Abstract
Genome-wide association studies (GWAS) have identified >300 loci associated with measures of adiposity including body mass index (BMI) and waist-to-hip ratio (adjusted for BMI, WHRadjBMI), but few have been identified through screening of the African ancestry genomes. We performed large scale meta-analyses and replications in up to 52,895 individuals for BMI and up to 23,095 individuals for WHRadjBMI from the African Ancestry Anthropometry Genetics Consortium (AAAGC) using 1000 Genomes phase 1 imputed GWAS to improve coverage of both common and low frequency variants in the low linkage disequilibrium African ancestry genomes. In the sex-combined analyses, we identified one novel locus (TCF7L2/HABP2) for WHRadjBMI and eight previously established loci at P < 5×10−8: seven for BMI, and one for WHRadjBMI in African ancestry individuals. An additional novel locus (SPRYD7/DLEU2) was identified for WHRadjBMI when combined with European GWAS. In the sex-stratified analyses, we identified three novel loci for BMI (INTS10/LPL and MLC1 in men, IRX4/IRX2 in women) and four for WHRadjBMI (SSX2IP, CASC8, PDE3B and ZDHHC1/HSD11B2 in women) in individuals of African ancestry or both African and European ancestry. For four of the novel variants, the minor allele frequency was low (<5%). In the trans-ethnic fine mapping of 47 BMI loci and 27 WHRadjBMI loci that were locus-wide significant (P < 0.05 adjusted for effective number of variants per locus) from the African ancestry sex-combined and sex-stratified analyses, 26 BMI loci and 17 WHRadjBMI loci contained ≤ 20 variants in the credible sets that jointly account for 99% posterior probability of driving the associations. The lead variants in 13 of these loci had a high probability of being causal. As compared to our previous HapMap imputed GWAS for BMI and WHRadjBMI including up to 71,412 and 27,350 African ancestry individuals, respectively, our results suggest that 1000 Genomes imputation showed modest improvement in identifying GWAS loci including low frequency variants. Trans-ethnic meta-analyses further improved fine mapping of putative causal variants in loci shared between the African and European ancestry populations.
Author summary
Genome-wide association studies (GWAS) have identified >300 genetic regions that influence body size and shape as measured by body mass index (BMI) and waist-to-hip ratio (WHR), respectively, but few have been identified in populations of African ancestry. We conducted large scale high coverage GWAS and replication of these traits in 52,895 and 23,095 individuals of African ancestry, respectively, followed by additional replication in European populations. We identified 10 genome-wide significant loci in all individuals, and an additional seven loci by analyzing men and women separately. We combined African and European ancestry GWAS and were able to narrow down 43 out of 74 African ancestry associated genetic regions to contain small number of putative causal variants. Our results highlight the improvement of applying high density genome coverage and combining multiple ancestries in the identification and refinement of location of genetic regions associated with adiposity traits.
Introduction
Obesity is a worldwide public health epidemic, with current US estimates of 37.9% obese and 7.7% morbidly obese adults [1]. Disparities in obesity rates, as well as rates of comorbidities and mortality, are evident across sex and racial/ethnic groups. Estimates from NHANES for 2013–2014 [1] show that obesity is more prevalent among African Americans (48.5%) than among non-Hispanic Whites (37.1%). In addition, obesity rates are higher among African American women (57.2%) than among African American men (38.2%). For comparison, the obesity rates in non-Hispanic Whites were 38.7% and 35.4%, respectively, for women and men.
Genome-wide association studies (GWAS) in diverse populations have identified > 300 loci associated with measures of adiposity including body mass index (BMI) and waist-to-hip ratio (adjusted for BMI, WHRadjBMI) in populations of European [2–9], African [10–12], and East Asian ancestry [13–15]. The majority of associated variants are common (MAF >5%) with small effect size, and jointly explain only a fraction of the phenotypic variances [7–8]. It has long been hypothesized that low frequency (MAF = 0.5–5%) and rare (MAF < 0.5%) variants may also contribute to variability in complex traits. However, these variants are not well captured in previous GWAS imputed to the HapMap reference panel [16–17]. The availability of higher density reference panels such as the 1000 Genomes Project (38M variants in 1092 individuals from phase 1) [18] has demonstrated improved imputation quality in European populations particularly for low frequency variants (aggregate R2 ~0.6 for MAF = 0.5%). However its impact is less clear for non-European populations [19]. We took this opportunity to use higher density imputation to reevaluate our previous GWAS for associations with anthropometric traits in individuals of African ancestry (AA) including African Americans and Africans.
The African Ancestry Anthropometry Genetics Consortium (AAAGC) previously identified seven genome-wide significant loci for BMI in up to 71,412 AA individuals, and an additional locus when combined with European ancestry (EA) data from the Genetic Investigation of ANthropometric Traits (GIANT) consortium using GWAS imputed to the HapMap Phase 2 reference panel [11]. No genome-wide significant loci were identified for WHRadjBMI in a GWAS of up to 27,350 AA individuals [12]. The low yield of discovery in AA studies is likely due to their relatively smaller sample sizes in comparison to EA studies [7–8], as well as their lower degree of linkage disequilibrium (LD) and thus poorer imputation quality. Here, we extended our previous work in the AAAGC to perform meta-analyses and replication of GWAS imputed to the 1000 Genomes reference panel in up to 52,895 AA individuals for BMI and up to 23,095 AA individuals for WHRadjBMI. We aimed to 1) discover novel variants, 2) fine map established loci, and 3) evaluate the coverage and contribution of low frequency variants in genetic associations in AA populations.
Results
Study overview
We conducted sex-combined and sex-stratified meta-analyses of GWAS summary statistics across 17 studies for BMI (N = 42,752) and 10 studies for WHRadjBMI (N = 20,384) in AA individuals in stage 1 discovery (S1 and S2 Tables, S1 Fig). Missing genotypes in individual studies were imputed to the 1000 Genomes Project cosmopolitan reference panel (Phase I Integrated Release Version 3, March 2012) [18] using MaCH/minimac [20] or SHAPEIT2/IMPUTEv2 [21–22] (S3 Table). Among all variants with MAF ≥ 0.1% in the largest Women’s Health Initiative (WHI) study, the average info score was 0.81 and 90.5% had imputation info score ≥ 0.3 (S4 Table). Genomic control corrections were applied to each study and after meta-analysis (λ = 1.07 for BMI, 1.01 for WHRadjBMI) (S3 Table, S2–S5 Figs). Association results for ~18M variants for BMI and ~21M variants for WHRadjBMI were subsequently interrogated further.
From stage 1 meta-analyses, variants associated with BMI (3,241 in all, 1,498 in men, 2,922 in women) and WHRadjBMI (2,496 in all, 1,408 in men, 2,827 in women) at P < 1×10−4 were carried forward for replication in AA and EA. Stage 2 included 10,143 AA (2,458 men and 7,685 women) for BMI and 2,711 AA (981 men and 1,730 women) for WHRadjBMI analyses. Stage 3 included 322,154 EA (152,893 men and 171,977 women) for BMI and 210,086 EA (104,079 men and 116,742 women) for WHRadjBMI analyses by imputing HapMap summary statistics results [7–8] to 1000 Genomes [23] (S1 Fig). Meta-analyses were performed to combine either sex-combined or sex-specific results from AA (stages 1+2, N ≤ 57,895 for BMI, ≤ 23,095 for WHRadjBMI in sex-combined analyses) and both AA and EA (stages 1+2+3, N ≤ 380,049 for BMI, ≤ 233,181 for WHRadjBMI in sex-combined analyses, S6–S9 Figs). Variants that reached genome-wide statistical significance (P < 5×10−8) were assessed for generalization of associations with BMI to children in two additional AA cohorts (N = 7,222).
Genome-wide significant loci in meta-analyses
Sex-combined analyses
In the sex-combined meta-analysis of BMI in AA, seven previously established European or African ancestry-derived loci in/near SEC16B, TMEM18, GNPDA2, GALNT10, KLHL32, FTO and MC4R reached genome-wide significance (P < 5×10−8) (Table 1, S6 and S10A Figs). The rs7708584 variant at GALNT10 had the lowest P-value (P = 4.2×10−14) and was the same lead variant as reported in our previous AA study (S5 Table) [11]. The association at KLHL32 was specific to the AA population as the lead variant was not statistically significant in EA (P > 0.05), consistent with our previous finding (S5 Table) [11]. No additional novel BMI loci were identified after meta-analysis of AA and EA data. Two previously reported loci in AA, ADCY3 and MIR148A-NFE2L3 [11], did not reach genome-wide significance in the present study. The previously reported lead variant at ADCY3, rs7586879, showed weaker effect and association in AA (effect = 0.047, P = 3.60×10−8 [11] vs. effect = 0.032, P = 1.05×10−4, this study). On the other hand, a moderately correlated (r2 = 0.52 in AFR) variant, rs10203482, show stronger association at P = 3.35×10−7 (S5 Table). At MIR148A/NFE2L3, the previously reported lead variant identified by meta-analysis of AA and EA, rs10261878, also showed weaker association in the current study primarily due to weak association in EA (P = 4.10×10−5 in AA, 4.69×10−3 in EA and 2.10×10−5 in AA+EA) (S5 Table). For WHRadjBMI, an established locus (ADAMTS9-AS2) (S10B Fig) and a novel locus (TCF7L2/HABP2) (Fig 1A) showed significant associations, the latter lead variant rs116718588 was low frequency (MAF = 0.045, Table 1). Meta-analyses including both AA and EA individuals revealed an additional novel locus at SPRYD7/DLEU2 for WHRadjBMI (Table 1, Fig 1A and S7 Fig). Overall, all the BMI associated lead variants were present in HapMap and were in high LD (r2 > 0.5 in 1000 Genomes AFR population) with the lead variants in our previous HapMap imputed data, except for TMEM18 rs62105306 (r2 = 0.17) which is absent in HapMap. In contrast, all three WHRadjBMI lead variants were absent in HapMap and the lead variants at ADAMTS9-AS2 and TCF7L2-HABP2 were in low LD (r2 < 0.5) with the lead variants in our previous HapMap imputed data [11–12]. We used conditional and joint association analyses to examine the genome-wide significant locus for secondary signals, but no additional independent signals were found.
