Abstract
Following primary infection, whether Human Cytomegalovirus (HCMV) enters either the latent or lytic lifecycle is dependent on the phenotype of the cell type infected. Multiple cell types are permissive for lytic infection with HCMV whereas, in contrast, latency is restricted to a very specific population of CD34+ cells resident in the bone marrow. It is becoming increasingly clear that one of the mechanisms that promote HCMV latency involves the recruitment of histone proteins to the major immediate early promoter (MIEP) which are heavily methylated to promote a transcriptionally inactive state. Integral to this, is the role of cellular transcriptional repressors that interact with histone modifying enzymes that promote and maintain this repressed state during latency. Crucially, the chromatin associated with the MIEP is dynamically regulated – myeloid cell differentiation triggers the acetylation of histones bound to the MIEP which is concomitant with the reactivation of IE gene expression and re-entry into lytic infection.
Interestingly, this dynamic regulation of the MIEP by chromatin structure in latency extends not only into lytic infection but also for the regulation of multiple viral promoters in all phases of infection. HCMV lytic infection is characterised by a timely and co-ordinated pattern of gene expression that now has been shown to correlate with active re-modelling of the histones associated with Early and Late promoters. These effects are mediated by the major IE products (IE72 and IE86) which physically and functionally interact with histone modifying enzymes resulting in the efficient activation of viral gene expression. Thus chromatin appears to play an important role in gene regulation in all phases of infection. Furthermore, these studies are highly suggestive that an intrinsic cellular anti-viral response to incoming viral genomes is to promote chromatinisation into a transcriptionally repressed state which the virus must overcome to establish a lytic infection. What is becoming evident is that chromatin structure is becoming as increasingly important for the regulation of viral gene expression as it is for cellular gene expression and thus understanding the mechanisms employed by HCMV to modulate chromatin function could have broader implications on our understanding of the control of gene expression in general.
Introduction
Two hallmark, albeit not unique, characteristics of herpes virus infections are the temporal regulation of lytic viral gene expression and the ability of the virus to establish latent infections with periodic reactivation events occurring during the lifetime of the host. Although both primary infection and reactivation events in healthy hosts are usually asymptomatic this is in stark contrast to the clinical outcome in a number of patient populations with impaired immune responses where Human cytomegalovirus (HCMV) remains a significant cause of morbidity and mortality in these individuals (Crough and Khanna, 2009; Legendre and Pascual, 2008; Limaye et al., 2008).
It is becoming increasingly clear that timely and regulated viral gene expression in all phases of infection is co-ordinated by an intricate interplay of viral and cellular factors. Viral gene expression during lytic infection occurs in 3 broad phases termed immediate-early (IE), Early (E) and Late (L) – a classification based on the effects on expression of protein synthesis and viral DNA replication inhibitors during infection – whereby IE gene expression occurs independent of de novo protein expression, E&L gene expression is dependent on IE proteins (predominantly IE72 and IE86) and L gene expression is predominant during and post DNA replication. Thus, given the pivotal role of the major IE proteins in orchestrating viral gene expression and, consequently, the progress of infection a number of laboratories have studied intensively the complex regulation of the major immediate-early promoter (MIEP). Through these studies, we have begun to understand the key role cellular mechanisms of gene regulation play in the control of the MIEP which, on further study, have been extrapolated into the control of early and late gene expression as well as the regulation of gene expression in other herpes viruses.
In contrast to lytic infection, latent gene expression is far more restricted with only a few transcripts having been identified in natural latency (Slobedman et al., 2010). In the main these transcripts are not latency-specific (Bego et al., 2005; Lunetta and Wiedeman, 2000; Petrucelli et al., 2009) suggesting that multiple mechanisms control expression during different phases of infection. A key event in latency, however, is the repression of the MIEP by cellular transcription factors and also higher order chromatin structure (Reeves and Sinclair, 2008; Sinclair, 2010). Furthermore, studies of cells that show differentiation-dependent permissiveness for lytic infection have correlated permissiveness with the specific modifications on histones bound to the MIEP. This review will focus on the control of the MIEP as a paradigm for the regulation of viral promoters by chromatin and, subsequently, how this work has been used to explore the regulation of all phases of HCMV gene expression.
Chromatin mediated regulation of gene expression and the Histone Code Hypothesis
It is approaching 40 years since the first seminal observations regarding chromatin structure were made (Kornberg, 1974; Oudet et al., 1975). Nuclease digestion of cellular DNA resulted in the generation of c.200bp fragments that were resistant to digestion (Hewish and Burgoyne, 1973). Biochemical studies subsequently elucidated the presence of a repeating protein complex on chromosomal DNA which was labelled the nucleosome – the repeating structure of chromatin structure responsible for the classical ‘beads on a string’ description of cellular DNA (Kornberg, 1974; Kornberg and Thomas, 1974; Oudet et al., 1975). The accepted interpretation of this structure is an octamer of histone proteins H2A, H2B, H3 and H4 with histone H1 acting as a linker protein to provide stability (Chung et al., 1978). Indeed, one contention of early studies on the regulation of viral gene expression by chromatin was whether histones were recruited to the viral genome in a manner analogous to that observed on cellular promoters. Recent data suggests that this may be the case: Nitzche et al performed a comprehensive analysis of histone occupancy throughout productive infection and observed that the stoichiometry of the recruitment of different histone proteins to viral DNA would be consistent with the assembly of the classical nucleosome (Nitzsche et al., 2008). A caveat of this study was that histone occupancy was lower and the distribution not uniform throughout the genome suggesting that differences could exist – particularly during those initial events of infection.
The recruitment of the nucleosome to DNA was shown to promote compaction of cellular DNA and thus play an important role in packaging (Lilley and Pardon, 1979). However, it was also observed that the structure of the nucleosome was dynamic and that modification of component histones could significantly alter the architecture and accessibility of the DNA region and thus it was hypothesized that this could also provide a mechanism for transcriptional control of gene expression (Weintraub and Groudine, 1976; Weintraub et al., 1976). Our current understanding of chromatin biology suggests that this does appear to be the case as a number of reversible modifications to histones (e.g. acetylation, methylation, phosphorylation, sumoylation and ubiquitination) have been associated with the function and activity of a genomic region (Doenecke and Gallwitz, 1982; Lopez-Rodas et al., 1993; Lusser, 2002; Shiio and Eisenman, 2003; Zhang and Reinberg, 2001) and these modifications form the basis of the ‘histone code hypothesis’ (Strahl and Allis, 2000).
Histone acetylation as a marker of transcriptionally active chromatin
The modification of histone proteins, and particularly histones H3 and H4, have been extensively characterised for their relationship with transcription appears to define the function of the nucleosome. To date, it is generally accepted that acetylation of the lysine tails of histones H3 and H4 correlates strongly with transcriptionally active regions of the genome (Doenecke and Gallwitz, 1982; Lusser, 2002; Mizzen and Allis, 1998). Consistent with these observations, histone acetyltransferases (HATs) and histone deacetylases (HDACs) act antagonistically to promote or repress gene expression, respectively (Khochbin et al., 2001; Kuo and Allis, 1998). Indeed, some of the first instructive observations regarding the regulation of viral gene expression by chromatin were that histone deacetylases inhibitors (i.e. trichostatin A (TSA) and valproic acid) significantly enhanced infection in both murine and human CMV infections (Kuntz-Simon and Obert, 1995; Tang and Maul, 2003).
