Abstract
The pineal gland plays a central role in the photoneuroendocrine system and acts as a photosensory organ in lower vertebrates. The orphan nuclear receptor Rev-erbα (NR1D1) has previously been shown to be expressed in the pineal and to be regulated with a robust circadian rhythm during zebrafish embryogenesis. This early pineal expression is under the control of the transcription factor Orthodenticle homeobox 5 (Otx5). In this paper, we show that Otx5 regulates the second zfRev-erbα promoter, ZfP2. Despite the absence of a classical Otx-binding site within ZfP2, this regulation depends on the integrity of the Otx5 homeodomain. Mapping experiments as well as EMSAs show that this interaction between Otx5 and ZfP2 depends on a noncanonical bipartite Otx-binding site (GANNCTTA and TAAA) that we called pineal expression related element (PERE). We showed that PERE is necessary for pineal expression in vivo by injecting zebrafish embryos with wild type and mutated versions of zfRev-erbα promoter fused to green fluorescent protein. Interestingly, PERE is found upstream of other genes expressed in the pineal gland, suggesting that it may play an important role in governing pineal expression. Our data establish that PERE is a novel cis-acting element contributing to pineal-specific gene expression and to Otx target gene regulation.
LIVING ORGANISMS USE environmental light signals for multiple physiological functions such as vision and photoentrainment of circadian rhythms. These diverse functions are mediated by the retina and by extraocular photoreceptive organs, such as the pineal gland. The pineal gland, sharing morphological and biochemical similarities with the retina, thus plays a unique and central role in the photoneuroendocrine system. The primary role of the pineal gland is the rhythmic production of circulating melatonin, which regulates numerous physiological activities (1). Pineal gland and retina probably arose via divergence from a common ancestral photoreceptive organ (2, 3, 4). In the course of vertebrate evolution, the physiological role of the pineal gland has been changed from a photosensory and photoendocrinal organ in teleosts fish and amphibians to a neuroendocrinal organ in mammals (5, 6).
The unique development of the pineal gland is directed by a specific combination of genes. Several research groups identified a pineal regulatory element (PIRE, TAATC/T), which is recognized by Crx, a divergent member of the Otx (orthodenticle homeobox) family of homeodomain transcription factors (7, 8). This element is present in the 5′-flanking regions of several pineal genes, such as rat arylalkylamine-N-acetyltransferase and human hydroxyindole-O-methyltransferase genes (7). A similar element, called photoreceptor-conserved element (TAATT), has been shown to be recognized by both Otx5 and Crx and to synergize with the CLOCK-BMAL (circadian locomoter output cycles kaput-brain and muscle ARNT-like) heterodimer, a major regulator of circadian rhythm (9). Using the zebrafish, another element called pineal expression-promoting element (TGACCCCAATCT) has been identified in the extraocular rhodopsin (exorh) promoter (10). In combination with binding sites for the Otx/Crx family of homeodomain transcription factors, pineal expression-promoting element contributes to pineal-specific expression.
These observations are in accordance with the recent observation that circadian gene expression in the zebrafish pineal complex requires Otx/Crx factors (9, 11). All the vertebrate Otx family proteins that share the repetition of a C-terminal amino acid motif can be split into three orthology classes: Otx1, Otx2, and Otx5/Crx (12). Recently, Appelbaum et al. (13, 14) revealed that the photoreceptor conserved element and E box mediated the action of Otx5 and CLOCK-BMAL, respectively.
Transcription of several genes is both circadian and developmentally regulated in zebrafish, providing an excellent model with which to decipher the mechanisms implicated in these complex regulations. This is also the case for the orphan nuclear receptor Rev-erbα (NR1D1), an important actor of the pacemaker that controls circadian rhythm in vertebrates. During zebrafish embryogenesis, rev-erbα expression is robustly circadian and has been observed to start at 24 h post fertilization (hpf) specifically in the pineal gland and then to extend at 48 hpf to the retina and, after one more day (72 hpf) to the optic tectum (15). In the zebrafish pineal, but not in the retina, rev-erbα gene expression is under the control of Otx5, suggesting that this factor may play a role in the early specific pineal expression of rev-erbα (11). We previously characterized the regulatory regions governing rev-erbα gene expression in mammals and zebrafish (16). In each case, rev-erbα expression is controlled by two promoters (Ref.17 ; see supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). The first one, ZfP1, is highly conserved between mammals and fish and is directly activated by the CLOCK-BMAL1 heterodimer (16). In addition this promoter is also repressed both in vitro and in vivo by its own product, REV-ERBα, whereas it is activated by the closely related orphan receptors (17). Interestingly, the second promoter, ZfP2 is strongly divergent between mammals and zebrafish and consequently is also regulated differently in mammals and zebrafish.
In this paper we present the mechanisms by which Otx5 controls rev-erbα expression. We found that Otx5 regulates ZfP2 through a previously uncharacterized bipartite sequence GANNCTTA followed by TAAA, which we call pineal expression related element (PERE). In addition, we show that in vivo ZfP2 is critical for rev-erbα early pineal expression in a PERE-dependant fashion. Taken together, our findings define a new Otx-binding site that is important for expression of specific genes in the pineal gland.