Table 1. Novel and previously identified BMI and WHRadjBMI loci at P < 5×10−8 in African ancestry discovery and replication samples, and European ancestry replication samples.
| Lead variant by locus | AA Discovery | AA Replication | AA Discovery + Replication | EA | AA + EA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Lead SNP | Chr | Position (b37/hg19) | Known Locus (if Yes, lead published variant) | Locus | Effect/Other alleles | EAF | Effect (SE) | P | HetISq | N | Effect (SE) | P | HetISq | N | Effect (SE) | P | P | N | P | |
| BMI | rs543874 | 1 | 177,889,480 | Yes | rs543874 | SEC16B | G/A | 0.248 | 0.055 (0.008) | 5.75E-11 | 0 | 42,681 | 0.057 (0.017) | 6.59E-04 | 28.9 | 10,143 | 0.055 (0.008) | 1.76E-13 | 4.36E-35 | 322,008 | 6.35E-46 |
| BMI | rs62105306 | 2 | 633,660 | Yes | rs13021737 | TMEM18 | T/C | 0.751 | 0.056 (0.01) | 1.55E-08 | 0 | 41,492 | 0.04 (0.02) | 4.40E-02 | 49.4 | 10,143 | 0.053 (0.009) | 2.17E-09 | 6.10E-21 | 244,176 | 3.04E-28 |
| BMI | rs10938397 | 4 | 45,182,527 | Yes | rs10938397 | GNPDA2 | G/A | 0.243 | 0.053 (0.008) | 3.76E-10 | 3.6 | 42,752 | 0.011 (0.017) | 5.40E-01 | 54 | 10,143 | 0.044 (0.008) | 3.95E-09 | 1.87E-38 | 320,955 | 5.60E-46 |
| BMI | rs7708584 | 5 | 153,543,466 | Yes | rs7715256; rs7708584 a | GALNT10 | A/G | 0.307 | 0.059 (0.008) | 1.05E-13 | 4.6 | 42,750 | 0.034 (0.016) | 3.93E-02 | 6.2 | 10,143 | 0.054 (0.007) | 4.21E-14 | 3.80E-07 | 234,015 | 4.35E-15 |
| BMI | rs17057164 | 6 | 97,410,536 | Yes | rs974417 a | KLHL32 | T/C | 0.659 | 0.043 (0.008) | 1.75E-08 | 0 | 42,751 | 0.025 (0.015) | 9.97E-02 | 35.2 | 10,143 | 0.04 (0.007) | 6.08E-09 | 7.44E-01 | 233,997 | 5.43E-03 |
| BMI | rs17817964 | 16 | 53,828,066 | Yes | rs1558902 | FTO | T/C | 0.117 | 0.067 (0.011) | 5.48E-09 | 0 | 42,750 | 0.08 (0.025) | 1.19E-03 | 49.2 | 10,143 | 0.069 (0.01) | 2.72E-11 | 2.40E-139 | 321,602 | 1.13E-146 |
| BMI | rs6567160 | 18 | 57,829,135 | Yes | rs6567160 | MC4R | C/T | 0.197 | 0.062 (0.009) | 2.74E-11 | 35.3 | 42,750 | 0.044 (0.019) | 1.99E-02 | 33.5 | 10,143 | 0.059 (0.008) | 2.29E-12 | 8.23E-54 | 321,958 | 2.09E-64 |
| WHRadjBMI | rs66815886 | 3 | 64,703,394 | Yes | rs2371767 | ADAMTS9-AS2 | G/T | 0.457 | 0.07 (0.01) | 3.90E-12 | 0 | 20,383 | 0.005 (0.033) | 8.75E-01 | 42.1 | 2,711 | 0.064 (0.01) | 2.46E-11 | 5.17E-19 | 145,257 | 9.13E-27 |
| WHRadjBMI | rs116718588 | 10 | 115,189,239 | No | TCF7L2/HABP2 | A/G | 0.955 | 0.114 (0.025) | 5.88E-06 | 0 | 20,384 | 0.348 (0.084) | 3.82E-05 | 45.6 | 2,711 | 0.134 (0.024) | 3.22E-08 | NA | NA | NA | |
| WHRadjBMI | rs2472591 | 13 | 50,536,360 | No | SPRYD7/DLEU2 | T/A | 0.206 | 0.05 (0.013) | 9.72E-05 | 0 | 20,371 | 0.06 (0.049) | 2.21E-01 | 0 | 2,160 | 0.05 (0.012) | 4.36E-05 | 1.69E-05 | 140,431 | 3.53E-08 | |
AA: African ancestry; BMI: body mass index; Chr: chromosome; EA: European ancestry; EAF: effect allele frequency; HetISq: heterogeneity measured by I-square; SE: standard error; WHRadjBMI: waist-to-hip ratio adjusted for BMI
a lead published variants reported in African ancestry
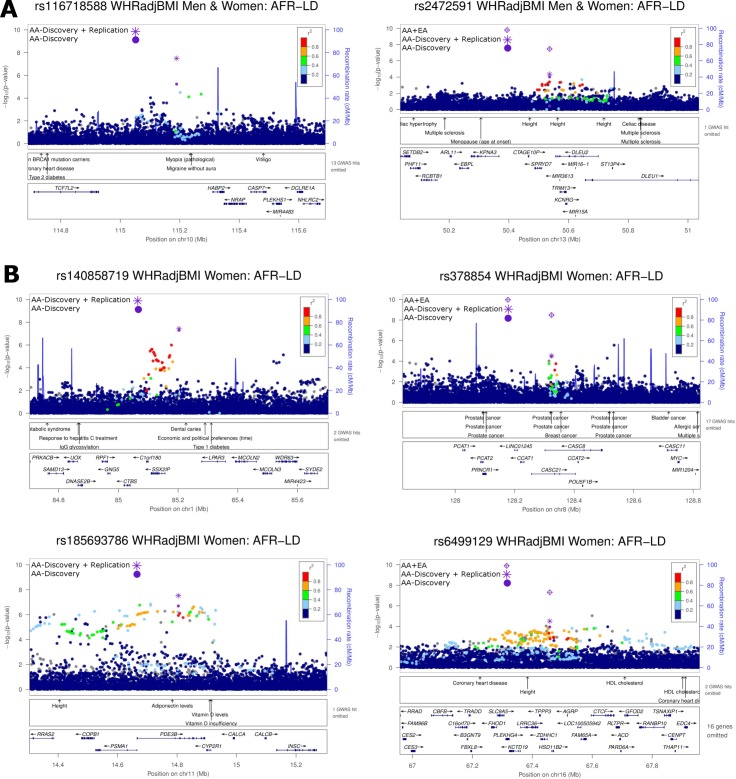
Fig 1.
Locuszoom plots of six novel waist-to-hip ratio adjusted for BMI (WHRadjBMI) loci: (A) TCF7L2/HABP2 and SPRYD7/DLEU2 in men and women combined; and (B) SSX2IP, PDE3B, CASC8, and ZDHHC1/HSD11B2 in women only. All plots use AFR LD from the 1000 Genomes phase 1 reference panel. In each plot, the most significant variant within a 1Mb regional locus is highlighted. P-values for all variants including the most significant variant are based on the African ancestry discovery phase only (AA-Discovery). In addition, for the most significant variant, P-values are annotated and illustrated from the African ancestry discovery and replication phases (AA-Discovery+Replication). SNP rs2472591 was available in the Europeans from the GIANT consortium effort and combined with the African ancestry discovery and replication phases (AA+EA).