It is worth noting that the correlation between histone acetylation and gene expression is just that – i.e. correlative. Some promoters are active even in the absence of significant changes in histone acetylation (Dudley et al., 1999) or increased DNase hypersensitivity of the promoter (Urnov et al., 2000; Wong et al., 1998) - by definition, the looser association of acetylated histones with DNA makes DNA less protected to the action of nucleases. However, and particularly in the context of HCMV infection, histone acetylation status has been a strong and reliable indicator of the transcriptional activity of a promoter and thus has been used extensively in CMV research.
Histone methylation as a marker of transcriptionally repressed chromatin
As well as acetylation, histone methylation is the other major modification that has been studied intensively (Zhang and Reinberg, 2001). Historically, methylation has been associated with transcriptionally inactive regions of the genome (Razin, 1998) and, as always, there are exceptions to the rule. The first characterised marks were di- and tri-methylation modifications on H3 histones at lysine residues 9 and 27 (K9 and K27) (Cao et al., 2002; Peters et al., 2002). Still regarded as bona fide markers of transcriptional repression their function is augmented by the recruitment of heterochromatin protein 1 (HP-1) to promoters through a high affinity interaction with K9 and K27 methylated H3 histones (Bannister et al., 2001; Nielsen et al., 2002). This interaction serves to further impact a transcriptionally repressive environment at the region of the genome and is regarded as a marker of transcriptionally inactive regions (James et al., 1989).
However, caveats exist. Later studies identified that methylation of lysine 4 (K4) or arginine 3 (R3) actually correlated with transcriptional activation. Methylation of R3 promotes histone acetylation and subsequent transcriptional activation and K4 methylation is now an established marker of a recently active promoter (Wang et al., 2001). Subsequently, the methylation of H3-K4 is now used to address whether a promoter has recently been transcriptionally active although an important caveat to interpreting such analyses becomes a key point: H3-K4 methylation appears to be indicative of a transcriptionally active promoter but not necessarily of gene expression per se. Provocative data from analyses of stem and differentiated cells suggests that a number of unexpressed genes have H3-K4 methylation marks associated with their promoters (Guenther et al., 2007). This suggests that chromatinised promoters once believed to be in a fixed repressed state actually maybe more dynamic than once thought and also highlights the importance of a more measured and integrated approach to chromatin analyses in the context of cellular and viral gene expression.
The profound role chromatin plays in the regulation of eukaryotic genes as well as the rapid advancement of the molecular approaches to address these functions has made it possible to sensibly address more precisely the role of chromatin and histone proteins in not just the regulation of cellular, but also viral gene expression. Given the obligate requirement of a virus on the host cell to survive it is perhaps not surprising that viruses are subject to similar mechanisms of gene control as eukaryotic cells and this, perhaps, is no more overt than in viruses that establish lifelong latent infections in the host.
The regulation of the MIEP in non-permissive cells
The regulation of the MIEP during both latent and lytic infection has been the focus of intensive research since the first definitions of this region (Boshart et al., 1985; Lubon et al., 1989; Thomsen et al., 1984). The IE genes hijack a number of cellular processes to prepare the cell for efficient DNA replication and progeny production (Castillo and Kowalik, 2002; Stinski and Petrik, 2008). Consequently, the mechanisms that govern activation of the MIEP are of direct consequence not only to the outcome of infection but also, of course, have potential therapeutic applications.
HCMV lytically infects a large number of cell types including endothelial cells, epithelial cells, fibroblasts, smooth muscle cells, and terminally differentiated dendritic cells (DCs) and macrophages (Sinzger et al., 2008). In contrast, cells that establish a latent HCMV infection are far more restricted (Sinclair and Sissons, 2006). Latency is characterised by the carriage of the viral genome in the absence of IE gene expression (Mendelson et al., 1996; Taylor-Wiedeman et al., 1991) but, crucially, IE gene expression can be re-activated when a favourable cellular environment is encountered (Hahn et al., 1998; Soderberg-Naucler et al., 1997; Taylor-Wiedeman et al., 1994; Zhuravskaya et al., 1997). One well studied site of HCMV latency is the haematopoietic progenitor cell population resident in the bone marrow (Bego and St Jeor, 2006; Mendelson et al., 1996; Reeves et al., 2005b; Sinclair and Sissons, 2006; Sindre et al., 1996). Furthermore, early myeloid progenitors (Goodrum et al., 2002; Hahn et al., 1998; Khaiboullina et al., 2004; Kondo et al., 1994; Kondo et al., 1996; Minton et al., 1994; Slobedman et al., 2002) and monocytes (Bevan et al., 1991; Taylor-Wiedeman et al., 1991) also support latent HCMV infections and thus a highly restricted cell pool promotes and carries the latent infection. Clearly there is something unique about these cell types that promote a repressive environment. Whether this is intrinsic to the cell, due to the engagement of a unique entry pathway in latent infection or, as recently hypothesized, due to a defect in trafficking of an essential virion transactivator to the nucleus (Penkert and Kalejta, 2010; Saffert et al., 2010) is still an area of debate. Although none of these mechanisms can be discounted the observation that the transfected MIEP is less active in undifferentiated myeloid cells still argues that a major reason is that the cellular environment of non-permissive cells is intrinsically inhibitory to the activity to the promoter (Sinclair et al., 1992).
The MIEP contains binding sites for multiple cellular transcription factors and thus is subject to complex regulation (Meier and Stinski, 1996). Multiple binding sites for the transcriptional activators CREB/ATF, AP1 and NF-kB are evident and are likely responsible for the prodigious activity of the MIEP in permissive cells and hence its application in multiple mammalian expression vectors. However, the full length MIEP also contains multiple binding sites for Ying Yang 1 (YY1), Ets-2 repressor factor (ERF) and growth factor independent 1 (Gfi-1) which have been shown to exert potent transcriptional repression of the MIEP (Bain et al., 2003; Liu et al., 1994; Zweidler-Mckay et al., 1996). Clearly, the relative balance of these transcription factors could dictate the outcome of infection and, indeed, it has been hypothesized that high levels of repressors in non-permissive cells drive a latency phenotype whereas in permissive cells high levels of activators favour a lytic infection or promote reactivation from latency upon cellular differentiation to either a DC or macrophage phenotype.
The observation that YY1 and ERF could exert their biological effects through an interaction with cellular enzymes that promote the post-translational modification of histones (Thomas and Seto, 1999; Wright et al., 2005) suggested that higher order chromatin structure may play a role in the regulation of the MIEP. Indeed, the observation that the addition of HDAC inhibitor, TSA, to the normally non-permissive NT2D1 human teratocarcinoma cell line rendered them permissive for HCMV infection (as measured by IE expression) was a highly suggestive of a role for histone proteins (Meier, 2001; Murphy et al., 2002). Further studies provided more direct evidence. Following exogenous infection of non-permissive NT2D1 cells or primary monocytes and, subsequently, haematopoietic CD34+ cells, the MIEP was clearly associated with HP1 protein when analysed by Chromatin Immunoprecipitation (ChIP) assays (Murphy et al., 2002; Reeves et al., 2005a). Thus in non-permissive cells the incoming viral genomes MIEPs are sequestered in a repressive chromatin structure by 24-72 hours post infection. Crucially, this appeared to be a phenomenon specific to non-permissive cells as the same analysis 24 hours post infection of permissive differentiated NT2D1 cells or macrophages showed that the MIEP was predominantly associated with acetylated histones (Murphy et al., 2002) – consistent with the known permissiveness of these cell types for IE gene expression (Gonczol et al., 1984; Lathey and Spector, 1991; Murphy et al., 2002).
