Lb-3 junction | Tam3Lc left junction | Tam3Lc retroelement junction | Tam3Lb/c right junction | Vacant Helitron 1 site (Wc progenitor) | Vacant Helitron 1 site (teosinte variant) | OH43 Helitron insertion | B73 Helitron 1 insertion site |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B73:MZ | Y | TT | 1 | X | ||||||||||
| B97:MZ | Y | TT | 1 | X | ||||||||||
| BKN009:MZ | n.d. | CC | 1 | |||||||||||
| BKN010:MZ | n.d. | CC | 1 | |||||||||||
| BKN011:MZ | n.d. | TT | 1 | |||||||||||
| BKN014:MZ | n.d. | CC | 1 | X | ||||||||||
| BKN015:MZ | W | CC | 16 | X | X | X | X | X | X | |||||
| BKN016:MZ | Y | TT | 1 | X | ||||||||||
| BKN017:MZ | n.d. | CC | 3 | X | X | X | ||||||||
| BKN018:MZ | W | CC | 8 | X | X | X | X | X | X | |||||
| BKN019:MZ | n.d. | TT | 1 | |||||||||||
| BKN020:MZ | n.d. | TT | 1 | |||||||||||
| BKN022:MZ | n.d. | CC | 11 | X | X | X | X | X | X | X | X | |||
| BKN023:MZ | n.d. | CC | 1 | X | ||||||||||
| BKN025:MZ | n.d. | ./. | 13 | X | X | X | X | X | ||||||
| BKN026:MZ | n.d. | CC | 21 | X | X | X | X | X | X | X | ||||
| BKN027:MZ | n.d. | TT | 1 | X | ||||||||||
| BKN029:MZ | n.d. | CC | 15 | X | X | X | X | X | X | X | X | |||
| BKN030:MZ | n.d. | CC | 2 | X | ||||||||||
| BKN031:MZ | n.d. | CC | 4 | X | X | X | X | X | X | X | X | X | ||
| BKN032:MZ | n.d. | TT | 1 | X | ||||||||||
| BKN033:MZ | n.d. | CC | 1 | |||||||||||
| BKN034:MZ | n.d. | CC | 1 | |||||||||||
| BKN035:MZ | n.d. | CC | 23 | X | X | X | X | X | X | X | X | |||
| BKN040:MZ | W | CC | 14 | X | X | X | X | X | X | X | ||||
| CAU178:MZ | Y | TT | 1 | X | ||||||||||
| CAU478:MZ | Y | TT | 1 | X | ||||||||||
| CAU5003:MZ | Y | TT | 1 | |||||||||||
| CAUCHANG72:MZ | Y | TT | 1 | |||||||||||
| CAUMO17:MZ | Y | TT | 1 | X | ||||||||||
| CAUZHENG58:MZ | Y | TT | 1 | |||||||||||
| CML103:MZ | W | CC | 13 | X | X | X | X | X | X | X | X | |||
| CML133:MZ | W | CC | 1 | |||||||||||
| CML192:MZ | Y | TT | 1 | |||||||||||
| CML202:MZ | W | CC | 12 | X | X | X | X | X | ||||||
| CML206:MZ | W | ./. | 1 | |||||||||||
| CML228:MZ | Y | TT | 1 | Xd | X | |||||||||
| CML247:MZ | W | ./. | 1 | |||||||||||
| CML277:MZ | W | CC | 8 | X | X | X | X | X | X | |||||
| CML312SR:MZ | W | CC | 17 | X | X | X | X | X | X | |||||
| CML322:MZ | W | CC | 9 | X | X | X | X | X | X | X | ||||
| CML330:MZ | W | CC | 3 | X | X | X | X | X | ||||||
| CML333:MZ | W | CC | 2 | X | X | X | X | X | ||||||
| CML341:MZ | W | CC | 10 | X | X | X | X | |||||||
| CML411:MZ | Y | TT | 1 | X | ||||||||||
| CML418:MZ | W | CC | 10 | X | X | X | X | X | X | |||||
| CML479:MZ | Y | TT | 1 | X | ||||||||||
| CML504:MZ | W | CC | 16 | X | X | X | X | X | X | X | ||||
| CML505:MZ | W | CC | 10 | X | X | X | X | X | X | X | ||||
| CML511:MZ | W | CC | 16 | X | X | X | X | X | ||||||
| CML52:MZ | Y | TT | 1 | X | ||||||||||
| CML52R:MZ | Y | ./. | 1 | |||||||||||
| CML69:MZ | Y | TT | 2 | X | X | X | X | X | ||||||
| CML84:MZ | W | CC | 11 | X | X | X | X | X | ||||||
| CML85:MZ | W | CC | 1 | |||||||||||
| CML96:MZ | W | CC | 1 | |||||||||||
| CML99:MZ | W | CC | 11 | X | X | X | X | X | X | X | ||||
| H16:MZ | W | CC | 1 | |||||||||||
| HP301:MZ | Y | TT | 1 | |||||||||||
| IL14H:MZ | W | ./. | 1 | X | ||||||||||
| KI11:MZ | Y | TT | 1 | |||||||||||
| KI3:MZ | Y | TT | 1 | X | ||||||||||
| KY21:MZ | W | CC | 13 | X | X | X | X | |||||||
| M162W:MZ | W | CC | 7 | X | X | X | X | X | X | X | ||||
| M37W:MZ | W | CC | 1 | |||||||||||
| MO17:MZ | Y | TT | 1 | X | ||||||||||
| MO18W:MZ | W | CC | 6 | X | X | X | X | X | X | X | ||||
| MS71:MZ | Y | TT | 1 | X | ||||||||||
| NC350:MZ | Y | TT | 8 | X | X | X | X | X | X | X | ||||
| NC358:MZ | Y | TT | 1 | X | ||||||||||
| OH43:MZ | Y | TT | 1 | Xd | X | |||||||||
| OH7B:MZ | Y | ./. | 1 | X | ||||||||||
| P1:MZ | W | CC | 16 | X | X | X | X | X | X | X | X | |||
| P39:MZ | Y | TT | 1 | X | ||||||||||
| TIL01:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL02:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL03:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL04-TIP285:TEO | n.d. | CC | 1 | |||||||||||
| TIL05:TEO | n.d. | CC | 1 | |||||||||||
| TIL06:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL07:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL08:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL09:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL10:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL11:TEO | n.d. | CC | 1 | |||||||||||
| TIL12:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL15:TEO | n.d. | CC | 1 | |||||||||||
| TIL16:TEO | n.d. | CC | 1 | |||||||||||
| TIL17:TEO | n.d. | TT | 1 | X | ||||||||||
| TIL25:TEO | n.d. | CC | 1 | X | ||||||||||
| TIL04-TIP454:TEO | n.d. | CC | 1 | |||||||||||
| TIL06:TEO | n.d. | CC | 1 | |||||||||||
| TIL14:TEO | n.d. | CC | 1 | X | ||||||||||
| TX303:MZ | Y | TT | 1 | X | ||||||||||
| TZI8:MZ | W | CC | 12 | X | X | X | X | X | X | X | ||||
| VL0512447:MZ | W | CC | 1 | |||||||||||
| VL05128:MZ | W | CC | 12 | X | X | X | X | X | X | |||||
| VL054178:MZ | W | ./. | 12 | X | X | X | X | X | X | X | ||||
| VL05610:MZ | W | CC | 1 | |||||||||||
| VL056883:MZ | W | CC | 17 | X | X | X | X | X | X | X | ||||
| VL062784:MZ | W | CC | 14 | X | X | X | X | X | ||||||
| W22:MZ | Y | TT | 1 | X | ||||||||||
| W64A:MZ | Y | TT | 1 | X |
Y, yellow; W, white.