Sex-stratified analyses
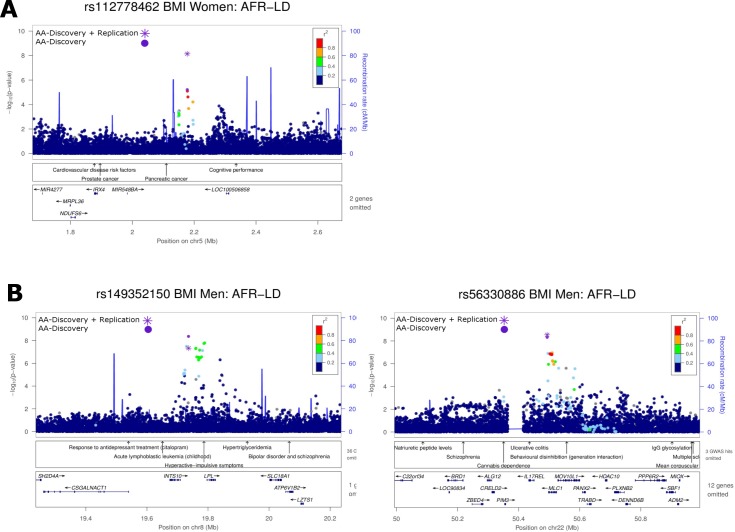
In the sex-stratified meta-analysis in AA, four established BMI loci (SEC16B, GALNT10, FTO and MC4R) and one established WHRadjBMI locus (ADAMTS9-AS2) were genome-wide significant among women (S6 Table, S8 and S9 Figs). ADAMTS9-AS2 showed a stronger association with WHRadjBMI among women than among men (Phet = 0.02) (S6 Table), consistent with findings among EA [9]. On the other hand, although our observed SEC16B rs543874 effect size differences (0.064 vs. 0.038, Phet = 0.08) for BMI in women compared to men were similar to those previously observed among EA (0.060 vs. 0.034, Phet = 5.23×10−5) [7], we did not observe statistically significant differences in effect size, likely due to a much smaller sample size and thus lower statistical power in our study. All these five loci were also genome-wide significant in the sex-combined meta-analyses. They were not further examined in subsequent sex-stratified analyses given their smaller sample sizes compared to the sex-combined analyses. In AA, additional novel loci were observed for association with BMI; these were variants in IRX4/IRX2 among women, variants in INTS10/LPL and MLC1 among men (Fig 2), and for WHRadjBMI, variants in SSX2IP and PDE3B among women (Fig 1B, Table 2). In meta-analyses including both AA and EA, two additional novel loci at CASC8 and ZDHHC1/HSD11B2 were identified for WHRadjBMI in women (Table 2, Fig 1B). Among all loci, the effect sizes of six variants (IRX4/IRX2, INTS10/LPL, MLC1, ADAMTS9-AS2, PDE3B and CASC8) were nominally significant different between men and women in AA (Phet < 0.05) (S6 Table).
Fig 2.
Locuszoom plots for three novel BMI loci: (A) IRX4/IRX2 in women only; and (B) INTS10/LPL and MLC1 in men only. All plots use AFR LD from the 1000 Genomes phase 1 reference panel. In each plot, the most significant variant within a 1Mb regional locus is highlighted. P-values for all variants including the most significant variant are based on the African ancestry discovery phase only (AA-Discovery). In addition, for the most significant variant, P-values are annotated and illustrated from the African ancestry discovery and replication phases (AA-Discovery+Replication).
Table 2. Additional novel BMI and WHRadjBMI loci at P < 5×10−8 in sex-stratified analyses of African ancestry discovery and replication samples.
| Lead variant by locus | AA Discovery | AA Replication | AA Discovery + Replication | EA | AA + EA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Cohort | Lead SNP | Chr | Position (b37/hg19) | Locus | Effect/Other alleles | EAF | Effect (SE) | P | HetISq | N | Effect (SE) | P | HetISq | N | Effect (SE) | P | N | P | N | P |
| BMI | Women | rs112778462 | 5 | 2,177,693 | IRX4/ IRX2 | A/G | 0.023 | 0.159 (0.035) | 6.06E-06 | 35.4 | 25,792 | 0.219 (0.059) | 2.11E-04 | 47.6 | 6,984 | 0.175 (0.03) | 7.21E-09 | 32,776 | NA | NA | NA |
| BMI | Men | rs149352150 | 8 | 19,736,154 | INTS10/ LPL | G/A | 0.013 | 0.341 (0.058) | 4.29E-09 | 0 | 15,179 | -0.034 (0.163) | 8.35E-01 | 1.0 | 2,147 | 0.299 (0.055) | 4.68E-08 | 17,326 | NA | NA | NA |
| BMI | Men | rs56330886 | 22 | 50,493,427 | MLC1 | G/T | 0.189 | 0.096 (0.016) | 4.81E-09 | 3.3 | 15,721 | 0.063 (0.052) | 2.21E-01 | 0.2 | 2,147 | 0.093 (0.016) | 2.88E-09 | 17,868 | NA | NA | NA |
| WHRadjBMI | Women | rs140858719 | 1 | 85,203,061 | SSX2IP | G/A | 0.994 | 0.506 (0.093) | 5.07E-08 | 0 | 11314 | 0.403 (0.487) | 4.08E-01 | 0 | 834 | 0.502 (0.091) | 3.69E-08 | 12,148 | NA | NA | NA |
| WHRadjBMI | Women | rs378854 | 8 | 128,323,819 | CASC8 | C/T | 0.787 | 0.062 (0.015) | 3.34E-05 | 4.5 | 15,600 | 0.034 (0.054) | 5.29E-01 | 73.2 | 1,730 | 0.060 (0.014) | 2.99E-05 | 17,330 | 3.70E-06 | 85325 | 3.26E-09 |
| WHRadjBMI | Women | rs185693786 | 11 | 14,804,296 | PDE3B | G/A | 0.930 | 0.122 (0.023) | 2.01E-07 | 0 | 15,601 | 0.17 (0.085) | 4.50E-02 | 21.6 | 1,730 | 0.125 (0.023) | 2.98E-08 | 17,331 | NA | NA | NA |
| WHRadjBMI | Women | rs6499129 | 16 | 67,458,251 | ZDHHC1/ HSD11B2 | A/C | 0.434 | 0.045 (0.012) | 1.12E-04 | 30.9 | 15,588 | 0.071 (0.042) | 9.29E-02 | 41.2 | 1,730 | 0.047 (0.011) | 3.07E-5 | 17,318 | 4.13E-05 | 86328 | 4.84E-08 |
AA: African ancestry; BMI: body mass index; Chr: chromosome; EA: European ancestry; EAF:effect allele frequency; HetISq: heterogeneity measured by I-square; SE: standard error; WHRadjBMI: waist-to-hip ratio adjusted for BMI
Replication in children
We evaluated the seven sex-combined and three sex-specific genome-wide significant BMI loci for associations in 7,222 AA children (3,552 boys and 3,670 girls). All lead variants displayed directional consistency, and five of these including SEC16B, TMEM18, GNPDA2, GALNT10 and MC4R showed nominal associations with BMI (P < 0.05, Pbinomial = 4.70×10−8) (S7 Table), supporting the role of these loci in modulating adiposity in AA children. WHRadjBMI data were not available in the cohorts of children.
Functional characterization of novel loci
We used multiple complementary approaches to elucidate the putative causal genes and/or variants associated with the nine novel BMI and WHRadjBMI loci from the sex-combined and sex-stratified analyses, including annotating nearby coding variants, cis-expression quantitative trait loci (cis-eQTL) analyses, and functional regulatory genomic element analyses. One missense variant in PLEKHG4, rs8044843, was in high LD (r2 = 0.75 in AFR) with rs6499129 associated with WHRadjBMI in women (S8 Table). We did not identify any coding variants in high LD (r2>0.7) with other lead variants within the flanking 1Mb-regions. Regulatory element analyses using RegulomeDB [24] and HaploReg [25] revealed that proxies (r2 = 0.73–0.84) to lead variants at three WHRadjBMI loci (SPRYD7/DLEU2, PDE3B, and ZDHHC1/HSD11B2) were associated with transcription factor binding, DNase peak, promoter or enhancer histone marks (S8 Table). In addition, the lead variant rs2472591 at SPRYD7/DLEU2 was in high LD (r2 = 0.85) with rs790943, a cis-eQTL associated with expression of the nearby gene, TRIM13, in blood dendritic cells in tuberculosis patients [26] (S9 Table), suggesting the associations at the SPRYD7/DLEU2 locus may be involved in the regulation of nearby gene expression at TRIM13.
Cross-trait associations of novel loci
We searched the NHGRI-EBI GWAS [27] and Genome-Wide Repository of Associations Between SNPs and Phenotypes (GRASP) [28] catalogs to assess if any of the nine novel lead variants were in high LD with variants that were genome-wide significantly (P < 5×10−8) or nominally (P < 0.05) associated with related anthropometric and cardiometabolic traits or gene expression in prior studies. Although a few lead variants were physically close (<500 kb) to GWAS loci for related traits in the NHGRI-EBI GWAS Catalog (Figs 1 and 2), none of our lead variants were in high LD with the previously associated lead variants. Additionally, there were no nearby associations for novel BMI loci in the GRASP Catalog. Of the novel variants associated with WHRadjBMI, rs2472591 at SPRYD7/DLEU2, rs378854 near MYC, and rs6499129 near ZDHHC1/HSD11B2 were in high LD (r2 > 0.7) with previously-reported WHRadjBMI variants, but they did not reach genome-wide significance (P > 2×10−5) [3] (S9 Table). Other nearby associations with related cardiometabolic traits include chronic kidney disease (CKD), high density lipoprotein cholesterol (HDL-C), anthropometric traits (BMI, height, and birth weight), blood pressure (systolic blood pressure and hypertension), diabetes-related traits (blood glucose and HOMA-IR), and gene expression of several genes (e.g. ATP6V0D1, ZDHHC1, DUS2L, AGRP, GFOD2 and LRRC29).
Evaluation of established European loci in African ancestry populations
Conditional analysis in GWAS loci
Among the six BMI (SEC16B, TMEM18, GNPDA2, GALNT10, FTO and MC4R) and one WHRadjBMI (ADAMTS9-AS2) genome-wide significant loci in AA that were previously reported in EA [7–8], we tested whether the African derived lead variants were independent of the reported European signals by conditioning on the European lead variants or their surrogates. For three of the BMI loci (SEC16B, GNPDA2 and MC4R), our lead variants are the same as those reported in the previous literature [7]. For all other loci, the lead variants demonstrated substantially lower significance upon conditional analysis, suggesting that the African ancestry results represented the same association signals as previously reported in GWAS performed predominantly in EA populations (S10 Table).