Clearly, these data suggested that the control of natural latency could be subject to the same mechanisms of regulation. Consistent with this hypothesis, the chromatin signature of histones associated with the MIEP in genomes isolated from the cells of healthy seropositives correlated directly with the phenotype of the virus infection (Reeves et al., 2005b). In both CD34+ cells and CD14+ monocytes the MIEP was associated with HP-1, consistent with the establishment and carriage of latent viral genomes in these cells. However, differentiation to a DC phenotype – which promoted the reactivation of IE gene expression – was concomitant with the histones becoming predominantly acetylated (Reeves et al., 2005b). Thus in natural latency, the viral genomes were associated with histones and the post-translational modification of these histones was dynamic. Furthermore, these observations in HCMV natural latency can be re-capitulated not only in experimental latency (Reeves et al., 2005a) but also in MCMV studies that have characterised the regulation of viral IE gene expression in the latency and reactivation phases of the virus lifecycle (Liu et al., 2008; Reddehase et al., 2008). Thus it appears that higher order chromatin structure is an intrinsic mechanism for regulation of CMV gene expression during latency. A likely scenario is that high levels of cellular transcriptional repressors such as YY1 and ERF in non-permissive results in transcriptional repression which is augmented by the recruitment of HDACs and histone methyltransferases (HMTs) to establish long term repression. Recently, it has been shown that Gfi-1 also plays a critical role in epigenetic regulation of haematopoietic gene expression via interactions with histone demethylase LSD1 and class I HDACs (Saleque et al., 2007) which also suggests that it could also contribute to the regulation the MIEP in a similar manner.
The regulation of latent gene expression in non-permissive cells
One of the defining characteristics of latency is the absence of lytic IE gene expression. These data suggested that higher order chromatin structure was playing a role in the regulation of the MIEP during latency but what about other promoters? Since IE72 and IE86 play a critical role in the expression of the E and L genes it is possible that the control of the MIEP is alone sufficient to prevent significant expression of the E and L gene transcripts. However, it is becoming increasingly evident that there is a limited, but distinct, viral transcriptional program in latency with expression from 4 gene loci having been confirmed in natural latency. For instance, a number of transcripts arising from both strands in the MIE region of HCMV have been reported from analyses of Granulocyte/macrophage- progenitors (GM-Ps) – an early haematopoietic cell type exhibiting cell surface markers of myeloid cell commitment (Kondo et al., 1996). Using the same cell population, an array analysis identified that a viral interleukin (IL) -10 homologue UL111A (subsequently shown to be a splice variant of the lytic vIL-10) is expressed during latency also (Jenkins et al., 2004). An independent microarray analysis also identified a number of potential candidates (Goodrum et al., 2002) but, to date, only UL138 and LUNA have been confirmed in natural latency (Bego et al., 2005; Goodrum et al., 2007; Goodrum et al., 2002; Reeves and Sinclair, 2010). The identification of LUNA was slightly serendipitous insofar Bego et al were characterising whether UL81 (which was suggested as a potential latent transcript by the analysis by Goodrum et al., 2002) was expressed in natural latency. However, careful molecular characterisation of the potential UL81 transcript actually identified that the transcript was being expressed from the opposite strand and in fact represented a novel RNA that was expressed in latent and lytic infections (Bego et al., 2005; Reeves et al., 2010; Reeves and Sinclair, 2010).
The identification of latently expressed gene products begged the question are the regulated by chromatin as well. Interestingly, in the case of the LUNA promoter there did appear to be a direct correlation between transcriptional activity and the histone modifications. In naturally latent CD34+ cells expressing LUNA but not IE, the LUNA promoter was associated predominantly with acetylated histones (Reeves and Sinclair, 2010). Intriguingly, during lytic infection the LUNA promoter appears to behave like an E promoter and expression is entirely dependent on the expression of IE72 (Reeves et al., 2010). Clearly, since IE72 is not expressed CD34+ cells and monocytes this suggests that other mechanisms (most likely cellular) are active during viral latency. Indeed, the presence of GATA 1 and 2 binding sites, transcription factors highly expressed in primitive myeloid cells (Ferreira et al., 2005; Weiss and Orkin, 1995), suggests that GATA could be driving latent gene expression. The LUNA promoter is GATA-2 responsive (Reeves and Sinclair, 2010) and the GATAs interacts with HATs (Blobel et al., 1998; Boyes et al., 1998). It remains to be determined the molecular mechanisms governing the expression of the other latency associated genes but one would predict that their promoters exhibit similar architectures to the LUNA promoter (i.e they will encode binding sites for cellular transcription factors expressed at high levels in haematopoietic cells) in order for latent gene expression to occur in primary myeloid cell precursors.
The regulation of the viral gene expression during lytic infection
The prevailing view of HCMV lytic infection is that viral transactivators promote IE gene expression which in turn drives early and then finally late gene expression (Kalejta, 2008; Spector, 1996). The whole process generally occurs over a period of 3-5 days and results in progeny virus production. The model of infection argued that the action of virion transactivators was augmented by high levels of cellular transcriptional factors that drove IE gene expression and, indeed, subsequent work that has characterised the role of histones does not lessen the importance of these mechanisms during infection. However, it is becoming evident from a number of studies that histone modification is playing a marked role in the regulation of the MIEP in lytic infection as well (Cuevas-Bennett and Shenk, 2008; Groves et al., 2009; Ioudinkova et al., 2006; Murphy et al., 2002; Reeves et al., 2006; Woodhall et al., 2006).
As previously stated, a number of studies have shown both MCMV and HCMV IE gene expression was elevated in the presence of HDAC inhibitors (Groves et al., 2009; Kuntz-Simon and Obert, 1995; Michaelis et al., 2004; Michaelis et al., 2005; Tang and Maul, 2003). Although suggestive of a role for chromatin, data from studies using chemical inhibitors can be attributed to off-target effects. Therefore, the first direct physical evidence of functional bound histones during lytic infection was the identification of acetylated histones bound to the MIEP in infected macrophages (Murphy et al., 2002). An intriguing aspect of this study was the detection of some MIEPs also associated with HP-1 in these cells at 24 hours post infection. Two explanations were immediately apparent: either this represented a proportion of infected cells with continually repressed viral genomes, or it suggested a more dynamic regulation of the MIEP? Since active regulation of the MIEP by histones would be consistent with the dynamic regulation of IE gene expression observed in infected cells this was addressed further. It is a long-standing observation that auto-repression of the MIEP is mediated by the binding of IE86 to the cis repression sequence (crs) at later times of infection (Cherrington et al., 1991; Liu et al., 1991). What became evident was that IE86 may be mediating this effect via interactions with HDACs and HMTs such that at late times of infection the majority of MIEPs were associated with markers of repressed chromatin (Reeves et al., 2006). However, deletion of the crs from the virus resulted in aberrant and elevated IE gene expression which correlated with the detection of acetylated histones on the MIEP throughout infection (Reeves et al., 2006).