n.d., not determined.
HapMap2 SNP genotype data (Chia et al. 2012).
Copy number rounded to nearest positive integer.
Features detected by single sequence reads in CML228 and OH43, respectively. Absence of corroborating evidence of other Wc features in these inbreds suggested that the single reads were most likely of spurious origin.
Wc contains a Tam3L macro-transposon
In the bac sequence, a 6573-bp region to the right of Tam3Lb is colinear with genomic sequence upstream of Ccd1r in the teosinte genome (Figure 2c). This region includes a copy of a neighboring gene, Ribosomal-large-subunit-protein-21 (Rpl21, GRMZM2G089421). This region of colinearity with the ancestral Ccd1r haplotype is bordered on the right by a second Tam3L element (Tam3La). Although the Tam3La transposon is intact, two features of its flanking sequences indicated that Tam3La likely forms one boundary of a macro-transposon. First, Tam3La is not flanked by an 8-bp direct duplication. Second, sequences on the left and right sides of Tam3La are not contiguous in the B73 reference genome. Instead, sequence to the right of Tam3La aligns to a position 1.9 Mb proximal to the Ccd1r locus in the B73 genome.
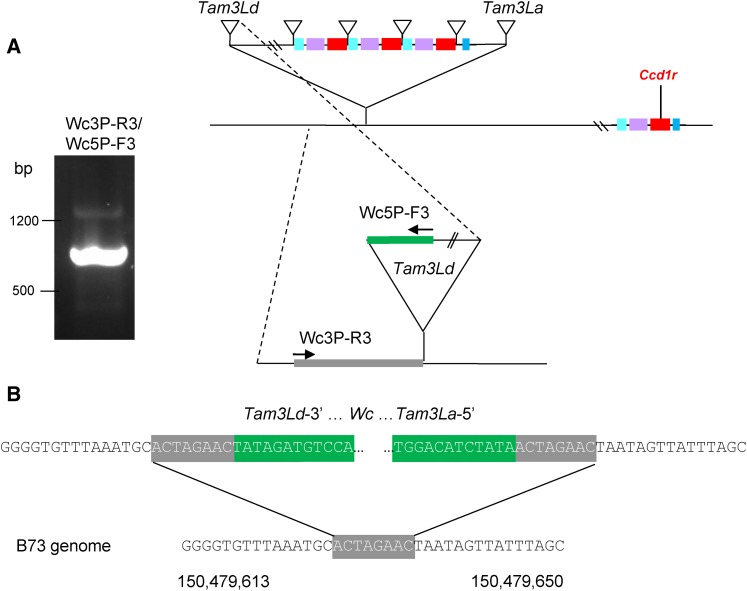
On this basis, we hypothesized that the other boundary of the Wc locus would be delimited by another Tam3L transposon (Tam3Ld). We further anticipated that Tam3Ld would have a left flanking sequence that (1) was contiguous in the reference genome with sequence flanking the right side of Tam3La (Figure 2a), and (2) shared a matching 8-bp host site duplication with Tam3La. As hypothesized, we were indeed able to identify the predicted Tam3Ld junction sequence that defines the 3′ (with respect to the Tam3La transposase coding sequence) boundary of the Wc macro-transposon in genomic DNA of Wc plants. We amplified the Tam3Ld-3′ junction using a combination of TAIL-PCR and PCR with primers specific for the 3′ arm of Tam3L and for the expected flanking sequence (Figure 3). In addition, we detected the Tam3Ld-3′ junction sequence in wgs data from multiple Wc inbreds (Table 3).
Figure 3.
Identification of the Tam3Ld insertion marking the proximal border of the Wc macro-transposon. (a) PCR amplification of the Tam3Ld-3′ junction from Wc plants. Candidate Tam3L-3′ junction sequences were amplified by TAIL-PCR anchored by locus-specific primers in the predicted location of Tam3Ld (see Materials and Methods). The TAIL-PCR sequences were used to design a pair of sequence-specific primers, Wc3P-R3 and Wc3P-F3, that amplified a 794-bp junction fragment (agarose gel electrophoresis image). The locations of sequence-specific primers are diagramed on the right. Green indicates transposon sequence. The 8-bp target site duplication is gray. (b) Confirmation of the Wc macro-transposon insertion site. The Tam3Ld junction sequence aligned to the B73 reference genome at a position that was contiguous with the sequence flanking Tam3La-5′ and included a matching 8-bp target site duplication (gray). The Tam3Ld and Tam3La 12-base inverted terminal repeats are colored green. B73 chromosome 9 genome coordinates (version 3) are indicated below the sequence.