SNP transferability
We further examined all sex-combined and sex-stratified BMI and WHRadjBMI loci identified from previous EA studies [7–9] in our AA data. Among 176 EA lead variants from 170 BMI loci, 119 variants displayed directionally consistent associations with BMI in our data, 31 of these were nominally significant at P < 0.05 (Pbinomial = 2.2×10−18 among 176 variants). Among 84 EA lead variants from 65 WHRadjBMI loci, 69 variants displayed directionally consistent associations with WHRadjBMI, and 23 of these were nominally significant (Pbinomial = 5.3×10−19 among 84 variants) (S11 Table). EA lead variants in 11 BMI and 3 WHRadjBMI loci showed directional consistency and significant associations after correction for multiple comparisons (P < 1.92×10−4). Among the 54 nominally transferable lead variants for BMI and WHRadjBMI, 45% and 43% of the effect sizes, respectively, were larger in the EA than the AA populations. In addition, 65% of the frequencies of the trait-raising alleles were higher in the EA populations for both traits. The correlations of both effect sizes and allele frequency of the transferable variants were high (0.74 and 0.79, respectively) for BMI but weak (0.19 and 0.37, respectively) for WHRadjBMI (S11 Fig). The significant but low proportion of lead variants that were transferable from EA to AA (18% for BMI and 27% for WHRadjBMI) suggests either that many loci are not implicated in AA or population differences in LD mask the detection of associated variants in AA. On the other hand, those variants that were transferable explain similar levels of variances for BMI in both populations, but not for WHRadjBMI.
Locus transferability
We further investigated locus transferability in EA loci derived from sex-combined and sex-stratified analyses by considering varying LD between EA and AA populations. S12 Table reports the most significant lead regional variants in our AA sex-combined and sex-stratified data within 0.1cM region of the previously published EA loci (from 176 BMI and 84 WHRadjBMI lead variants) [7–8]. Forty-five (26%) lead regional variants from BMI loci remained significant (Plocus < 0.05) after adjustment for the number of independent variants tested at each locus. Sixteen (36%) and 22 (49%) of these 45 lead regional variants are in LD (r2>0.2) with the EA BMI lead variants using 1000 Genomes AFR and CEU LD, respectively. Twenty-five of these variants are highly correlated with EA lead variants(r2>0.7 in CEU) or had ≥1 standard error decrease in effect sizes after conditional analyses, representing same association signals as in EA populations. Twenty-one (32%) lead regional variants for WHRadjBMI loci remained significant. Nine (43%) and seven (33%) of the 21 lead regional variants was in LD with the EA lead variant using 1000 Genomes AFR and CEU LD, respectively. Seven of these variants represented the same EA association in conditional analyses (S12 Table).
Fine mapping of novel AA loci and EA-AA transferable established loci
Among the locus-wide significant established loci (44 for BMI given two of 45 lead regional variants were identical in two loci, and 21 for WHRadjBMI), and novel loci (three for BMI and six for WHRadjBMI) derived from the sex-combined and sex-stratified analyses, we performed fine mapping to localize putative causal variants. We constructed 99% credible sets containing variants that jointly account for 99% posterior probability of driving the association in a locus using the corresponding sex-combined or sex-stratified meta-analysis results from AA, EA and combined ancestry (S13 Table). A smaller number of variants in a credible set represent a higher resolution of fine mapping and we considered a credible set containing ≤ 20 variants as “tractable’ for follow up. The credible sets in the EA analyses were generally smaller than those in the AA given their larger sample size. As compared to the EA analyses, the number of tractable loci in the meta-analyses of AA and EA increased from 23 to 26 for BMI, and from 14 to 17 for WHRadjBMI.
Among these 43 tractable loci, the lead variants in the combined ancestry analyses had posterior probability ≥ 0.95 in six BMI loci (SEC16B, TLR4, STXBP6, NLRC3, FTO and MC4R) and seven WHRadjBMI loci (DCST2, PPARG, ADAMTS9, SNX10, KLF13, CMIP and PEMT) (S13 Table). Functional characterization of variants within the tractable credible sets revealed two loci contain nonsynonymous variants (ADCY3: rs11676272 S107P; SH2B1: rs7498665 T484A from the ATP2A1 locus), but they had low posterior probability to drive the respective associations (0.02 and 0.15, respectively) (S14 Table). On the other hand, the ADCY3 non-coding variants rs10182181 and rs6752378 had higher posterior probability (0.26–0.72) and are cis-eQTLs of ADCY3 and nearby genes. Several BMI loci including MTCH2, MAP2K5, NLRC3 and ATP2A1, and WHRadjBMI loci including TBX15-WARS2 and FAM13A, also contained cis-eQTL variants regulating nearby gene expression in subcutaneous and/or visceral adipose tissue (S14 Table).
Discussion
In our large-scale meta-analyses of GWAS in up to 52,895 and 23,095 individuals of African ancestry for BMI and WHRadjBMI, respectively, we identified three novel (IRX4/IRX2, INTS10/LPL and MLC1) and seven established (SEC16B, TMEM18, GNPDA2, GALNT10, KLHL32, FTO and MC4R) BMI loci, as well as three novel (TCF7L2/HABP2, SSX2IP and PDE3B) and one established (ADAMTS9-AS2) WHRadjBMI loci in either sex-combined or sex-stratified analyses. By employing a recently developed method [23] to impute European GWAS summary statistics to the denser 1000 Genomes reference panel, followed by meta-analyses of both African and European ancestry individuals, we also identified three additional novel loci (SPRYD7/DLEU2, CASC8 and ZDHHC1/ HSD11B2) for WHRadjBMI. While all lead variants from established loci are common (MAF ≥ 5%), four of the nine lead variants from novel loci were low frequency (0.5% ≤ MAF < 5%). In addition, the lead variants from established loci including TMEM18 and ADAMTS9-AS2 were absent in HapMap. Overall, these results suggest the deeper genome coverage and/or improved imputation quality using 1000 Genomes, and complemented with additional sex-stratified analyses, facilitate the discovery of novel loci and identification of variants with stronger effects in established loci.
Among the novel sex-specific BMI loci (IRX4/IRX2, INTS10/LPL and MLC1), we did not identify any putative coding variants or regulatory regions underlying our association signals. Additionally, no associations have been reported with other metabolic traits in these novel BMI-associated signals. The first lead variant rs112778462 is located between the IRX4 and IRX2 genes which are members of the Iroquois homeobox gene family. IRX2 expression has been associated with deposition of fat in the subcutaneous abdominal adipose tissue but no sex difference was observed [29–30]. Irx4 knock out mice demonstrated cardiomyopathy with compensated increased Irx2 expression [31]. The second lead variant rs149352150 is located between the INTS10 and LPL genes. LPL encoded lipoprotein lipase is expressed in several tissues including adipose to mediate triglyceride hydrolysis and lipoprotein uptake. The serum LPL mass [32] and LPL activity and fat cell size of adipose tissues at gluteus and thigh [33] have been reported to be higher in women than in men. Previous GWAS demonstrated association of LPL with triglycerides and HDL cholesterol [34–35]. However, the reported lead variant rs12678919 was not in strong LD with rs149352150 (r2 = 0.005 in AFR and 0.006 in EUR). The third lead variant rs56330886 is located in a gene-rich region on chromosome 22q13 including MLC1. No biological candidates are identified in this region, therefore further analyses may be needed to explain the causative mechanism for this association signal.
Among the novel WHRadjBMI loci, rs116718588 is located between TCF7L2 and HABP2. TCF7L2 is the most significant type 2 diabetes locus in African Americans [36] and other populations [37]. However, rs116718588 was not in LD (r2 < 0.01 in AFR) with the reported type 2 diabetes associated variants. The second lead variant rs2472591 is located near SPRYD7, DLEU2 and TRIM13. This locus was associated with height in previous GWAS [6], but rs2472591 was not associated with height in our study (P > 0.05), suggesting different variants in this locus regulate different measures of body size. In addition, a surrogate of rs2472591, rs790943, is a cis-eQTL for TRIM13 [26] suggesting it may be the target gene. TRIM13 encodes an E3 ubiquitin-protein ligase involved in endoplasmic reticulum-associated degradation. The third lead variant rs140858719 is located between SSX2IP and LPAR3. LPAR3 is a plausible candidate as it encodes a receptor for lysophosphatidic acid (LPA). The autotaxin/LPA pathway mediates diverse biological actions including activation of preadipocyte proliferation [38], suppression of brown adipose differentiation [39], and promotion of systematic inflammation [40] which lead to increased risk for cardiometabolic diseases including obesity and insulin resistance [41–42]. LPA receptor 1 which is highly expressed in adipocytes and the gut primarily mediates these effects [43]. It has also been reported that LPA, via LPA1 and LPA3 receptors, mediated leukocytes recruitment and pro-inflammatory chemokine secretion during inflammation [44]. The fourth lead variant rs185693786 is located at intron 2 of PDE3B. The association signal spanned a large genomic region and harbors GWAS loci for adiponectin and height. Phosphodiesterase 3B is critical for mediating insulin/IGF-1 inhibition of cAMP signaling in adipocytes, liver, hypothalamus and pancreatic β cells [45]. Pde3b-knockout mice exhibited multiple alterations in regulation of lipolysis, lipogenesis, and insulin secretion, as well as signs of peripheral insulin resistance [46]. PDE3B expression has been reported to be higher in microvascular endothelial cell culture derived from skeletal muscles from male rats than in female rats [47]. The fifth lead variant rs6499129 is located intergenic between ZDHHC1 and HSD11B2. HSD11B2 encodes 11β-hydroxysteroid dehydrogenase type 2 which converts the active glucocorticoids to inactive metabolites. HSD2 activity was elevated in severe obesity and negatively associated with insulin sensitivity [48]. HSD2 expression is higher in omental than abdominal subcutaneous adipose tissue which may contribute to adipocyte hypertrophy and visceral obesity [49]. The sixth lead variant rs378854 is located at the long non-coding RNA CASC8. Associations of variants at CASC8 have been reported for various cancers [50–52] but no association was reported for cardiometabolic traits.