Clearly the MIEP is subject to dynamic regulation during lytic infection which is mediated, at least in part, by the known interactions between IE86 and chromatin remodelling enzymes (Bryant et al., 2000; Hsu et al., 2004; Park et al., 2007; Reeves et al., 2006). However, not only IE86 is known to target these enzymes. IE72, the most abundant IE gene product, is a potent transactivator of viral gene expression and, at low MOIs, is essential for virus growth (Greaves and Mocarski, 1998; Mocarski et al., 1996). In a subsequent study by Nevels et al, they identified that the mechanism by which IE72 activates E and L gene expression during infection is via an interaction with class I HDACs (Nevels et al., 2004). This study suggested that physical sequestration of HDACs away from E and L promoters by IE72 was sufficient to promote viral gene expression. Consistent with this, addition of TSA to cells infected with an IE72-deletion virus was sufficient to rescue a wild type growth phenotype. These data were highly suggestive of the dynamic regulation of all viral promoters by histone modifications.
Subsequently, it has been shown by a number of laboratories that the MIEP, Early and Late promoters are subject to a co-ordinated pattern of regulation by histone modification during the course of HCMV infection (Cuevas-Bennett and Shenk, 2008; Groves et al., 2009; Ioudinkova et al., 2006; Nitzsche et al., 2008). Detailed analyses of the E and L promoters studied showed that upon infection these promoters were associated predominantly with methylated histones at H3-K9. However, as the infection proceeded there was increased histone H3 and H4 acetylation. Consistent with transcriptional activation viral gene expression, H3-methylated K4 marks could be detected at E and L promoters as the infection proceeded.
Although the studies principally reported very similar findings, some differences were evident. Notably, one study reported the recruitment of methylated histones (H3-K9) to all promoters analysed immediately post infection (Groves et al., 2009). However, a preceding study suggested that the histones associated with the MIE and UL37 promoters were heavily acetylated at the same timepoint (Cuevas-Bennett and Shenk, 2008). It is likely that the differences are due to a difference in the multiplicity of infection (MOI) used. Indeed, it is a possibility that the recruitment of repressive chromatin represents an anti-viral response to incoming viral genomes by the cell. By shutting down viral gene expression the cell attempts to block infection and thus the differential seen with different MOIs is probably illustrating a dose-dependent effect. For instance, at lower MOIs the cellular response is more effective hence the initial repression observed. In contrast, at higher MOIs viral functions that antagonise this response are more abundant and thus overcome repression of the MIEP faster. Consistent with this, the repressive effects of hDaxx over-expression on HCMV infection could be overcome by the titration of more viruses into the system (Woodhall et al., 2006).
The exact contribution histone mediated regulation of viral gene expression makes to lytic infection is hard to quantify. A significant proportion of viral genomes are associated with histones during infection (a minimum of 20%) and the detection of H3-K4 methylation of histones bound to the MIEP is suggestive that they are transcriptionally active (Groves et al., 2009). Perhaps, more definitive evidence would be a ChIP re-ChIP strategy where the binding of RNA polymerase II to the MIEP could be correlated directly with the recruitment of modified histones to the same MIEPs. However, the published data does strongly suggest that during CMV infection histone modifications play a prominent role in the regulation of viral gene expression.
Virion transactivators must overcome a pre-immediate early environment repressive for viral gene expression
Whether this pre-immediate early environment observed at low MOIs suggests that the default pathway upon infection is latency is an intriguing hypothesis (Penkert and Kalejta, 2010; Saffert et al., 2010; Sinclair, 2010). Indeed, the concerted effect of pp71 on hDaxx and ATRX (and thus potentially associated chromatin modifying functions) (Cantrell and Bresnahan, 2006; Hofmann et al., 2002; Ishov et al., 2002; Lukashchuk et al., 2008; Saffert and Kalejta, 2006; Saffert and Kalejta, 2007), the increased ‘permissiveness’ in TSA treated cells (Groves et al., 2009), the abrogation of hDaxx-mediated repression by TSA (Woodhall et al., 2006), and the more pronounced growth defects of mutant viruses at low MOIs (Bresnahan and Shenk, 2000; Mocarski et al., 1996) as well as the continued targeting of PML, hDaxx and histone modifying enzymes by IE functions (Ahn et al., 1998; Ishov et al., 1997; Nevels et al., 2004; Reeves et al., 2006; Reeves et al., 2010; Tavalai et al., 2006; Wilkinson et al., 1998) all support the hypothesis that there is a pre-immediate early environment involving histone proteins that the virus has to overcome to initiate an efficient infection.
Whether the cellular environment encountered upon infection is a bona fide latent environment, however, is still unclear. The transcriptional profile of myeloid progenitor cells differs markedly from that of fibroblasts upon infection with HCMV which, in itself, will impact on the outcome of infection (Browne et al., 2001; Slobedman et al., 2004). One intriguing observation recently reported is that pp71 fails to translocate to the nucleus and degrade hDaxx, a key player during lytic infection, during latent infection (Penkert and Kalejta, 2010; Saffert et al., 2010). However, siRNA mediated depletion of hDaxx from normally non-permissive T2 cells did not render them permissive for infection in a separate study (Groves and Sinclair, 2007). Whilst this does not necessarily preclude any role for hDaxx it does lessen the argument against hDaxx being the sole determinant of latency. Indeed, the observation that the transfected MIEP is less active in undifferentiated myeloid cells suggests that the longstanding hypothesis that the differential expression of intrinsic cellular repressors in these cells is crucial (Sinclair and Sissons, 2006) and, to date, there is no evidence that hDaxx expression, for instance, is elevated in myeloid progenitor cells. Similarly, the addition of Vasoactive Peptide to normally non-permissive T2 cells promoted IE expression with no discernible effects on hDaxx levels (Yuan et al., 2009). Finally, clinical isolates have been suggested to establish a latent infection more efficiently than laboratory strains suggesting the presence of a viral determinant (UL138) specific to clinical isolates also playing a role (Goodrum et al., 2007).
Whether other virion proteins play a role is less well characterised. Interestingly, the UL69 protein, which has been extensively characterised as an RNA-binding protein (Toth and Stamminger, 2008), has also shown to interact with hSPT6 (Winkler et al., 2000) - an important mediator of nucleosome assembly on naked DNA (Bortvin and Winston, 1996). UL69 transactivates IE gene expression and thus, in the context of the data presented here, it has been hypothesized that this interaction could be important for UL69 function. However, the specific chromatin function of SPT6 is for the efficient assembly of nucleosomes on DNA and in fact UL69 competes with the interaction of SPT6 with histone H3 (Winkler et al., 2000) which theoretically could de-stabilize chromatin. Thus the exact effect, if any, of UL69 on the recruitment of chromatin to the viral genome is thus unclear and indeed the interaction of UL69 with hSPT6 may actually be important for the recruitment of a second function of hSPT6 to the MIEP – hSPT6 enhancement of RNA polymerase II activity (Endoh et al., 2004). However, it is a possibility that an interaction of UL69 with SPT6 at late stages of infection may de-stabilize chromatinisation of newly replicated genomes which could provide one explanation for the lack of histone proteins associated with packaged viral genomes in progeny virus (Groves et al., 2009; Nitzsche et al., 2008).