Wc repeats are punctuated by a composite Tam3L sequence
Each 28-kb repeat is bordered by a 9980-bp transposon-like sequence (Tam3Lc) that has two Tam3L-3′ termini with two unrelated sequence fragments sandwiched between them (Figure 2d and Figure S3 in File S1). The leftward Tam3L-3′ terminal fragment is 2058 bp long and right arm is 1582 bp. One of the two internal sequences is a 4835-bp fragment of an uncharacterized retrotransposon. The other is a 1508-bp segment that aligns to genomic sequence immediately upstream of P450 in the B73 reference genome. Sequence derived from the P450 flanking region is nearly contiguous with the right junction of Tam3Lc, except for a deletion of 16 bp at the insertion site. These features are consistent with an abortive transposition that inserted a single Tam3L end upstream of P450. The right end of Tam3Lc has several polymorphisms in common with Tam3La-3′ (Figure S2 in File S1) that distinguish it from Tam3Lb-3′, whereas the opposite end of Tam3Lc is derived from the 3′-terminus of Tam3Lb.
Creation of Wc by macro-transposition and gene amplification
The structural features described above indicated that the Wc locus was created by interactions between Tam3L elements that (1) transposed a copy of the Ccd1r locus to a position 1.9 Mb upstream of the progenitor locus, and (2) initiated amplification of a 28-kb segment of the transposed copy. A proposed series of events that account for the structure depicted in Figure 2 is outlined in Figure S4 in File S1. In this scenario, creation of the Wc locus was preceded by a series of Tam3L insertions in the ancestral Ccd1r locus. This resulted in a pair of Tam3L elements that flanked a region containing Ccd1r and neighboring Rpl21, Tglu, and P450 genes. As a replication fork moved through this segment, Tam3Ld-3′ and Tam3La-5′ then formed a macro-transposon that inserted at an unreplicated site 1.9 Mb proximal to the Ccd1r locus. We suggest that a subsequent interaction of Tam3L elements at the new locus duplicated a segment containing Ccd1, thus enabling expansion of repeat-number by unequal crossing over. In some configurations, Ac is capable of creating tandem duplications by initiating rolling-circle replication or rereplication (Ralston et al. 1989; Zhang et al. 2014). In instances where Ac has induced rereplication of DNA, Zhang et al. (2014) observed that stalling of the replication fork prevented extensive rolling-circle replication. While a similar mechanism may have created the initial tandem repeat in Wc, the precise origin of the composite Tam3Lc element is unclear. The incorporation of an interior sequence derived from the upstream flanking region of the P450 gene suggests that Tam3Lc was formed in part by an abortive transposition that inserted one arm of a transposon – possibly derived from Tam3La-3′ – upstream of P450. One speculative possibility is that a macro-transposition involving Tam3La-3′ and Tam3Lb-5′ aborted during replication, resulting in a fractured chromosome with one or more double-stranded breaks. Formation of Tam3Lc by repair reactions that fused nearby Tam3La-3′ and Tam3Lb-3′ fragments could plausibly have created a circular template that initiated transient rereplication of Ccd1.
Independent diversification of Ccd1r and the Wc locus
Subsequent to creation of Wc, the Ccd1r progenitor and Wc continued along separate paths of evolution and diversification (Figure 2c). Comparison of the Ccd1r sequences from B73 and ancestral teosinte revealed that the B73 haplotype contains helitron transposon insertions near the transcription start sites of both the Ccd1r and Rpl21 genes. The helitron insertions displaced upstream flanking sequences of both genes, potentially altering their regulation. A search of wgs data detected the B73 Hel1 junction sequence in at least 13 of 83 maize inbreds and one of 19 teosinte accessions (Table 3). At least four Wc inbreds also carried Hel1 (B73 type) Ccd1r alleles. As shown in Figure 2a, the progenitor of Wc lacked the Hel1 insertion. As expected, this Hel1-free promoter variant is detected in the majority of Wc inbreds. Its presence in at least eight non-Wc maize inbreds and eight of 19 teosinte accessions further indicates that Ccd1r alleles resembling the Wc progenitor exist in both maize and teosinte. Evidence of a second, independent helitron insertion located in a similar position upstream of Ccd1r was detected in OH43 (data not shown). The OH43 promoter variant was found in at least 11 of 83 maize accessions, but in none of the teosinte accessions (Table 3).
Wc alleles account for extensive Ccd1 copy number variation in maize
To evaluate Ccd1 copy number variation in maize, we analyzed k-mer frequencies in wgs data from 102 maize and teosinte genomes represented in the HapMap2 resource (Chia et al. 2012). We adapted JELLYFISH (Marçais and Kingsford 2011) for this purpose. Ccd1 copy number per genome was estimated by determining the frequencies of Ccd1-specific 22-mers in each data set. The counts of Ccd1 22-mers in wgs data were normalized to a set of control 22-mers. The control set consisted of 124 M, single-copy 22-mers derived from the B73 filtered gene set (gramene.org). Effective depth of coverage obtained for each genome is summarized in Table S1.
Of the 83 maize genomes represented in the HapMap2 collection, 36 (43%) had an estimated Ccd1 copy number of two or greater (Table 3, rounding to nearest integer). The highest copy number detected, 23 copies per genome in landrace accession BKN035, was comparable to the qPCR-based estimate of 24 copies in the Wc reference stock (Figure 1c). All accessions contained at least one Ccd1 copy, which we attributed to the Ccd1r locus. In contrast, with one exception, all maize genomes that had two or more Ccd1 copies were also confirmed to carry a suite of sequence features specific to the Wc locus (Table 3). The exception was landrace BKN030, which had an estimated two copies of Ccd1, but no evidence of Wc-specific features. Therefore, in nearly all cases examined, presence of additional Ccd1 copies in maize inbreds could be attributed to the Wc locus.