In our SNP and locus transferability analyses, a moderate number of EA-derived BMI and WHRadjBMI associated variants shared the same trait-raising alleles and displayed nominally significant associations in AA individuals, similar to previous findings [11–12]. While the BMI variants were similar in terms of their effect sizes and frequencies of trait-raising alleles between EA and AA populations, there were more discrepancies for WHRadjBMI variants. In addition, a substantial proportion of lead regional variants in AA were not in strong LD with EA lead variants, suggesting AA populations either have different association signals or the results may be spurious. Taken together, only <30% of EA loci were associated with BMI and WHRadjBMI in AA.
Trans-ethnic fine mapping improved resolution to refine putative causal variant(s) in some loci as compared to using EA studies alone. In the meta-analyses of AA and EA GWAS, four BMI loci (SEC16B, STXBP6, FTO and MC4R) and six WHRadjBMI loci (PPARG, ADAMTS9, SNX10, KLF13, CMIP and PEMT) only contained one variant in the 99% credible sets. Among 16 BMI and 3 WHRadjBMI loci that were examined in both the previous trans-ethnic meta-analysis studies using HapMap imputation [7–8] and the present study, the number of variants and the interval of credible sets were either the same or lower in the present study for 13 and 15 loci, respectively. The majority of credible variants are non-coding in those sets containing ≤ 20 variants. Several of them located at the MTCH2, MAP2K5, NLRC3, ATP2A1, TBX15-WARS2 and FAM13A loci are cis-eQTL variants regulating nearby gene expression in subcutaneous and/or visceral adipose tissue, suggesting the putative causal variants may have a regulatory role instead of directly altering protein structure and function. Despite the low posterior probabilities, the coding changes of credible variants at ADCY3 and SH2B1 suggest that they may be the causal genes in the respective loci modulating BMI. Further studies are warranted to delineate putative causal variants including functional annotation in trans-ethnic fine mapping efforts [53].
Our large-scale GWAS meta-analyses in African ancestry individuals imputed to the 1000 Genomes reference panel, complemented by imputation of European GWAS using summary statistics and additional sex-stratified analyses, boosts the study power and improves resolution, leading to the identification of nine novel loci and fine mapping 37 loci with tractable credible sets. We observed significant associations for variants with MAF ≥ 0.5%, but rare variants were unlikely to be detected due to limited power and poor imputation quality. Large scale sequencing studies are needed to evaluate the contribution of rare variants in modulating complex traits such as BMI and WHR. Given the substantially larger sample size in European than in African ancestry samples, the trans-ethnic fine mapping results are largely driven by variants showing strong associations in Europeans. Future trans-ethnic studies including additional non-European populations will further improve the fine mapping effort.
Materials and methods
Study design
We used a three-stage design to evaluate genetic associations with BMI and WHRadjBMI in sex-combined and sex-stratified samples (S1 Fig). Stage 1 included GWAS meta-analyses in AA individuals and stage 2 included replication of top associations from stage 1. Stage 3 included meta-analysis of top associations from stages 1 and 2 AA studies and EA meta-analysis results. In the discovery stage 1 of AAAGC, 17 GWAS of up to 42,752 AA individuals (16,559 men and 26,193 women; 41,696 African Americans and 1,056 Africans) were included for the BMI analyses. A total of 10 GWAS of up to 20,384 AA individuals (4,783 men and 15,601 women; all African Americans) were included for the WHRadjBMI analyses. For variants with P < 1×10−4 in either the sex-combined or the sex-stratified meta-analyses, stage 2 replication was performed in additional AA individuals from AAAGC (N = 10,143 for BMI, N = 2,711 for WHRadjBMI), followed by meta-analysis with EA individuals from the GIANT consortium (322,154 for BMI, 210,086 for WHRadjBMI). Variants that reached genome-wide significance (P < 5×10−8) were assessed for associations with BMI in two cohorts of children (N = 7,222). All AA participants in these studies provided written informed consent for the research, and approval for the study was obtained from the ethics review boards at all participating institutions. Detailed descriptions of each participating study and measurement and collection of height, weight, waist and hip circumferences are provided in S1 Text, S1 and S2 Tables.
Genotyping, imputation and quality control
Genotyping in each study was performed with Illumina or Affymetrix genome-wide SNP arrays. Pre-phasing and imputation of missing genotypes in each study was performed using MaCH/ minimac [20] or SHAPEIT2/IMPUTEv2 [21–22] using the 1000 Genomes Project cosmopolitan reference panel (Phase I Integrated Release Version 3, March 2012) [18]. The details of the array, genotyping and imputation quality-control procedures and sample exclusions for each study are listed in S3 Table. In general, samples reflecting duplicates, low call rates, gender mismatch, or population outliers were excluded. Variants were excluded by the following criteria: call rate < 0.95, minor allele count (MAC) ≤ 6, Hardy-Weinberg Equilibrium (HWE) P < 1×10−4, imputation quality score < 0.3 for minimac or < 0.4 for IMPUTE, or absolute allele frequency difference > 0.3 compared with expected allele frequency (calculated as 1000 Genomes frequency of AFR × 0.8 + EUR × 0.2).
Performance of 1000 Genomes imputation in African ancestry
We evaluated the performance of 1000 Genomes imputation using the largest study, the Women’s Health Initiative (WHI) (N = 8,054). A total of 25.1 million variants with MAF ≥ 0.1% were imputed to the 1000 Genomes reference panel. Of these, 98.1% (8.8 million) common variants, 95.4% (9.3 million) low frequency variants (0.5% ≤ MAF < 5%), and 72.5% (4.6 million) rare variants (0.1% ≤ MAF < 0.5%) were well imputed with IMPUTE info scores ≥ 0.3 (S4 Table). Notably, these frequencies are slightly lower than those obtained by imputation using 1000 Genomes phase 1 interim reference panel in Europeans [54]. However, 72.6%, 95.5% and 99.5% of the common, low frequency and rare variants, respectively, from the 1000 Genomes reference panel were not present in the HapMap and therefore demonstrate deeper coverage of the genome, particularly for the low frequency and rare variants.
Study-level association analyses
At all stages, genome-wide association analyses were performed by each of the participating studies. BMI was regressed on age, age squared, principal components and study site (if needed) to obtain residuals, separately by sex and case-control status, if needed. WHR was regressed on age, age squared, principal components, BMI and study site to obtain residuals, separately by sex and case-control status. Principal components were included to adjust for admixture proportion and population structure within each study. Residuals were inverse-normally transformed to obtain a standard normal distribution with mean of zero and standard deviation of one. For studies with unrelated subjects, each variant was tested assuming an additive genetic model with each trait by regressing the transformed residuals on the number of copies of the variant effect allele. The analyses were stratified by sex and case-control status (if needed). For studies that included related individuals, family based association tests were conducted that took into consideration the genetic relationships among the individuals. Sex stratified, case-control stratified and combined analyses were performed. Association results with extreme values (absolute beta coefficient or standard error ≥ 10), primarily due to small sample sizes and/or low minor allele count, were excluded for meta-analysis.
Imputation of European GWAS summary statistics to 1000 Genomes
The latest summary statistics of sex-combined and sex-stratified meta-analyses of BMI and WHRadjBMI imputed to the HapMap reference panel in EA from the Genetic Investigation of ANthropometric Traits (GIANT) consortium were obtained from http://www.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files [7–8]. These association summary statistics were used to impute z-scores of unobserved variants at the 1000 Genomes Project EUR reference panel (Phase I Integrated Release Version 3) using the ImpG program [23]. In brief, palindromic variants (AT/CG) and variants with allele mismatch with the reference were removed from the data. Using the ImpG-Summary method, the z-score of an unobserved variant was calculated as a linear combination of observed z-scores weighted by the variance-covariance matrix between variants induced by LD within a 1 Mb window from the reference haplotypes. The sample size of each unobserved variant was also interpolated from the sample sizes of observed variants using the same weighting method for z-score as . Here, t = 1,2,….,T, where T is the number of observed variants, wi,t is the element of the covariance matrix Σi,t for the unobserved variant i and the observed variant t within window. The performance of imputation was assessed by r2pred, with similar characteristics as the standard imputation accuracy metric r2hat [20]. Results of variants with r2pred ≥ 0.6 were used in subsequent analyses.
Meta-analysis
In the discovery stage 1, association results were combined across studies in sex-combined and sex-stratified samples using inverse-variance weighted fixed-effect meta-analysis implemented in the program METAL [55]. The study-specific λ values of association ranged from 0.97 to 1.05 for BMI, and 0.98 to 1.05 for WHRadjBMI (S3 Table). Genomic control correction [56] was applied to each study before meta-analysis, and to the overall results after meta-analysis (λ = 1.07 for BMI, 1.01 for WHRadjBMI). Variants with results generated from < 50% of the total sample size for each trait were excluded. After filtering, the numbers of variants reported in the meta-analyses were 17,972,087 for BMI, and 20,502,658 for WHRadjBMI.