The regulation of viral gene expression is not solely due to chromatin
Despite strong evidence of a role for histone proteins in the regulation of viral gene expression the importance of other mechanisms should not be dismissed as a result. For instance, although the addition of TSA to non-permissive NT2D1 does render them permissive for HCMV infection (Murphy et al., 2002) – arguably by blocking the formation of repressive chromatin on the MIEP - the ability of TSA to reactivate IE gene expression in quiescently infected NT2D1 cells reduces over time (Meier, 2001). Thus, the action of other mechanisms (i.e. direct transcriptional repression of gene expression) clearly plays a major role also. Similarly, the addition of TSA to long-term latently infected myeloid progenitors does not trigger reactivation (unpublished observations; M. Reeves & J. Sinclair) – and again it is likely that chromatin mediated gene regulation is just one facet of a complex environment regulating latency and reactivation.
Further evidence for multiple levels of regulation was an analysis of the LUNA promoter (Reeves et al., 2010). The expression of LUNA during latency correlated with the recruitment of acetylated histones to the promoter during latency (Reeves and Sinclair, 2010). Similarly, by transfection the LUNA promoter is TSA-responsive (Reeves et al., 2010). These data suggested that the IE72 dependent regulation of LUNA expression during lytic infection was as reported for other early promoters – i.e. sequestration of HDAC activity away from the promoter (Nevels et al., 2004). However, this was not the case as in the absence of IE72, LUNA expression during infection was not rescued by TSA. Intriguingly, elevated LUNA gene expression was observed in wild type infected cells treated with TSA. The simplest explanation was that TSA increases IE72 expression which, in turn, drives increased LUNA gene expression. Another possibility relies on the premise that histone acetylation makes a promoter more accessible to positive effectors and the transcriptional machinery. However, unless the steric hindrance of the repressor complex is removed, transcriptional activators are not recruited and thus no positive effect of TSA on transcription is realised. A similar explanation can be used to explain the inability to reactivate latent HCMV from normally non-permissive cells with HDAC inhibitors. The acetylation of histones plays a role but, alone, is not sufficient unless other criteria are met.
Concluding Remarks
The regulation of viral gene expression by the recruitment and dynamic modification of histone proteins is by no means unique to CMV. Similar mechanisms of control are also evident in different phases of the lifecycles of other herpesviruses (Knipe and Cliffe, 2008; Tempera and Lieberman, 2010), SV40 virus (Georgiev et al., 1981) and retro/lentiviruses (Easley et al., 2010). In the case of the herpes viruses whether this chromatinisation is a default cellular response to naked DNA or, the far less likely alternative of being actively promoted by the virus is not known. No matter the mechanism, incoming CMV genomes appear to be extensively chromatinised upon infection (Sinclair, 2010).
The mechanisms specifically governing the regulation of the MIEP are pivotal events during latency, reactivation and lytic infection. A complex interplay between the relative levels of transcriptional repressors and activators, the recruitment of histone proteins and subsequent modification by histone modifying enzymes, as well as the cellular environment triggered by infection of the cell, likely dictate the outcome of infection. For instance, latently infected cells up-regulate a number of cellular repressors that are down-regulated upon infection of permissive cells suggesting that the outcome of HCMV infection may begin to be defined at the point of entry (Slobedman et al., 2004).
However, it is becoming increasingly clear that the regulation of viral gene expression during all phases of infection is dependent on the ability to modify the chromatin structure. During latency and reactivation, it is likely that changes in the modifications of histones associated with the MIEP are likely concomitant with the differentiation of the cells to mature DC or macrophage phenotypes. Terminal differentiation of myeloid precursors results in significant changes in the chromatin architecture of cellular promoters – particularly those of inflammatory genes (Saccani et al., 2002). Given the similarities between the MIEP and these inflammatory promoters it is not unlikely that it could be regulated by intra-cellular changes also.
In contrast, during lytic infection (and, plausibly, during events post IE gene expression during reactivation) HCMV gene products actively engage the gamut of histone modifying enzymes (Sinclair, 2010). Upon infection, HCMV makes a concerted effort to target Nuclear Domain (ND) 10 bodies, and their constituent parts, for dispersal (Maul, 2008). It is thought part of ND10 bodies’ intrinsic anti-viral activity is that they are sites of deposition of PML, hDaxx, HDACs and HMTs which all act to repress viral transcription. Initially the incoming tegument protein, pp71, and then subsequently IE72 counteract these effects by degradation or re-localisation of the ND10 constituents (Kalejta, 2008; Maul, 2008). Furthermore, both IE72 and IE86 physically interact with HATs, HDACs and HMTs in a complex pattern of cellular and viral gene regulation. These interactions alone highlight the ever growing role of chromatin during virus infection.
Although recent work as suggested the stoichemetric loading of histones onto viral DNA would be consistent with bona fide nucleosome formation this is still not formally proven (Nitzsche et al., 2008). Whether this is truly important is a moot point as there is clearly a functional outcome of histone recruitment to the viral genomes. What is less clear is whether the recruited histones play any other structural roles. For instance, does chromatin play a role in the replication and processing of viral DNA? Histones and chromatin could provide a template for the recruitment of DNA processing enzymes and, given, the reliance of viruses on the host cell to complete its’ lifecycle, it is possible that further roles for chromatinisation of the viral genome may become apparent. These questions are still unanswered.
Overall, it is hoped that research leading towards an understanding of the fundamental mechanisms underlying the regulation of viral gene expression could impact on strategies designed to generate novel therapeutic interventions as well as increase our understanding of the role higher order structure plays in the regulation of gene expression.
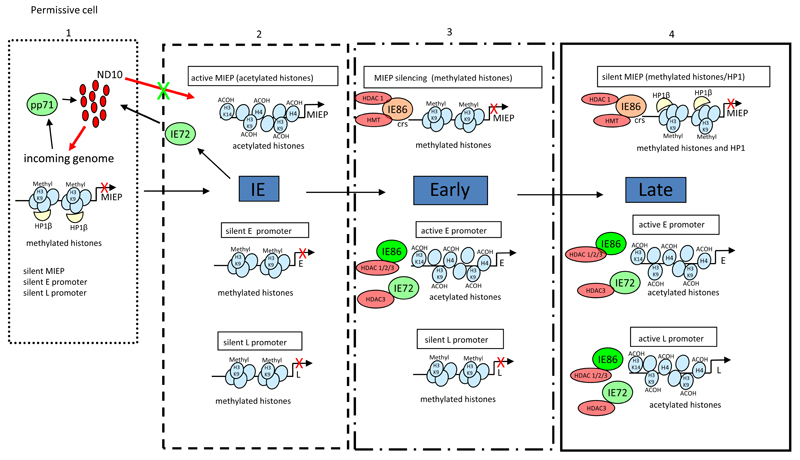
Figure 1. All classes of HCMV gene expression are subject to regulation by histone modifications during lytic infection.
Following infecton, the HCMV genome is localised to Nuclear Domain 10 bodies which may represent an anti-viral response. A number of constituents of ND10, including Daxx, PML and SP100, have been shown to interact with histone modifying enzymes and HP1 which could promote a ‘pre-immediate early’ environment that is transcriptionally repressive (1). This repression is overcome by the virion transactivator pp71 – which targets Daxx for degradation (1) – allowing IE72 expression and the dispersal of ND10 structures (2). The ensuing robust viral IE gene expression causes the accumulation of IE72 and IE86 which, via interactions with HDACs/HMTs relieve the transcriptional repression of Early (3) and then Late (4) promoters. Concomitant with the activation of Early and Late gene expression is the active silencing of the MIEP at late times by IE86 mediated auto-regulation which, in part, involves the recruitment of HDACs and HMTs to the MIEP via the cis repression sequence which drives methylation of histones bound to the MIEP(3 & 4). Figure reproduced with permission (Sinclair, 2010).