Wc is not detected in teosinte
Each of the 19 teosinte genomes represented in the wgs collection show 22-mer frequencies indicative of a single-copy Ccd1 at the progenitor Ccd1r locus (Table 3). In addition, no Wc-specific sequence features were detected in teosinte genomes. In line with these data, Southern blot analysis detected only a single Ccd1 copy in two Z. mays spp. parviglumis accessions and one accession of Z. mays spp. Mexicana (Figure S2 in File S1). Together these results suggest that the Wc locus is unique to maize.
Structural heterogeneity in Wc alleles
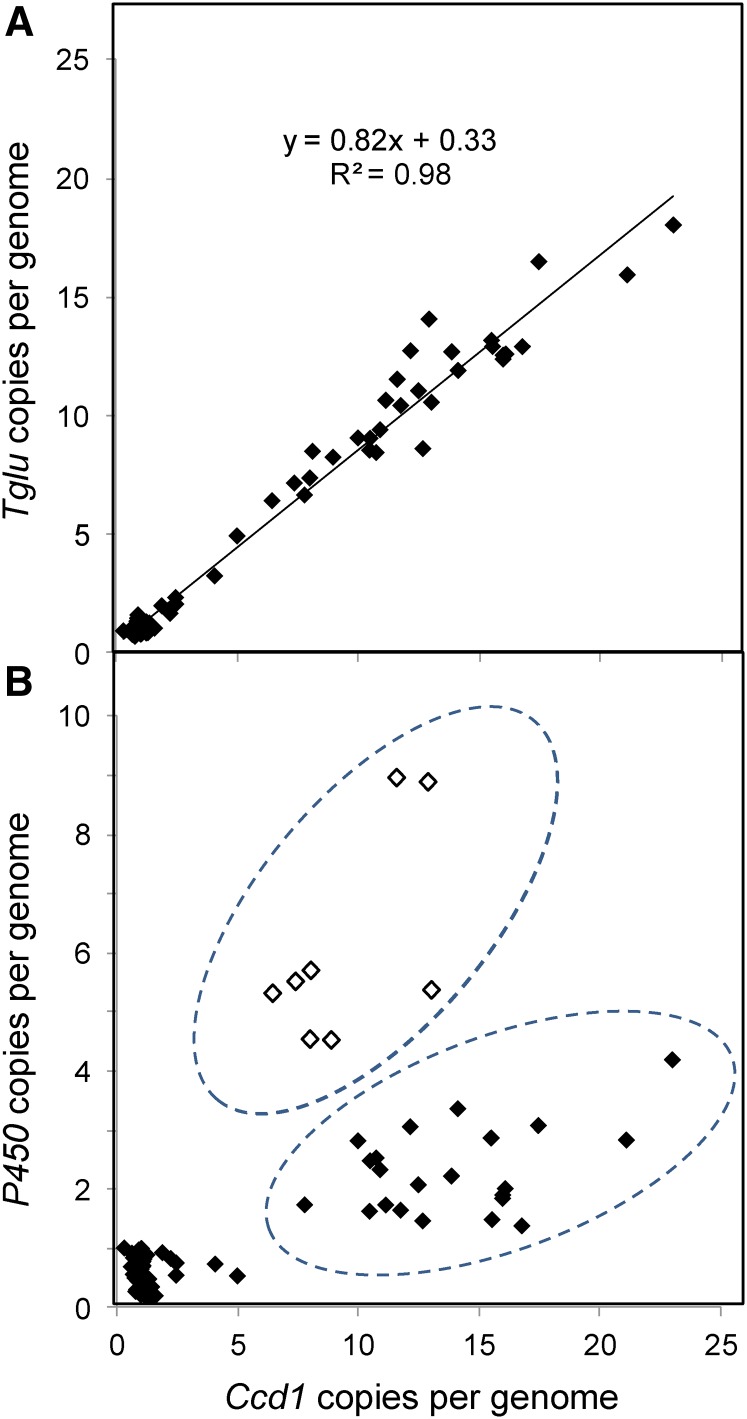
Our model for the Wc locus predicts that Ccd1 and Tglu copy number should vary among Wc alleles in a constant ratio. We found that Ccd1 and Tglu copies were indeed highly correlated (R2 = 0.98; Figure 4a), though the average ratio of Tglu to Ccd1 copies was somewhat less than the expected 1:1 ratio (0.82:1). This could be explained if the initial tandem duplication depicted in Figure S4d in File S1 ended between the Ccd1 and Tglu genes. On that basis, subtracting one copy of Ccd1 gives an average ratio in Wc inbreds of 1.06 ± 0.05 [i.e. Tglu copies/(Ccd1 copies − 1) = 1.06] as predicted if the terminal repeat is truncated.
Figure 4.
Structural heterogeneity in Wc repeats. (a) Ccd1 and Tglu copy number vary uniformly in Wc alleles. Ccd1 and Tglu copy numbers were estimated using k-mer frequency analysis. The line was determined by linear regression with the equation and R2 value indicated. (b) Variation in P450 copy number relative to Ccd1 reveals structural heterogeneity in Wc repeats. Gene copy number was estimated as described in Materials and Methods. Two groups of Wc alleles that differ in P450:Ccd1 copy number ratio are indicated by the dashed ovals. Open symbols, alleles having comparatively high P450:Ccd1 ratios.
In contrast to the uniform Tglu:Ccd1 copy number ratio, the P450:Ccd1 ratio varied among Wc alleles, indicating structural heterogeneity in the Wc repeats (Figure 4b). We postulate that alleles with higher P450:Ccd1 ratios (open symbols in Figure 4b) have a high proportion of repeats with a canonical structure (as delineated in Figure 2a), whereas alleles with lower P450:Ccd1 ratios include a subset of repeats lacking most or all of the P450 sequence. The exact structure of the truncated repeat could not be determined from these data.
The limited variation of Tglu and P450 copy number detected in non-Wc accessions is evident in Figure 4, a and b as tight clustering of copy number values in lines that have ∼1 Ccd1 copy. These data indicate that Wc accounts for nearly all of the copy number variation for these genes as well as Ccd1.