Variants with P < 1×10−4 in stage 1 sex-combined or sex-stratified meta-analyses were carried forward for replication in additional AA individuals (stage 2) and EA individuals (stage 3). For each of the replication AA studies, trait transformation and association were performed as in stage 1 and results were meta-analyzed using the inverse-variance method in METAL. For the replication study in EA, HapMap imputed summary statistics of each trait from the GIANT consortium were used to impute z-scores of unobserved variants at the 1000 Genomes.
In stages 1 and 2, meta-analysis results of AA studies were combined using the inverse-variance weighted method. In all stages including both AA and EA studies, meta-analysis results expressed as signed z-scores were combined using the fixed effect sample size weighted method in METAL due to the lack of beta and standard error estimates from the ImpG program [23]. Evidence of heterogeneity of allelic effects between males and females, within and across stages were assessed by the I2 statistic in METAL. Genome-wide significance was declared at P < 5×10−8 from each of the sex-combined and sex-stratified meta-analysis including AA and/or combined AA and EA individuals. Difference in effects between men and women was assessed using Cochran’s Q test and nominal Phet < 0.05 declared as significant. A lead variant in a locus was defined as the most significant variant within a 1 Mb region. A novel locus was defined as a lead variant with distance > 500 kb from any established lead variants reported in previous studies. By convention, a locus was named by the closest gene(s) to the lead variant.
Conditional and joint analyses of summary statistics
For the genome-wide significant loci identified in sex-combined and sex-stratified analyses in AA (stages 1+2), we used the program GCTA [57–58] to select the top independent associated variants from summary statistics of the meta-analyses. This method uses the LD correlations between variants estimated from a reference sample to perform an approximate conditional association analysis. We used 8,054 unrelated individuals of African ancestry from the WHI cohort with ~15.7M variants available as the reference sample for LD estimation. To select the top independent variants in the discovery and replication meta-analysis results, we first selected all variants that had P < 5×10−8 and conducted analysis conditioning on the selected variants to search for the top variants iteratively via a stepwise model to select the independent variants from this list. Then we proceeded to condition the rest of the variants that had P > 5×10−8 on the list of independent variants in the same fashion until no variant had conditional P that passed the significance level P < 5×10−8. Finally, all the selected variants were fitted jointly in the model for effect size estimation.
We also tested if the genome-wide significant variants identified from sex-combined GWAS in AA and the locus-wide significant variants identified from sex-combined and sex-specific locus transferability studies in AA were independent from nearby established loci identified from EA studies [7–8]. First, the published lead variants from EA studies were used to search for all surrogate variants that were in high LD (r2>0.8 in 1000 Genomes Project EUR population). Second, these variants were pruned to select only variants in low LD in AA (r2<0.3 in the 1000 Genomes Project AFR population) to avoid collinearity in conditional analysis. Third, association analysis was conducted on the AA significant variants conditioned on the selected EA lead and surrogate variants, using the program GCTA and estimated LD correlation from the WHI cohort. For genome-wide significant loci, an AA derived association signal is considered as independent from the established EA signals when the difference in–logP <3 and difference in effect size < 1 standard error after conditional analysis. For locus-wide significant loci, given the lower level of significance, independence is only considered as difference in effect size < 1 standard error after conditional analysis.
SNP and locus transferability analyses
We investigated the transferability of EA BMI and WHR associated variants and loci in AA individuals from stage 1 sex-combined and sex-stratified meta-analyses. First, we tested for replication of lead variants previously reported to be associated with BMI (176 variants from 170 loci) and WHRadjBMI (84 variants from 65 loci) at genome-wide significance in sex-combined and sex-stratified analyses from the GIANT consortium studies [7–9]. We defined SNP transferability as an EA lead variant sharing the same trait-raising allele at nominal P < 0.05 in AA individuals. To account for differences in local LD structure across populations, we also interrogated the flanking 0.1cM regions of the lead variants to search for the best variants with the smallest association P in AA individuals. Locus-wide significance was declared as Plocus < 0.05 by Bonferroni correction for the effective number of tests within a locus, estimated using the Li and Ji approach [59].
Fine mapping analyses
We compared the credible set intervals of established loci that showed locus-wide significance (Plocus < 0.05) in the sex-combined or sex-specific analyses from this study in summary statistics datasets including the 1000 Genomes imputed results from GIANT, AAAGC and meta-analysis of GIANT and AAAGC. In each dataset, a candidate region is defined as the flanking 0.1cM region of the lead variant reported by the GIANT consortium. Under the assumption of one causal variant in a region of M variants, the posterior probability of a variant j with association statistics Z driving the association, P(Cj|Z), was calculated using the formula . A 99% credible set was constructed by ranking all variants by their posterior probability, followed by adding variants until the credible set has a cumulative posterior probability > 0.99 [53].
Bioinformatics
Functional annotation of novel variants
To determine whether any of our nine novel GWAS lead variants identified in the sex-combined and sex-specific analyses might be tagging potentially functional variants, we identified all variants within 1 Mb and in LD (r2 > 0.7, 1000 Genomes AFR) with our lead variants. As such, we identified 137 variants and annotated each of them using ANNOVAR [60]. The predicted functional impact for coding variants were assessed via the Exome Variant Server (http://evs.gs.washington.edu/EVS/) for PhastCon, GERP [61], and PolyPhen [62], as well as SIFT [63].
We further characterized the variants that were in LD with the novel variants using the web-based tool RegulomeDB (http://regulomedb.org/) [24]. The variants that were likely to affect binding and linked to expression of a gene target (scores 1a-1f) based on “eQTL, transcription factor (TF) binding, matched TF motif, matched DNase footprint and DNase peak” or were only likely to affect binding (scores 2a-2c) based on “TF binding, matched TF motif, matched DNase footprint and DNase peak” were selected. For these variants, the sequence conservation (GERP and SiPhy [64]), the epigenomic data from the Roadmap Epigenomic project (ChromHMM states corresponding to enhancer or promoter elements, histone modification ChIP-seq peaks, and DNase hypersensitivity data peaks), the regulatory protein binding from the ENCODE project, the regulatory motifs based on commercial, literature and motif-finding analysis of the ENCODE project, and the eQTLs from Genotype-Tissue Expression (GTEx) project [65] were extract from web-based HaploReg v4 [25]. For variants within the tractable credible sets in the fine mapping analyses, similar analyses were also conducted.
Cross-trait associations
To assess whether the novel loci identified in the sex-combined and sex-specific analyses were associated with any related cardiometabolic and anthropometric traits, or may be in high LD with known eQTLs, we examined the NHGRI-EBI GWAS Catalog [27] and the GRASP (Genome-Wide Repository of Associations Between SNPs and Phenotypes) catalog [28] for reported variant-trait associations near our lead variants. We supplemented the catalogs with additional genome-wide significant associations of interest from the literature [7–9,66]. We used PLINK to identify variants within 1 Mb of lead variants. All variants within the specified regions with r2 > 0.7 (1000 Genomes AFR) were retained from the catalogs for further evaluation.
Power analysis
Given our sample sizes in the discovery and replication stages in our African ancestry populations, we have >80% power to detect variants explaining 0.08% variance for BMI that corresponds to effect sizes of 0.09 and 0.20 SD units for MAF of 0.05 and 0.01, respectively. For WHRadjBMI, we have >80% power to detect variants explaining 0.18% variance that corresponds to effect sizes of 0.14 and 0.30 SD units for MAF of 0.05 and 0.01, respectively.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Locuszoom plots using discovery results for established loci that reached genome-wide significance: (A) SEC16B, TMEM18, GNPDA2, GALNT10, KLHL32, FTO and MC4R for BMI in men and women combined; and (B) ADAMTS9-AS2 for waist-to-hip ratio adjusted for BMI (WHRadjBMI) in men and women combined. All plots use AFR LD from the 1000 Genomes phase 1 reference panel. In each plot, the most significant variant within a 1Mb regional locus is highlighted. P-values for all variants including the most significant variant are based on the African ancestry discovery phase only (AA-Discovery). In addition, for the most significant variant, P-values are annotated and illustrated from the African ancestry discovery and replication phases (AA-Discovery+Replication).
(PDF)
Correlation of effect sizes for (A) BMI and (B) WHRadjBMI, and effect allele frequencies for (C) BMI and (D) WHRadjBMI in European and African ancestry studies in SNP transferability analyses.
(PDF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
This table lists all previously-reported associations within 1 Mb (+/- 500 kb) and in high LD (r2 >0.7) with our lead novel SNPs along with relevant annotation (e.g. miRNA target binding site, variant location relevant to nearest gene, gene function prediction) reported in the GRASP Catalog.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
We gratefully acknowledge the contribution of all participants from the various studies that contributed to this work.
Data Availability
All relevant data are within the paper and its Supporting Information files. Summary association statistics from meta-analyses are available from dbGAP at accession number phs000930.