Figure 2. Does the pre-immediate early environment seen in lytic infection contribute to latency?
At least two models, not necessarily mutually exclusive, can be hypothesized to promote HCMV latency. The first model assumes that the cellular response to incoming viral genomes generates the same pre-immediate early environment as observed in lytic infection (1). Subsequently, the failure of pp71 to translocate to the nucleus to inhibit the repressive function of constituents of ND10 bodies results in continued transcriptional repression of the MIEP (2). The second model hypothesizes that the pre-immediate early environment upon infection is either augmented by the comparably high levels of transcriptional repressors to activators in these cells (3). These repressors can interact with HDACs and HMTs to promote the long term silencing of the MIEP in latency irrespective of the effects of pp71 on ND10 bodies components. Whether these repressors augment or actually promote the repression of the MIEP upon infection, and thus repression is potentially ND10-independent, is unclear.
References
- Ahn JH, Brignole EJ, 3rd, Hayward GS. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol Cell Biol. 1998;18(8):4899–913. doi: 10.1128/mcb.18.8.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain M, Mendelson M, Sinclair J. Ets-2 Repressor Factor (ERF) mediates repression of the human cytomegalovirus major immediate-early promoter in undifferentiated non-permissive cells. J Gen Virol. 2003;84(Pt 1):41–9. doi: 10.1099/vir.0.18633-0. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bego M, Maciejewski J, Khaiboullina S, Pari G, St Jeor S. Characterization of an antisense transcript spanning the UL81-82 locus of human cytomegalovirus. J Virol. 2005;79(17):11022–34. doi: 10.1128/JVI.79.17.11022-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bego MG, Jeor S. Human cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp Hematol. 2006;34(5):555–70. doi: 10.1016/j.exphem.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Bevan IS, Daw RA, Day PJ, Ala FA, Walker MR. Polymerase chain reaction for detection of human cytomegalovirus infection in a blood donor population. Br J Haematol. 1991;78(1):94–9. doi: 10.1111/j.1365-2141.1991.tb04388.x. [DOI] [PubMed] [Google Scholar]
- Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc Natl Acad Sci U S A. 1998;95(5):2061–6. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272(5267):1473–6. doi: 10.1126/science.272.5267.1473. [DOI] [PubMed] [Google Scholar]
- Boshart M, Weber F, Jahn G, Dorsch-Hasler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41(2):521–30. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396(6711):594–8. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- Bresnahan WA, Shenk TE. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc Natl Acad Sci U S A. 2000;97(26):14506–11. doi: 10.1073/pnas.97.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne EP, Wing B, Coleman D, Shenk T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J Virol. 2001;75(24):12319–30. doi: 10.1128/JVI.75.24.12319-12330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant LA, Mixon P, Davidson M, Bannister AJ, Kouzarides T, Sinclair JH. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J Virol. 2000;74(16):7230–7. doi: 10.1128/jvi.74.16.7230-7237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell SR, Bresnahan WA. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J Virol. 2006;80(12):6188–91. doi: 10.1128/JVI.02676-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298(5595):1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Castillo JP, Kowalik TF. Human cytomegalovirus immediate early proteins and cell growth control. Gene. 2002;290(1–2):19–34. doi: 10.1016/s0378-1119(02)00566-8. [DOI] [PubMed] [Google Scholar]
- Cherrington JM, Khoury EL, Mocarski ES. Human cytomegalovirus ie2 negatively regulates alpha gene expression via a short target sequence near the transcription start site. J Virol. 1991;65(2):887–96. doi: 10.1128/jvi.65.2.887-896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SY, Hill WE, Doty P. Characterization of the histone core complex. Proc Natl Acad Sci U S A. 1978;75(4):1680–4. doi: 10.1073/pnas.75.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22(1):76–98. doi: 10.1128/CMR.00034-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Bennett C, Shenk T. Dynamic histone H3 acetylation and methylation at human cytomegalovirus promoters during replication in fibroblasts. J Virol. 2008;82(19):9525–36. doi: 10.1128/JVI.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenecke D, Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem. 1982;44(2):113–28. doi: 10.1007/BF00226895. [DOI] [PubMed] [Google Scholar]
- Dudley AM, Rougeulle C, Winston F. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 1999;13(22):2940–5. doi: 10.1101/gad.13.22.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley R, Van Duyne R, Coley W, Guendel I, Dadgar S, Kehn-Hall K, Kashanchi F. Chromatin dynamics associated with HIV-1 Tat-activated transcription. Biochim Biophys Acta. 2010;1799(3–4):275–85. doi: 10.1016/j.bbagrm.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M, Zhu W, Hasegawa J, Watanabe H, Kim DK, Aida M, Inukai N, Narita T, Yamada T, Furuya A, Sato H, et al. Human Spt6 stimulates transcription elongation by RNA polymerase II in vitro. Mol Cell Biol. 2004;24(8):3324–36. doi: 10.1128/MCB.24.8.3324-3336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25(4):1215–27. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev GP, Bakayev VV, Nedospasov SA, Razin SV, Mantieva VL. Studies on structure and function of chromatin. Mol Cell Biochem. 1981;40(1):29–48. doi: 10.1007/BF00230186. [DOI] [PubMed] [Google Scholar]
- Gonczol E, Andrews PW, Plotkin SA. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984;224(4645):159–61. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- Goodrum F, Reeves M, Sinclair J, High K, Shenk T. Human cytomegalovirus sequences expressed in latently infected individuals promote a latent infection in vitro. Blood. 2007;110(3):937–45. doi: 10.1182/blood-2007-01-070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrum FD, Jordan CT, High K, Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc Natl Acad Sci U S A. 2002;99(25):16255–60. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves RF, Mocarski ES. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J Virol. 1998;72(1):366–79. doi: 10.1128/jvi.72.1.366-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves IJ, Reeves MB, Sinclair JH. Lytic infection of permissive cells with human cytomegalovirus is regulated by an intrinsic 'pre-immediate-early' repression of viral gene expression mediated by histone post-translational modification. J Gen Virol. 2009;90(Pt 10):2364–74. doi: 10.1099/vir.0.012526-0. [DOI] [PubMed] [Google Scholar]
- Groves IJ, Sinclair JH. Knockdown of hDaxx in normally non-permissive undifferentiated cells does not permit human cytomegalovirus immediate-early gene expression. J Gen Virol. 2007;88(Pt 11):2935–40. doi: 10.1099/vir.0.83019-0. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130(1):77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci U S A. 1998;95(7):3937–42. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish DR, Burgoyne LA. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973;52(2):504–10. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hofmann H, Sindre H, Stamminger T. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J Virol. 2002;76(11):5769–83. doi: 10.1128/JVI.76.11.5769-5783.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CH, Chang MD, Tai KY, Yang YT, Wang PS, Chen CJ, Wang YH, Lee SC, Wu CW, Juan LJ. HCMV IE2-mediated inhibition of HAT activity downregulates p53 function. EMBO J. 2004;23(11):2269–80. doi: 10.1038/sj.emboj.7600239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioudinkova E, Arcangeletti MC, Rynditch A, De Conto F, Motta F, Covan S, Pinardi F, Razin SV, Chezzi C. Control of human cytomegalovirus gene expression by differential histone modifications during lytic and latent infection of a monocytic cell line. Gene. 2006;384:120–8. doi: 10.1016/j.gene.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Ishov AM, Stenberg RM, Maul GG. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J Cell Biol. 1997;138(1):5–16. doi: 10.1083/jcb.138.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov AM, Vladimirova OV, Maul GG. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J Virol. 2002;76(15):7705–12. doi: 10.1128/JVI.76.15.7705-7712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TC, Eissenberg JC, Craig C, Dietrich V, Hobson A, Elgin SC. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol. 1989;50(1):170–80. [PubMed] [Google Scholar]