Ccd1 expression is directly proportional to Ccd1 copy number
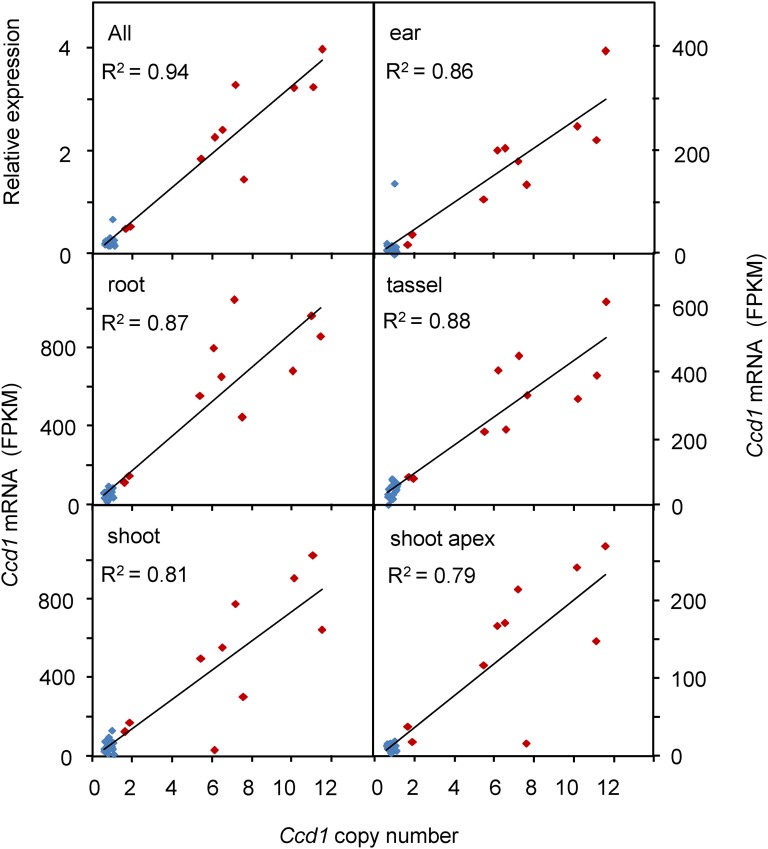
To determine whether the extensive copy number variation at Wc is correlated with gene expression, we analyzed RNAseq data from the 27 diverse nested association mapping (NAM) inbreds (Yu et al. 2008) available at the QTELLER.org database. Based on our analysis (Table 3), 10 inbreds in the NAM collection carry Wc alleles with Ccd1 copy numbers ranging from 2 to 12 copies per genome. As shown in Figure 5, Ccd1 mRNA levels are highly correlated with Ccd1 copy number in root, ear, tassel, shoot, and shoot apex transcriptomes. Nearly linear relationships are revealed for each of the diverse tissues analyzed (R2 values ranging from 0.79 to 0.88 within tissues; R2 = 0.94 for relative expression normalized over all tissues). By contrast, expression of the adjacent Tglu gene shows no discernible relationship to gene dosage despite having a copy number range comparable to that of Ccd1 (R2 = 0.05 for relative expression overall, data not shown).
Figure 5.
Gene expression is proportional to Ccd1 copy number. The relationship between Ccd1 copy number and gene expression in five tissues (root, shoot, ear, tassel, and shoot apex as indicated) was determined for the 27 inbred parents of the NAM population (Yu et al. 2008) using RNAseq data obtained from QTELLER.org. The top left panel shows the correlation of copy number with relative expression averaged over all tissues using values normalized to the means of each tissue. Lines and R2 values were determined by linear regression. Red diamonds, inbreds that have the Wc locus; blue diamonds, non-Wc inbreds.
Wc is often associated with recessive y1 in white-grain maize
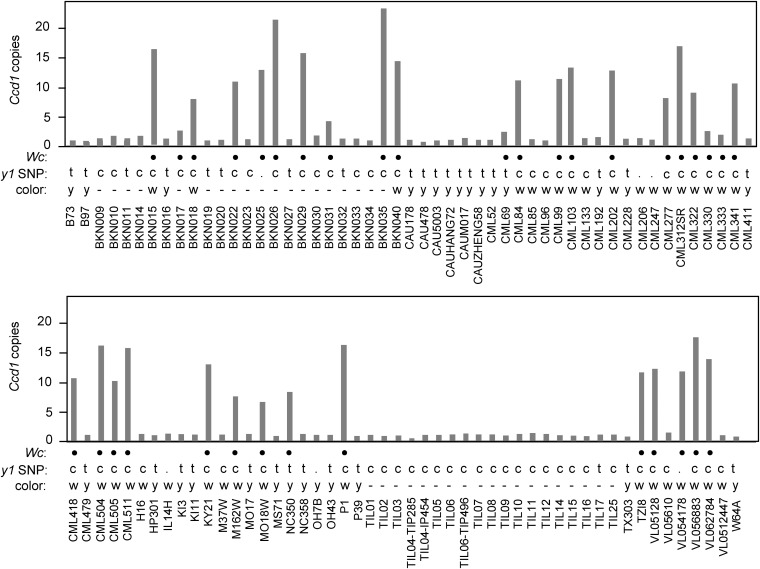
The presence of Wc in “Silver Queen” sweetcorn (Figure 1), inbred A188 (Figure S2 in File S1), and other white inbreds (Stinard 2010) indicates that Wc often occurs in modern inbreds that also carry recessive y1 alleles. A priori, Wc would not be expected to have an obvious phenotype in y1 endosperm due to a low capacity for synthesis of CCD1 substrates. However, evidence indicates that Wc can enhance the white-endosperm phenotype of y1 in backgrounds that carry dominant alleles of Brown aleurone-1 (Bn1) (Stinard 2010 and Figure S6 in File S1). A Southern blot survey of inbreds and landraces from diverse geographical locations (Table 2) confirms that Wc is present in the majority of white accessions (16 of 25, 64%). Wc is not limited to white-kernel maize. At least six yellow or mixed-color landraces from Central and South America contain Wc alleles. Grain color phenotype data were available for an additional 65 maize inbreds in the HapMap2 collection (Figure 6). Consistent with survey results in Table 2, at least 26 of 36 white-grain accessions (72%) contain Wc alleles, whereas only two of 29 yellow-grain inbreds carry Wc. The two exceptions are CML69 (two Ccd1 copies) and NC350 (eight Ccd1 copies). In addition, we noted that within this set of 65 inbreds, white- and yellow-grain color phenotypes, respectively, correlate with C and T variants of SNP PZE0680879922 (Chia et al. 2012). PZE0680879922 is located upstream of the y1 gene, indicating that it could be used as a marker for the y1 genotype. On that basis, we infer that as many as 31 of 35 Wc accessions (89%) in the HapMap2 collection carry both Wc and a recessive y1 allele (Table 3).