Funding Statement
AABC-MEC: The MEC and genotyping of samples for the GWAS of breast and prostate cancer was supported by National Institutes of Health grants CA63464, CA54281, CA164973, CA1326792, CA148085, HG004726 and a Department of Defense Breast Cancer Research Program Era of Hope Scholar Award to CAH (W81XWH-08-1-0383) and the Norris Foundation. AABC-CARE: CARE was supported by National Institute for Child Health and Development contract NO1-HD-3-3175. AABC-WCHS: WCHS is supported by a U.S. Army Medical Research and Material Command (USAMRMC) grant DAMD-17-01-0-0334, National Institutes of Health grant CA100598, and the Breast Cancer Research Foundation. AABC-SFBCS: SFBCS was supported by National Institutes of Health grant CA77305 and United States Army Medical Research Program grant DAMD17-96-6071. AABC-NC-BCFR: The Breast Cancer Family Registry (BCFR) was supported by the National Cancer Institute, National Institutes of Health under RFA CA-06-503 and through cooperative agreements with members of the Breast Cancer Family Registry and Principal Investigators, including the Cancer Prevention Institute of California (U01 CA69417). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government or the BCFR. AABC-CBCS: CBCS is supported by National Institutes of Health Specialized Program of Research Excellence in Breast Cancer grant CA58223 and Center for Environmental Health and Susceptibility, National Institute of Environmental Health Sciences, National Institutes of Health, grant ES10126. AABC-PLCO: Genotyping of the PLCO samples was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, the National Institutes of Health. The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the screening center investigators and staff of the PLCO Cancer Screening Trial, Mr. Thomas Riley and staff at Information Management Services, Inc., and Ms. Barbara O’Brien and staff at Westat, Inc. for their contributions to the PLCO Cancer Screening Trial. AABC-NBHS: NBHS is support by National Institutes of Health grant CA100374. NBHS sample preparation was conducted at the Biospecimen Core Lab that is supported in part by the Vanderbilt-Ingram Cancer Center (CA68485). AABC-WFBC: WFBC is supported by National Institutes of Health grant CA73629. AAPC-CPS-II: CPSII is supported by the American Cancer Society. AAPC-KCPCS: KCPCS was supported by National Institutes of Health grants CA056678, CA082664 and CA092579, with additional support from the Fred Hutchinson Cancer Research Center. AAPC-LAAPC: LAAPC was funded by grant 99-00524V-10258 from the Cancer Research Fund, under Interagency Agreement #97-12013 (University of California contract #98-00924V) with the Department of Health Services Cancer Research Program. AAPC-DCPC: DCPC was supported by National Institutes of Health grant S06GM08016 and Department of Defense grants DAMD W81XWH-07-1-0203 and DAMD W81XWH-06-1-0066. AAPC-MEC: The MEC and genotyping of samples for the GWAS of prostate cancer was supported by National Institutes of Health grants CA63464, CA54281, CA164973, CA1326792, CA148085 and HG004726. AAPC-SELECT trial: The SELECT trial is supported by National Cancer Institute grants U10CA37429 and 5UM1CA182883. AAPC-PLCO: See AABC-PLCO. AAPC-GECAP: GECAP was supported by National Institutes of Health grant ES011126. AAPC-SCCS: SCCS is funded by National Institutes of Health grant CA092447. SCCS sample preparation was conducted at the Biospecimen Core Lab that is supported in part by the Vanderbilt-Ingram Cancer Center (CA68485). AAPC-MDA: MDA was support by grants CA68578, ES007784, DAMD W81XWH-07-1-0645, and CA140388. AAPC-CaP Genes: CaP Genes was supported by CA88164 and CA127298. ARIC: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. BioMe: NIH/NHGRI U01HG007417. BMDCS: We thank the investigators Heidi Kalkwarf, Joan Lappe, Sharon Oberfield, Vicente Gilsanz, John Shepherd and Andrea Kelly for their contribution. BMDCS is supported by NIH: R01 HD58886, HD076321, DK094723-01, DK102659-01; NICHD N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333; CTSA program Grant 8 UL1 TR000077. CARDIA: The Coronary Artery Risk Development in Young Adults (CARDIA) study is supported by the NHLBI (contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C); the Intramural Research Program of the National Institute on Aging (NIA); and an intra-agency agreement between the NIA and the NHLBI (grant AG0005). CFS: The Cleveland Family Study (CFS) is supported by the National Institutes of Health: HL046380, HL113338, RR000080. Dr. Cade has been supported by HL007901. CHOP: This research was financially supported by an Institute Development Award from the Children’s Hospital of Philadelphia, a Research Development Award from the Cotswold Foundation, NIH grant R01 HD056465 and the Daniel B. Burke Endowed Chair for Diabetes Research (SFAG). CHS: This CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants U01HL080295, R01HL085251, R01HL087652, R01HL105756, R01HL103612, R01HL120393 and HL130114 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Participants provided informed consent for participation in DNA studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. FamHS: NIH/NIDDK R01DK089256, and NIH/NHLBI R01HL117078. GeneSTAR: NIH/NHLBI U01 HL72518; NIH/NHLBI HL087698; NIH/NHLBI 58625-01A1; NIH/NHLBI HL071025-01A1; NIH/NINR NR0224103; NIH/NCRR M01-RR000052. HANDLS: Healthy Aging in Neighborhoods of Diversity across the Life Span Study (HANDLS) was supported by the Intramural Research Program of the NIH, National Institute on Aging and the National Center on Minority Health and Health Disparities (project # Z01-AG000513 and human subjects protocol # 2009-149). HealthABC: Health, Aging, and Body Composition Study (Health ABC Study) was supported by NIA contracts N01AG62101, N01AG62103, and N01AG62106. The genome-wide association study was funded by NIA grant 1R01AG032098-01A1 to Wake Forest University Health Sciences and genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. HRS: HRS is supported by the National Institute on Aging (NIA U01AG009740). The genotyping was funded separately by the National Institute on Aging (RC2 AG036495, RC4 AG039029), and the analysis was funded in part by R03 AG046389. Our genotyping was conducted by the NIH Center for Inherited Disease Research (CIDR) at Johns Hopkins University. Genotyping quality control and final preparation of the data were performed by the Genetics Coordinating Center at the University of Washington. HUFS: The HUFS (Howard University Family Study) was supported by grants S06GM008016-380111 to AA and S06GM008016-320107 to CR, both from the NIGMS/MBRS/SCORE Program. Participant enrollment for the HUFS was carried out at the Howard University General Clinical Research Center (GCRC), which was supported by grant 2M01RR010284 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This research was supported in part by the NIH Intramural Research Program in the Center for Research on Genomics and Global Health (CRGGH) with support from the National Human Genome Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Center for Information Technology, and the Office of the Director at the National Institutes of Health (Z01HG200362). HyperGEN: The HyperGEN network is funded by cooperative agreements (U10) with NHLBI: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515, and R01 HL55673 from the National Heart, Lung, and Blood Institute. The study involves: University of Utah: (Network Coordinating Center, Field Center, and Molecular Genetics Lab); Univ. of Alabama at Birmingham: (Field Center and Echo Coordinating and Analysis Center); Medical College of Wisconsin: (Echo Genotyping Lab); Boston University: (Field Center); University of Minnesota: (Field Center and Biochemistry Lab); University of North Carolina: (Field Center); Washington University: (Data Coordinating Center); Weil Cornell Medical College: (Echo Reading Center); National Heart, Lung, & Blood Institute. For a complete list of HyperGEN Investigators: http://www.biostat.wustl.edu/hypergen/Acknowledge.html. JHS: The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. JGW is supported by U54GM115428 from the National Institute of General Medical Sciences. Maywood: NIH: R37-HL045508, R01-HL074166, R01-HL086718, R01-HG003054; analysis also funded through R01-DK075787. MESA: The Multi-Ethnic Study of Atherosclerosis study (MESA) was supported by the Multi-Ethnic Study of Atherosclerosis (MESA) contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. Funding for MESA SHARe genotyping was provided by NHLBI Contract N02-HL-6-4278. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Nigeria: NIH: R37-HL045508, R01-HL053353, R01-DK075787 and U01-HL054512; analysis also funded through R01-DK075787. ROOT: NIH: R01-CA142996, P50-CA125183, R01-CA89085, and U01-CA161032. American Cancer Society: MRSG-13-063-01-TBG and CRP-10-119-01-CCE. SAPPHIRE: NIH/NHLBI R01HL118267; NIH/NIAID R01AI079139; NIH/NIDDK R01DK064695 and R01 DK113003; American Asthma Foundation. SIGNET-REGARDS: The Sea Islands Genetics Network (SIGNET) was supported by R01 DK084350 (MM Sale). The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This study (REGARDS) was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS). WFSM: Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSC268200782096C. This work was supported by National Institutes of Health grants R01 DK087914, R01 DK066358, R01 DK053591, R01 HL56266, R01 DK070941 and by the Wake Forest School of Medicine grant M01 RR07122 and Venture Fund 20543. WHI: Funding support for the “Epidemiology of putative genetic variants: The Women’s Health Initiative” study is provided through the NHGRI PAGE program (U01HG004790 and its NHGRI ARRA supplement). The WHI program is funded by the National Heart, Lung, and Blood Institute; NIH; and U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whiscience.org/publications/ WHI_investigators_shortlist.pdf. CAH: R01 DK101855-01. CL: R01 DK089256. KEN: R01 DK089256, R01 DK101855-01, 15GRNT25880008. KLY: KL2TR001109. LAC: R01 DK089256. RJFL: R01 DK101855-01. YLi: R01HG006292 and R01HL129132. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL (2016) Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291. doi: 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42: 937–948. doi: 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, et al. (2010) Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42: 949–960. doi: 10.1038/ng.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherag A, Dina C, Hinney A, Vatin V, Scherag S, et al. (2010) Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet 6: e1000916 doi: 10.1371/journal.pgen.1000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, et al. (2012) A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet 44: 526–531. doi: 10.1038/ng.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, et al. (2013) Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 45: 501–512. doi: 10.1038/ng.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, et al. (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, et al. (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518: 187–196. doi: 10.1038/nature14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler TW, Justice AE, Graff M, Barata L, Feitosa MF, et al. (2015) The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet 11: e1005378 doi: 10.1371/journal.pgen.1005378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng MC, Hester JM, Wing MR, Li J, Xu J, et al. (2012) Genome-wide association of BMI in African Americans. Obesity (Silver Spring) 20: 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, et al. (2013) A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet 45: 690–696. doi: 10.1038/ng.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CT, Monda KL, Taylor KC, Lange L, Demerath EW, et al. (2013) Genome-wide association of body fat distribution in African ancestry populations suggests new loci. PLoS Genet 9: e1003681 doi: 10.1371/journal.pgen.1003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada Y, Kubo M, Ohmiya H, Takahashi A, Kumasaka N, et al. (2012) Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet 44: 302–306. doi: 10.1038/ng.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, et al. (2012) Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44: 307–311. doi: 10.1038/ng.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen W, Zheng W, Okada Y, Takeuchi F, Tabara Y, et al. (2014) Meta-analysis of genome-wide association studies in East Asian-ancestry populations identifies four new loci for body mass index. Hum Mol Genet 23: 5492–5504. doi: 10.1093/hmg/ddu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449: 851–861. doi: 10.1038/nature06258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, et al. (2010) Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58. doi: 10.1038/nature09298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65. doi: 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaneau O, Marchini J (2014) Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat Commun 5: 3934 doi: 10.1038/ncomms4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR (2010) MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 34: 816–834. doi: 10.1002/gepi.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaneau O, Marchini J, Zagury JF (2011) A linear complexity phasing method for thousands of genomes. Nat Methods 9: 179–181. doi: 10.1038/nmeth.1785 [DOI] [PubMed] [Google Scholar]