- Jenkins C, Abendroth A, Slobedman B. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J Virol. 2004;78(3):1440–7. doi: 10.1128/JVI.78.3.1440-1447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalejta RF. Functions of human cytomegalovirus tegument proteins prior to immediate early gene expression. Curr Top Microbiol Immunol. 2008;325:101–15. doi: 10.1007/978-3-540-77349-8_6. [DOI] [PubMed] [Google Scholar]
- Khaiboullina SF, Maciejewski JP, Crapnell K, Spallone PA, Dean Stock A, Pari GS, Zanjani ED, Jeor SS. Human cytomegalovirus persists in myeloid progenitors and is passed to the myeloid progeny in a latent form. Br J Haematol. 2004;126(3):410–7. doi: 10.1111/j.1365-2141.2004.05056.x. [DOI] [PubMed] [Google Scholar]
- Khochbin S, Verdel A, Lemercier C, Seigneurin-Berny D. Functional significance of histone deacetylase diversity. Curr Opin Genet Dev. 2001;11(2):162–6. doi: 10.1016/s0959-437x(00)00174-x. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6(3):211–21. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci U S A. 1994;91(25):11879–83. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Xu J, Mocarski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci U S A. 1996;93(20):11137–42. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184(139):868–71. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Thomas JO. Chromatin structure; oligomers of the histones. Science. 1974;184(139):865–8. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Kuntz-Simon G, Obert G. Sodium valproate, an anticonvulsant drug, stimulates human cytomegalovirus replication. J Gen Virol. 1995;76(Pt 6):1409–15. doi: 10.1099/0022-1317-76-6-1409. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20(8):615–26. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Lathey JL, Spector SA. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991;65(11):6371–5. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre C, Pascual M. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: late-onset disease and indirect consequences. Clin Infect Dis. 2008;46(5):732–40. doi: 10.1086/527397. [DOI] [PubMed] [Google Scholar]
- Lilley DM, Pardon JF. Structure and function of chromatin. Annu Rev Genet. 1979;13:197–233. doi: 10.1146/annurev.ge.13.120179.001213. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–22. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hermiston TW, Stinski MF. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J Virol. 1991;65(2):897–903. doi: 10.1128/jvi.65.2.897-903.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Baillie J, Sissons JG, Sinclair JH. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 1994;22(13):2453–9. doi: 10.1093/nar/22.13.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Yan S, Abecassis M, Hummel M. Establishment of murine cytomegalovirus latency in vivo is associated with changes in histone modifications and recruitment of transcriptional repressors to the major immediate-early promoter. J Virol. 2008;82(21):10922–31. doi: 10.1128/JVI.00865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodas G, Brosch G, Georgieva EI, Sendra R, Franco L, Loidl P. Histone deacetylase. A key enzyme for the binding of regulatory proteins to chromatin. FEBS Lett. 1993;317(3):175–80. doi: 10.1016/0014-5793(93)81271-z. [DOI] [PubMed] [Google Scholar]
- Lubon H, Ghazal P, Hennighausen L, Reynolds-Kohler C, Lockshin C, Nelson J. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1989;9(3):1342–5. doi: 10.1128/mcb.9.3.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashchuk V, McFarlane S, Everett RD, Preston CM. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J Virol. 2008;82(24):12543–54. doi: 10.1128/JVI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunetta JM, Wiedeman JA. Latency-associated sense transcripts are expressed during in vitro human cytomegalovirus productive infection. Virology. 2000;278(2):467–76. doi: 10.1006/viro.2000.0666. [DOI] [PubMed] [Google Scholar]
- Lusser A. Acetylated, methylated, remodeled: chromatin states for gene regulation. Curr Opin Plant Biol. 2002;5(5):437–43. doi: 10.1016/s1369-5266(02)00287-x. [DOI] [PubMed] [Google Scholar]
- Maul GG. Initiation of cytomegalovirus infection at ND10. Curr Top Microbiol Immunol. 2008;325:117–32. doi: 10.1007/978-3-540-77349-8_7. [DOI] [PubMed] [Google Scholar]
- Meier JL. Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. J Virol. 2001;75(4):1581–93. doi: 10.1128/JVI.75.4.1581-1593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JL, Stinski MF. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology. 1996;39(5–6):331–42. doi: 10.1159/000150504. [DOI] [PubMed] [Google Scholar]
- Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996;77(Pt 12):3099–102. doi: 10.1099/0022-1317-77-12-3099. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Kohler N, Reinisch A, Eikel D, Gravemann U, Doerr HW, Nau H, Cinatl J., Jr Increased human cytomegalovirus replication in fibroblasts after treatment with therapeutical plasma concentrations of valproic acid. Biochem Pharmacol. 2004;68(3):531–8. doi: 10.1016/j.bcp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Suhan T, Reinisch A, Reisenauer A, Fleckenstein C, Eikel D, Gumbel H, Doerr HW, Nau H, Cinatl J., Jr Increased replication of human cytomegalovirus in retinal pigment epithelial cells by valproic acid depends on histone deacetylase inhibition. Invest Ophthalmol Vis Sci. 2005;46(9):3451–7. doi: 10.1167/iovs.05-0369. [DOI] [PubMed] [Google Scholar]
- Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68(6):4017–21. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen CA, Allis CD. Linking histone acetylation to transcriptional regulation. Cell Mol Life Sci. 1998;54(1):6–20. doi: 10.1007/s000180050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Kemble GW, Lyle JM, Greaves RF. A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc Natl Acad Sci U S A. 1996;93(21):11321–6. doi: 10.1073/pnas.93.21.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JC, Fischle W, Verdin E, Sinclair JH. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 2002;21(5):1112–20. doi: 10.1093/emboj/21.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevels M, Paulus C, Shenk T. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc Natl Acad Sci U S A. 2004;101(49):17234–9. doi: 10.1073/pnas.0407933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature. 2002;416(6876):103–7. doi: 10.1038/nature722. [DOI] [PubMed] [Google Scholar]
- Nitzsche A, Paulus C, Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J Virol. 2008;82(22):11167–80. doi: 10.1128/JVI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P, Gross-Bellard M, Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Park JJ, Kim YE, Pham HT, Kim ET, Chung YH, Ahn JH. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J Gen Virol. 2007;88(Pt 12):3214–23. doi: 10.1099/vir.0.83171-0. [DOI] [PubMed] [Google Scholar]