Figure 6.
Ccd1 copy number variation in maize and teosinte populations. K-mer frequency analysis of wgs data (see Materials and Methods) was used to estimate CCD1 gene copy number in genomes of diverse maize landraces, inbreds, and teosinte accessions represented in the HapMap2 collection (Chia et al. 2012). Black filled circles, accessions that contain at least two sequence features that are specific to the Wc locus (Table 3). y1 SNP: homozygosity for T (t) and C (c) variants of PZE0680879922; (.), not determined. Color: grain-color phenotype; y, yellow; w, white; -, data not available.
Discussion
Our results indicate that a Ccd1 gene cluster at the Wc locus is the basis for a dominant white-endosperm phenotype that has likely contributed to human selection for grain color (Figure 1). Diversity among the Wc alleles accounts for the extensive Ccd1 copy number variation observed in maize. We show that the Wc locus was created by a Tam3L macro-transposon that duplicated a chromosome segment containing Ccd1 and several nearby genes. Subsequent tandem duplication of Ccd1 at the new locus likely set up further expansion and variation of Ccd1 copy number in Wc alleles through unequal crossing over. Remarkably, transcriptome data indicate that Ccd1 expression in diverse maize tissues is directly proportional to Ccd1 copy number over a range of at least 1–12 copies per genome. While Wc is thus far detected only in maize, its broad geographic distribution is consistent with creation of the locus prior to dispersal of maize from its center of origin in Central Mexico. Interestingly, in diverse landraces as well as modern inbreds, Wc is most often found in white-grain varieties that are also homozygous for recessive alleles of y1 that have little capacity for carotenoid biosynthesis in the endosperm. We suggest that Wc contributed to human selection for grain color by enhancing the y1 white-endosperm phenotype.
hAT transposons are a potent source of structural diversity in the maize genome
The Wc locus illustrates the potency of hAT family transposable elements in generating novel structural-genetic variation in the maize genome. Our model (Figure 2 and Figure S4 in File S1) for the creation of Wc and amplification of Ccd1 builds on previous analyses of the Ac/Ds system in maize (Ralston et al. 1989; Zhang and Peterson 1999; Huang and Dooner 2008; Zhang et al. 2013, 2014). These studies document a variety of chromosome rearrangements arising from interactions between compatible ends of nearby Ac/Ds elements. The capacity for macro-transposition in the Ac/Ds system is augmented by (1) preferential transposition of Ac during DNA replication and (2) the propensity for elements to transpose to nearby sites. Our results indicate that Tam3L has similar characteristics. The macro-transposon structure is confirmed by wgs data and PCR. These results together confirm presence of Tam3La-5′ and Tam3Ld-3′ junctions that share a matching 8-bp host site duplication (Figure 3). The mechanism responsible for tandem duplication of Ccd1 at Wc is less clear. While previously documented mechanisms for Ac-induced DNA rereplication (Zhang et al. 2014) do not account for all aspects of the Wc structure, presence of repeats punctuated by the composite Tam3Lc sequence implicates Tam3L transposons in their formation. Once formed, a partial duplication of the 28-kbp sequence (e.g., Figure S4d in File S1) would have enabled expansion of copy number by unequal recombination of Wc alleles. Overall, the Wc repeats are highly uniform indicating a relatively young age. However, variation in the Ccd1:P450 copy number ratio indicates that Wc alleles contain at least two classes of repeats that have diverged through partial or complete loss of P450 (Figure 4). Hence, the relatively young Wc complex will likely continue to evolve toward greater structural heterogeneity as individual repeats diverge in ways that may affect dynamics of recombination.
Quantitative variation in Ccd1 expression is proportional to copy number
Our results indicate that copy number variation at Wc causes proportional quantitative variation in Ccd1 expression. Remarkably, the gene dosage response is linear up to at least 12 copies per genome (Figure 5). By contrast, the adjacent gene in the Wc repeat Tglu showed no correlation between expression and gene dosage. Clearly, gene amplification alone is not sufficient to produce a stable, proportional dosage response. While the basis for this intriguing, qualitative difference in dosage dependence of the Ccd1 and Tglu genes is unclear, we speculate that the Tam3L insertion at the 5′-end results in more or less constitutive expression of Ccd1 gene copies. In any case, Wc offers a unique opportunity for investigating the effects of tandem duplication on chromatin structure and gene expression.
Haplotype diversity at Wc and selection for grain color
The Wc locus most likely originated shortly after the domestication of maize from teosinte, but prior to dispersal of maize from its center of origin in Mexico. In modern maize, the Wc locus is broadly distributed in white-grain inbreds and landraces from North, Central and South America as well as Africa (Tables 2 and 3). In contrast, the locus is not detected in any of the 22 teosinte accessions (19 Z. mays spp. parviglumis and 3 Z. mays spp. mexicana) surveyed in this study (Figure 6).