- 22.Howie BN, Donnelly P, Marchini J (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529 doi: 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasaniuc B, Zaitlen N, Shi H, Bhatia G, Gusev A, et al. (2014) Fast and accurate imputation of summary statistics enhances evidence of functional enrichment. Bioinformatics 30: 2906–2914. doi: 10.1093/bioinformatics/btu416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, et al. (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22: 1790–1797. doi: 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward LD, Kellis M (2016) HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res 44: D877–881. doi: 10.1093/nar/gkv1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barreiro LB, Tailleux L, Pai AA, Gicquel B, Marioni JC, et al. (2012) Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 109: 1204–1209. doi: 10.1073/pnas.1115761109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welter D, MacArthur J, Morales J, Burdett T, Hall P, et al. (2014) The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 42: D1001–1006. doi: 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie R, O'Donnell CJ, Johnson AD (2014) GRASP: analysis of genotype-phenotype results from 1390 genome-wide association studies and corresponding open access database. Bioinformatics 30: i185–194. doi: 10.1093/bioinformatics/btu273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, et al. (2013) Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab 98: 362–371. doi: 10.1210/jc.2012-2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, et al. (2014) Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes 63: 3785–3797. doi: 10.2337/db14-0385 [DOI] [PubMed] [Google Scholar]
- 31.Bruneau BG, Bao ZZ, Fatkin D, Xavier-Neto J, Georgakopoulos D, et al. (2001) Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol Cell Biol 21: 1730–1736. doi: 10.1128/MCB.21.5.1730-1736.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onat A, Hergenc G, Agirbasli M, Kaya Z, Can G, et al. (2009) Preheparin serum lipoprotein lipase mass interacts with gender, gene polymorphism and, positively, with smoking. Clin Chem Lab Med 47: 208–215. doi: 10.1515/CCLM.2009.018 [DOI] [PubMed] [Google Scholar]
- 33.Votruba SB, Jensen MD (2007) Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol Endocrinol Metab 292: E1823–1828. doi: 10.1152/ajpendo.00601.2006 [DOI] [PubMed] [Google Scholar]
- 34.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713. doi: 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45: 1274–1283. doi: 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng MC, Shriner D, Chen BH, Li J, Chen WM, et al. (2014) Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet 10: e1004517 doi: 10.1371/journal.pgen.1004517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, et al. (2014) Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 46: 234–244. doi: 10.1038/ng.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferry G, Tellier E, Try A, Gres S, Naime I, et al. (2003) Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem 278: 18162–18169. doi: 10.1074/jbc.M301158200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Federico L, Ren H, Mueller PA, Wu T, Liu S, et al. (2012) Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol Endocrinol 26: 786–797. doi: 10.1210/me.2011-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui DY (2016) Intestinal phospholipid and lysophospholipid metabolism in cardiometabolic disease. Curr Opin Lipidol 27: 507–512. doi: 10.1097/MOL.0000000000000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rancoule C, Dusaulcy R, Treguer K, Gres S, Attane C, et al. (2014) Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie 96: 140–143. doi: 10.1016/j.biochi.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 42.Reeves VL, Trybula JS, Wills RC, Goodpaster BH, Dube JJ, et al. (2015) Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity (Silver Spring) 23: 2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yung YC, Stoddard NC, Chun J (2014) LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 55: 1192–1214. doi: 10.1194/jlr.R046458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C, Sardella A, Chun J, Poubelle PE, Fernandes MJ, et al. (2011) TNF-alpha promotes LPA1- and LPA3-mediated recruitment of leukocytes in vivo through CXCR2 ligand chemokines. J Lipid Res 52: 1307–1318. doi: 10.1194/jlr.M008045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, et al. (2011) From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol 11: 676–682. doi: 10.1016/j.coph.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, et al. (2006) Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest 116: 3240–3251. doi: 10.1172/JCI24867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Bingaman S, Huxley VH (2010) Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. Am J Physiol Heart Circ Physiol 298: H1146–1154. doi: 10.1152/ajpheart.00252.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mussig K, Remer T, Haupt A, Gallwitz B, Fritsche A, et al. (2008) 11beta-hydroxysteroid dehydrogenase 2 activity is elevated in severe obesity and negatively associated with insulin sensitivity. Obesity (Silver Spring) 16: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 49.Lee MJ, Fried SK, Mundt SS, Wang Y, Sullivan S, et al. (2008) Depot-specific regulation of the conversion of cortisone to cortisol in human adipose tissue. Obesity (Silver Spring) 16: 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, et al. (2009) Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet 41: 1122–1126. doi: 10.1038/ng.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao K, Hua L, Wei L, Meng J, Hu J (2015) Correlation Between CASC8, SMAD7 Polymorphisms and the Susceptibility to Colorectal Cancer: An updated meta-analysis based on GWAS results. Medicine (Baltimore) 94: e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma G, Gu D, Lv C, Chu H, Xu Z, et al. (2015) Genetic variant in 8q24 is associated with prognosis for gastric cancer in a Chinese population. J Gastroenterol Hepatol 30: 689–695. doi: 10.1111/jgh.12801 [DOI] [PubMed] [Google Scholar]
- 53.Kichaev G, Pasaniuc B (2015) Leveraging functional-annotation data in trans-ethnic fine-mapping studies. Am J Hum Genet 97: 260–271. doi: 10.1016/j.ajhg.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horikoshi M, Mgi R, van de Bunt M, Surakka I, Sarin AP, et al. (2015) Discovery and fine-mapping of glycaemic and obesity-related trait loci using high-density imputation. PLoS Genet 11: e1005230 doi: 10.1371/journal.pgen.1005230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willer CJ, Li Y, Abecasis GR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26: 2190–2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devlin B, Roeder K, Wasserman L (2001) Genomic control, a new approach to genetic-based association studies. Theor Popul Biol 60: 155–166. doi: 10.1006/tpbi.2001.1542 [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Lee SH, Goddard ME, Visscher PM (2011) GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 88: 76–82. doi: 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Ferreira T, Morris AP, Medland SE, Madden PA, et al. (2012) Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet 44: 369–375, S361-363. doi: 10.1038/ng.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 95: 221–227. [DOI] [PubMed] [Google Scholar]
- 60.Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164 doi: 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper GM, Stone EA, Asimenos G, Green ED, Batzoglou S, et al. (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15: 901–913. doi: 10.1101/gr.3577405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. doi: 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, et al. (2012) SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 40: W452–457. doi: 10.1093/nar/gks539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garber M, Guttman M, Clamp M, Zody MC, Friedman N, et al. (2009) Identifying novel constrained elements by exploiting biased substitution patterns. Bioinformatics 25: i54–62. doi: 10.1093/bioinformatics/btp190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Consortium GTEx (2015) Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348: 648–660. doi: 10.1126/science.1262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, et al. (2014) Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 46: 1173–1186. doi: 10.1038/ng.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Locuszoom plots using discovery results for established loci that reached genome-wide significance: (A) SEC16B, TMEM18, GNPDA2, GALNT10, KLHL32, FTO and MC4R for BMI in men and women combined; and (B) ADAMTS9-AS2 for waist-to-hip ratio adjusted for BMI (WHRadjBMI) in men and women combined. All plots use AFR LD from the 1000 Genomes phase 1 reference panel. In each plot, the most significant variant within a 1Mb regional locus is highlighted. P-values for all variants including the most significant variant are based on the African ancestry discovery phase only (AA-Discovery). In addition, for the most significant variant, P-values are annotated and illustrated from the African ancestry discovery and replication phases (AA-Discovery+Replication).
(PDF)
Correlation of effect sizes for (A) BMI and (B) WHRadjBMI, and effect allele frequencies for (C) BMI and (D) WHRadjBMI in European and African ancestry studies in SNP transferability analyses.
(PDF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
This table lists all previously-reported associations within 1 Mb (+/- 500 kb) and in high LD (r2 >0.7) with our lead novel SNPs along with relevant annotation (e.g. miRNA target binding site, variant location relevant to nearest gene, gene function prediction) reported in the GRASP Catalog.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Summary association statistics from meta-analyses are available from dbGAP at accession number phs000930.