- Penkert RR, Kalejta RF. Nuclear localization of tegument-delivered pp71 in human cytomegalovirus infected cells is facilitated by one or more factor present in terminally differentiated fibroblasts. J Virol. 2010 doi: 10.1128/JVI.00500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, Mermoud JE, O'Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2002;30(1):77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- Petrucelli A, Rak M, Grainger L, Goodrum F. Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus. J Virol. 2009;83(11):5615–29. doi: 10.1128/JVI.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17(17):4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:315–31. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- Reeves M, Murphy J, Greaves R, Fairley J, Brehm A, Sinclair J. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin remodeling enzymes by IE86. J Virol. 2006;80(20):9998–10009. doi: 10.1128/JVI.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- Reeves M, Woodhall D, Compton T, Sinclair J. Human Cytomegalovirus IE72 protein interacts with the transcriptional repressor hDaxx to regulate LUNA gene expression during lytic infection. J Virol. 2010 doi: 10.1128/JVI.02231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MB, Lehner PJ, Sissons JG, Sinclair JH. An in vitro model for the regulation of human cytomegalovirus latency and reactivation in dendritic cells by chromatin remodelling. J Gen Virol. 2005a;86(Pt 11):2949–54. doi: 10.1099/vir.0.81161-0. [DOI] [PubMed] [Google Scholar]
- Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci U S A. 2005b;102(11):4140–5. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MB, Sinclair JH. Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. J Gen Virol. 2010;91(Pt 3):599–604. doi: 10.1099/vir.0.015602-0. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Saffert RT, Kalejta RF. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J Virol. 2006;80(8):3863–71. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffert RT, Kalejta RF. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J Virol. 2007;81(17):9109–20. doi: 10.1128/JVI.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffert RT, Penkert RR, Kalejta RF. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. J Virol. 2010;84(11):5594–604. doi: 10.1128/JVI.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27(4):562–72. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2003;100(23):13225–30. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection. Biochim Biophys Acta. 2010;1799(3–4):286–95. doi: 10.1016/j.bbagrm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87(Pt 7):1763–79. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- Sinclair JH, Baillie J, Bryant LA, Taylor-Wiedeman JA, Sissons JG. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J Gen Virol. 1992;73(Pt 2):433–5. doi: 10.1099/0022-1317-73-2-433. [DOI] [PubMed] [Google Scholar]
- Sindre H, Tjoonnfjord GE, Rollag H, Ranneberg-Nilsen T, Veiby OP, Beck S, Degre M, Hestdal K. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood. 1996;88(12):4526–33. [PubMed] [Google Scholar]
- Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- Slobedman B, Cao JZ, Avdic S, Webster B, McAllery S, Cheung AK, Tan JC, Abendroth A. Human cytomegalovirus latent infection and associated viral gene expression. Future Microbiol. 2010;5(6):883–900. doi: 10.2217/fmb.10.58. [DOI] [PubMed] [Google Scholar]
- Slobedman B, Mocarski ES, Arvin AM, Mellins ED, Abendroth A. Latent cytomegalovirus down-regulates major histocompatibility complex class II expression on myeloid progenitors. Blood. 2002;100(8):2867–73. doi: 10.1182/blood.V100.8.2867. [DOI] [PubMed] [Google Scholar]
- Slobedman B, Stern JL, Cunningham AL, Abendroth A, Abate DA, Mocarski ES. Impact of human cytomegalovirus latent infection on myeloid progenitor cell gene expression. J Virol. 2004;78(8):4054–62. doi: 10.1128/JVI.78.8.4054-4062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91(1):119–26. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- Spector DH. Activation and regulation of human cytomegalovirus early genes. Intervirology. 1996;39(5–6):361–77. doi: 10.1159/000150507. [DOI] [PubMed] [Google Scholar]
- Stinski MF, Petrik DT. Functional roles of the human cytomegalovirus essential IE86 protein. Curr Top Microbiol Immunol. 2008;325:133–52. doi: 10.1007/978-3-540-77349-8_8. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Tang Q, Maul GG. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J Virol. 2003;77(2):1357–67. doi: 10.1128/JVI.77.2.1357-1367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalai N, Papior P, Rechter S, Leis M, Stamminger T. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J Virol. 2006;80(16):8006–18. doi: 10.1128/JVI.00743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–64. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68(3):1597–604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempera I, Lieberman PM. Chromatin organization of gammaherpesvirus latent genomes. Biochim Biophys Acta. 2010;1799(3–4):236–45. doi: 10.1016/j.bbagrm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Seto E. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene. 1999;236(2):197–208. doi: 10.1016/s0378-1119(99)00261-9. [DOI] [PubMed] [Google Scholar]
- Thomsen DR, Stenberg RM, Goins WF, Stinski MF. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984;81(3):659–63. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth Z, Stamminger T. The human cytomegalovirus regulatory protein UL69 and its effect on mRNA export. Front Biosci. 2008;13:2939–49. doi: 10.2741/2899. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Yee J, Sachs L, Collingwood TN, Bauer A, Beug H, Shi YB, Wolffe AP. Targeting of N-CoR and histone deacetylase 3 by the oncoprotein v-erbA yields a chromatin infrastructure-dependent transcriptional repression pathway. EMBO J. 2000;19(15):4074–90. doi: 10.1093/emboj/19.15.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang ZQ, Xia L, Feng Q, Erdjument-Bromage H, Strahl BD, Briggs SD, Allis CD, Wong J, Tempst P, Zhang Y. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science. 2001;293(5531):853–7. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976;193(4256):848–56. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Worcel A, Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976;9(3):409–17. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Orkin SH. GATA transcription factors: key regulators of hematopoiesis. Exp Hematol. 1995;23(2):99–107. [PubMed] [Google Scholar]
- Wilkinson GW, Kelly C, Sinclair JH, Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J Gen Virol. 1998;79(Pt 5):1233–45. doi: 10.1099/0022-1317-79-5-1233. [DOI] [PubMed] [Google Scholar]
- Winkler M, aus Dem Siepen T, Stamminger T. Functional interaction between pleiotropic transactivator pUL69 of human cytomegalovirus and the human homolog of yeast chromatin regulatory protein SPT6. J Virol. 2000;74(17):8053–64. doi: 10.1128/jvi.74.17.8053-8064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Patterton D, Imhof A, Guschin D, Shi YB, Wolffe AP. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J. 1998;17(2):520–34. doi: 10.1093/emboj/17.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhall DL, Groves IJ, Reeves MB, Wilkinson G, Sinclair JH. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J Biol Chem. 2006;281(49):37652–60. doi: 10.1074/jbc.M604273200. [DOI] [PubMed] [Google Scholar]
- Wright E, Bain M, Teague L, Murphy J, Sinclair J. Ets-2 repressor factor recruits histone deacetylase to silence human cytomegalovirus immediate-early gene expression in non-permissive cells. J Gen Virol. 2005;86(Pt 3):535–44. doi: 10.1099/vir.0.80352-0. [DOI] [PubMed] [Google Scholar]
- Yuan J, Liu X, Wu AW, McGonagill PW, Keller MJ, Galle CS, Meier JL. Breaking human cytomegalovirus major immediate-early gene silence by vasoactive intestinal peptide stimulation of the protein kinase A-CREB-TORC2 signaling cascade in human pluripotent embryonal NTera2 cells. J Virol. 2009;83(13):6391–403. doi: 10.1128/JVI.00061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15(18):2343–60. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhuravskaya T, Maciejewski JP, Netski DM, Bruening E, Mackintosh FR, Jeor S. Spread of human cytomegalovirus (HCMV) after infection of human hematopoietic progenitor cells: model of HCMV latency. Blood. 1997;90(6):2482–91. [PubMed] [Google Scholar]
- Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16(8):4024–34. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]