The parallel and independent diversification of multi-copy Wc and single-copy Ccd1r haplotypes in maize is intriguing because the variation at these loci would potentially support selection for yellow- as well as white-grained maize. On the one hand, in a y1 background, selection for increased Ccd1 copy number at Wc potentially contributed to breeding of white-endosperm varieties. Conversely, helitron insertions in B73 and OH43 haplotypes that displace or disrupt upstream regulatory sequences of Ccd1r would possibly enhance carotenoid accumulation by attenuating carotenoid turnover in yellow endosperm. The B73 and OH43 variants together show evidence of enrichment in yellow inbreds relative to white inbreds (χ2, P = 0.0065).
Although the striking dominant white phenotype of Wc in yellow maize (Y1) was reported a century ago (White 1917), its contribution to selection and breeding of both traditional and modern white-grain maize has been largely unappreciated. Homozygous y1 progeny obtained from crossing white (y1) and yellow (Y1) inbreds often have off-white (“dingy”) phenotypes due to the presence of residual pigment (Poneleit 2001; Stinard 2010). The off-color phenotype is attributable, at least in part, to Brown aleurone-1 (Bn1, on chromosome 7), which causes accumulation of an unidentified yellow-brown pigment in aleurone (Stinard 2010). In a Bn1 y1 background, Wc alleles inhibit accumulation of the yellow-brown pigment, thus producing a more intense white-endosperm phenotype (Stinard 2010; Figure S6 in File S1). The broad-spectrum CCD1 activity could conceivably degrade the product of the Bn1 pathway.
Persistence of Wc in yellow maize
Wc is also occasionally found in yellow-grain maize (Y1 background). Examples include two modern inbreds, NC350 (eight Ccd1 copies) and CML69 (two Ccd1 copies), as well as landraces from Peru and Central Mexico (Table 2). The majority of these landraces segregate a mixture of white- and yellow-grain phenotypes. Other historically important groups of Wc Y1 maize include “White Cap Yellow Dent” and similar open-pollinated varieties that were grown widely in North America in the 19th and early 20th centuries (Brink 1930). The term “white-cap” was also widely applied to flint landraces grown by Native Americans in Northeastern United States and Eastern Canada (Brink 1930). While these varieties were not included in our survey, the long history of the “white-cap” phenotype implies that Wc Y1 genotypes have been utilized through centuries of human cultivation.
The relative rarity of Wc Y1 in modern yellow inbreds is likely due at least in part to active selection against Wc in breeding maize hybrids. Brink (1930) cited two explicit sources of bias against using Wc Y1 genotypes during formative years of the hybrid seed industry. First, by 1930 Wc Y1 “White Cap Yellow Dent” varieties were known to have reduced provitamin A content relative to yellow-dent (wc Y1) maize affecting their value as livestock feed (Russel 1930). Second, obtaining a uniform endosperm color in the grain harvested from hybrid plants was an important breeding objective. In promoting utilization and marketing of hybrids, uniform color was highlighted as a contrast with the variation typical of competing, open-pollinated varieties. Achieving uniform color in double-cross hybrids common at that time required that the four inbred parents all be either Wc or non-Wc.
Where Wc occurs in yellow maize, an increased rate of carotenoid turnover in endosperm is likely. This in turn could increase production of apo-carotenoid compounds that often contribute to grain quality. Notably, apo-carotenoid products of CCD1 are important determinants of food taste and aroma (Vogel et al. 2008). Some CCD1 products have also been implicated in other biological processes including formation of mycorrhizal symbioses in roots (Sun et al. 2008). The relative importance of aromatic/taste phenotypes of Wc would likely depend on how the maize crop was utilized. Peak expression of Ccd1 during midgrain fill could lead to production of volatiles with potential to increase quality of kernels harvested early for fresh consumption (e.g., sweet corn or Mexican etole). In New England, the preferred maize for preparation of “johnny cakes” is “Rhode Island White Cap,” a modern descendant of Native American white cap landraces (Thomas 1911). Because CCD1 protein is localized to the cytosol (Tan et al. 2003) the enzyme in vivo would normally be expected to have limited access to carotenoids that are located primarily in plastid membranes. However, substrate availability would likely increase as cells in the endosperm undergo desiccation during seed maturation, thus accounting for late onset of visible whitening in the Wc phenotype. As a result, the potential for apo-carotenoid production is likely to be comparatively high in freshly harvested grain. Together these considerations indicate a rich potential for interactions between Wc genotypes and the diverse cultural practices and culinary customs built around maize.
The agricultural genomics of Wc presented here shows how creation of this unusual locus provided a foundation for human selection of white- and yellow-grain maize. Molecular dissection of the Wc locus reveals a striking example of transposon remodeling that has altered a genome in a way historically important to humankind.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.198911/-/DC1.
Acknowledgments
We thank Daniel Ngu and Patrick Schnable (Iowa State University) for permission to use NAM inbred RNAseq data deposited at QTELLER.ORG. We are grateful to Phil Stinard and Marty Sachs at the Maize Cooperation Genetics Stock Center for drawing our attention to the interaction between Wc and Bn1, stimulating discussions, permission to cite Maize Genetics Newsletter notes, and provision of genetic stocks. This work was supported by National Science Foundation grants IOS:1116561 (D.R.M. and K.E.K.) and IOS:152100 (J.-C.G., K.E.K., and D.R.M.), United States Department of Agriculture Institute of Food and Agriculture grant 2011-67003-30215 (D.R.M. and K.E.K.), and Natural Science Foundation of China (91435201, BCT).
Footnotes
Communicating editor: J. A. Birchler
Literature Cited
- Brink R. A., 1930. Some problems in the utilization of inbred strains of corn (Zea mays). Am. Nat. 64: 525–539. [Google Scholar]
- Buckner B., Kelson T. L., Robertson D. S., 1990. Cloning of the y1 locus of maize, a gene involved in the biosynthesis of carotenoids. Plant Cell 2: 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner B., Miguel P. S., Janick-Buckner D., Bennetzen J. L., 1996. The Y1 gene of maize codes for phytoene synthase. Genetics 143: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J.-M., Song C., Bradbury P. J., Costich D., de Leon N., et al. , 2012. Maize HapMap2 identifies extant variation from a genome in flux. Nat. Genet. 44: 803–807. [DOI] [PubMed] [Google Scholar]
- DeBolt S., 2010. Copy number variation shapes genome diversity in Arabidopsis over immediate family generational scales. Genome Biol. Evol. 2: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Dooner H. K., 2002. Intraspecific violation of genetic colinearity and its implications in maize. Proc. Natl. Acad. Sci. USA 99: 9573–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M., 1987. Identification of a receptor for the morphogen retinoic acid. Nature 330: 624–629. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P. B., Puech-Pagès V., Dun E. A., et al. , 2008. Strigolactone inhibition of shoot branching. Nature 455: 189–194. [DOI] [PubMed] [Google Scholar]
- Han F., Lamb J. C., Yu W., Gao Z., Birchler J. A., 2007. Centromere function and nondisjunction are independent components of the maize B chromosome accumulation mechanism. Plant Cell 19: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L. C., McCarty D. R., 1991. The sweet corn “Silver Queen” contains two genes conditioning white seed. Maize Genet. Coop. News Lett. 65: 62. [Google Scholar]
- Hardigan M. A., Crisovan E., Hamilton J. P., Kim J., Laimbeer P., et al. , 2016. Genome reduction uncovers a large dispensable genome and adaptive role for copy number variation in asexually propagated Solanum tuberosum. Plant Cell 28: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. T., Dooner H. K., 2008. Macrotransposition and other complex chromosomal restructuring in maize by closely linked transposons in direct orientation. Plant Cell 20: 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Madan A., 1999. CAP3: a DNA sequence assembly program. Genome Res. 9: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempken F., Windhofer F., 2001. The hAT family: a versatile transposon group common to plants, fungi, animals, and man. Chromosoma 110: 1–9. [DOI] [PubMed] [Google Scholar]
- Koren S., Walenz B. P., Berlin K., Miller J. R., Phillippy A. M., 2016. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. bioRxiv https://doi.org/10.1101/071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. G., Chen Y., 2007. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43: 649–656. [DOI] [PubMed] [Google Scholar]
- Marçais G., Kingsford C. A., 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27: 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaisa K. A., Morgante M., Williams M., Rafalski A., 2003. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell 15: 1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaisa K., Morgante M., Tingey S., Rafalski A., 2004. Long-range patterns of diversity and linkage disequilibrium surrounding the maize Y1 gene are indicative of an asymmetric selective sweep. Proc. Natl. Acad. Sci. USA 101: 9885–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poneleit C. G., 2001. Breeding white endosperm corn, pp. 235–274 in Specialty Corns, Ed. 2, edited by Hallauer A. R. CRC, Boca Raton, FL. [Google Scholar]
- Ralston E., English J., Dooner H. K., 1989. Chromosome-breaking structure in maize involving a fractured Ac element. Proc. Natl. Acad. Sci. USA 86: 9451–9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel W. C., 1930. The vitamin A content of yellow and white-capped yellow dent corn. J. Nutr. 2: 265–268. [Google Scholar]
- Schnable P. S., Ware D., Fulton R. S., Stein J. C., Wei F., et al. , 2009. The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Schwartz S. H., Tan B. C., Gage D. A., Zeevaart J. A., McCarty D. R., 1997. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874. [DOI] [PubMed] [Google Scholar]
- Springer N. M., Ying K., Fu Y., Ji T., Yeh C. T., et al. , 2009. Maize inbreds exhibit high levels of copy number variation (CNV) and presence/absence variation (PAV) in genome content. PLoS Genet. 5: e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinard P. S., 1995. Three-point linkage data for Wc1 Bf1 bm4 on 9L. Maize Genet. Coop. News Lett. 69: 130. [Google Scholar]
- Stinard P. S., 2010. Isolation and characterization of a dominant inhibitor of Bn1. Maize Genet. Coop. News Lett. 84: 42–43. [Google Scholar]
- Studer A., Zhao Q., Ross-Ibarra J., Doebley J., 2011. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 43: 1160–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Hans J., Walter M. H., Matusova R., Beekwilder J., et al. , 2008. Cloning and characterisation of a maize carotenoid cleavage dioxygenase (ZmCCD1) and its involvement in the biosynthesis of apocarotenoids with various roles in mutualistic and parasitic interactions. Planta 228: 789–801. [DOI] [PubMed] [Google Scholar]
- Tan B. C., Schwartz S. H., Zeevaart J. A., McCarty D. R., 1997. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 94: 12235–12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. C., Joseph L. M., Deng W. T., Liu L., Li Q. B., et al. , 2003. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 35: 44–56. [DOI] [PubMed] [Google Scholar]
- Thomas E. K., 1911. Corn and its uses. J. Education. 74: 327. [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., et al. , 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200. [DOI] [PubMed] [Google Scholar]
- Vogel J. T., Tan B. C., McCarty D. R., Klee H. J., 2008. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 283: 11364–11373. [DOI] [PubMed] [Google Scholar]
- White O. E., 1917. Inheritance of endosperm color in maize. Am. J. Bot. 4: 396–406. [Google Scholar]
- Yu J. M., Holland J. B., McMullen M. D., Buckler E. S., 2008. Genetic design and statistical power of nested association mapping in maize. Genetics 178: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A., Heath T. G., Gage D. A., 1989. Evidence for a universal pathway of abscisic acid biosynthesis in higher plants from 18O incorporation patterns. Plant Physiol. 91: 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Peterson T., 1999. Genome rearrangements by nonlinear transposons in maize. Genetics 153: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zuo T., Peterson T., 2013. Generation of tandem direct duplications by reversed-ends transposition of maize ac elements. PLoS Genet. 9: e1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zuo T., Wang D., Peterson T., 2014. Transposition-mediated DNA re-replication in maize. eLife 3: e03724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Naqvi S., Breitenbach J., Sandmann G., Christou P., et al. , 2008. Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc. Natl. Acad. Sci. USA 105: 18232–18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Wc Ccd1 genomic and cDNA sequences are deposited in Genbank (accessions: DQ100348, DQ100347, and cDNA DQ100346). The Wc bac sequence is Genbank accession KX760165. Genetic strains used in this study are available by request.