RESULTS
Otx5 Activates rev-erbα ZfP2 Activity
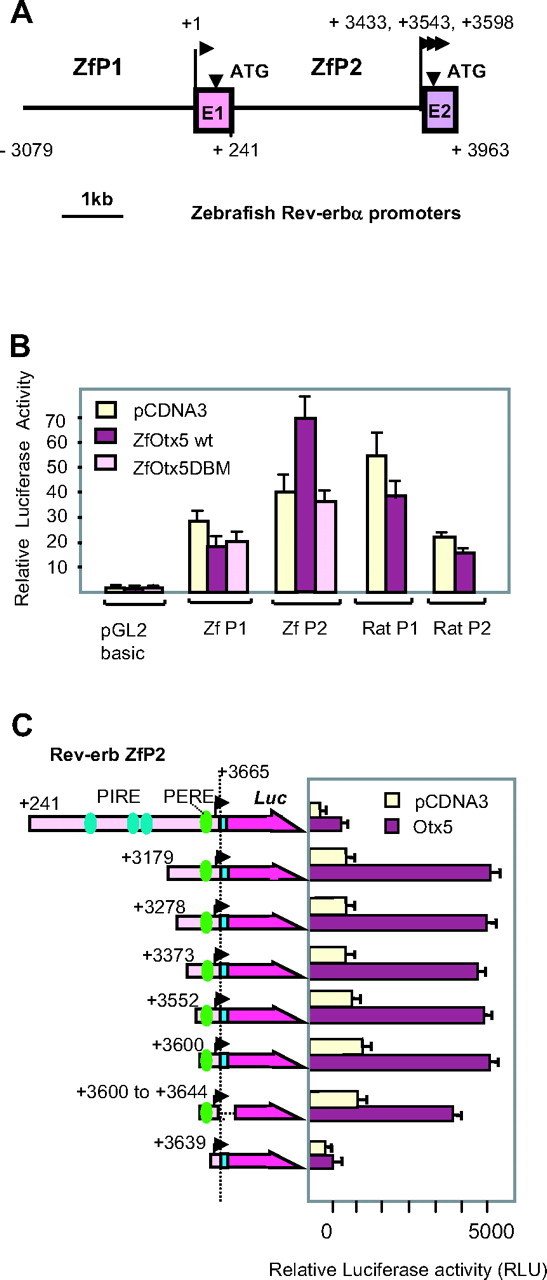
Rev-erbα gene expression in the zebrafish pineal gland requires Otx5 because otx5 knockdown shuts down rev-erbα expression (11). Each of the two zebrafish Rev-erbα promoters (Fig. 1A) contains putative sites for Otx/Crx factors (see supplemental Fig. 1). To determine which one is regulated by Otx5, ZfP1-Luc or ZfP2-Luc constructs were cotransfected into COS-1 cells with Otx5 expression vectors. Reporter gene expression driven by ZfP2 was significantly (P < 0.001) enhanced up to 1.7-fold in the presence Otx5 (Fig. 1B). In contrast, neither ZfP1 nor the mammalian Rev-erbα promoters (P1 and P2) were activated by Otx5 (Fig. 1B). Interestingly, ZfP2 was also regulated by mammalian Otx1 and Otx2, but not by mammalian or zebrafish Crx proteins (data not shown). We asked whether the effect of ZfOtx5 was dependent on a direct DNA binding by testing a DNA binding-deficient mutant of ZfOtx5 [Otx5 DNA-binding mutant (DBM)] containing three specific point mutations in the homeodomain (V84Y, K87E, and N88A). As expected, Otx5 DBM failed to transactivate ZfP2 (Fig. 1B).
Fig. 1.
OTX5 Binding Site Mapping within ZfRev-erbα
A, Genomic organization of the two zebrafish promoters of Rev-erbα. Transcription start sites are represented by arrows with their respective positions. B, Otx5 activates zfRev-erbα P2 in a DNA-binding dependent manner. Luciferase reporter (100 ng) (either ZfP1, ZfP2, RatP1, or RatP2) vector, and 50 ng zfOtx5 expression vector encompassing the WT or mutated DNA binding domain were cotransfected into COS-1 cells. C, Determination of the functional Otx5 binding site. Deletion constructs are shown on the left side of the figure with the following color code: pink box, ZfP2; blue box, exon 2, purple arrow, luciferase gene. PIRE and PERE are shown as dark blue and green spots, respectively. Dotted lines represent internal sequence deletions. The ZfP2 transcription start site is shown by an arrowhead. Several different zfRev-erbα luciferase reporter vectors (100 ng) with deleted regulatory regions and 100 ng zfOtx5 expression vector were cotransfected into COS-1 cells. For both panels, relative luciferase activities are presented after normalization with an internal control (β-gal activities). Each transfection was conducted in triplicate wells, and data represent the mean ± sd of at least three independent experiments.
To identify the region of the ZfP2 promoter involved in Otx5 binding, a series of ZfP2 deletion constructs were designed and tested for their ability to be activated by Otx5 in transient transfection assays in Cos-1 cells (Fig. 1C). A deletion of the three putative PIRE sequences, such as in construct +3179, does not impair activation by zfOtx5. Further deletions suggest that the very proximal region (+3600 to +3665) is still activated by Otx5 but not by the Otx5 DBM (Fig. 1C and data not shown). Interestingly, a shorter promoter region spanning +3639 bp to +3665 bp resulted in a marked reduction of Otx5 activation, whereas the promoter itself is still active albeit at a reduced level. In line with this observation, a short version of the promoter containing only 45 bp (3600–3644) is fully active and can be activated by Otx5 (Fig. 1C). These results suggest that the region of ZfP2 activated by Otx5 is located in this 45-bp region that contains no canonical Otx binding site or PIRE element. Of note, Otx2 is also able to activate ZfP2 through this element (data not shown). Taken together, these results suggest that Otx factors directly activate ZfP2 in a DNA binding-dependent manner through this short element.
Otx5 Binding to a New Element
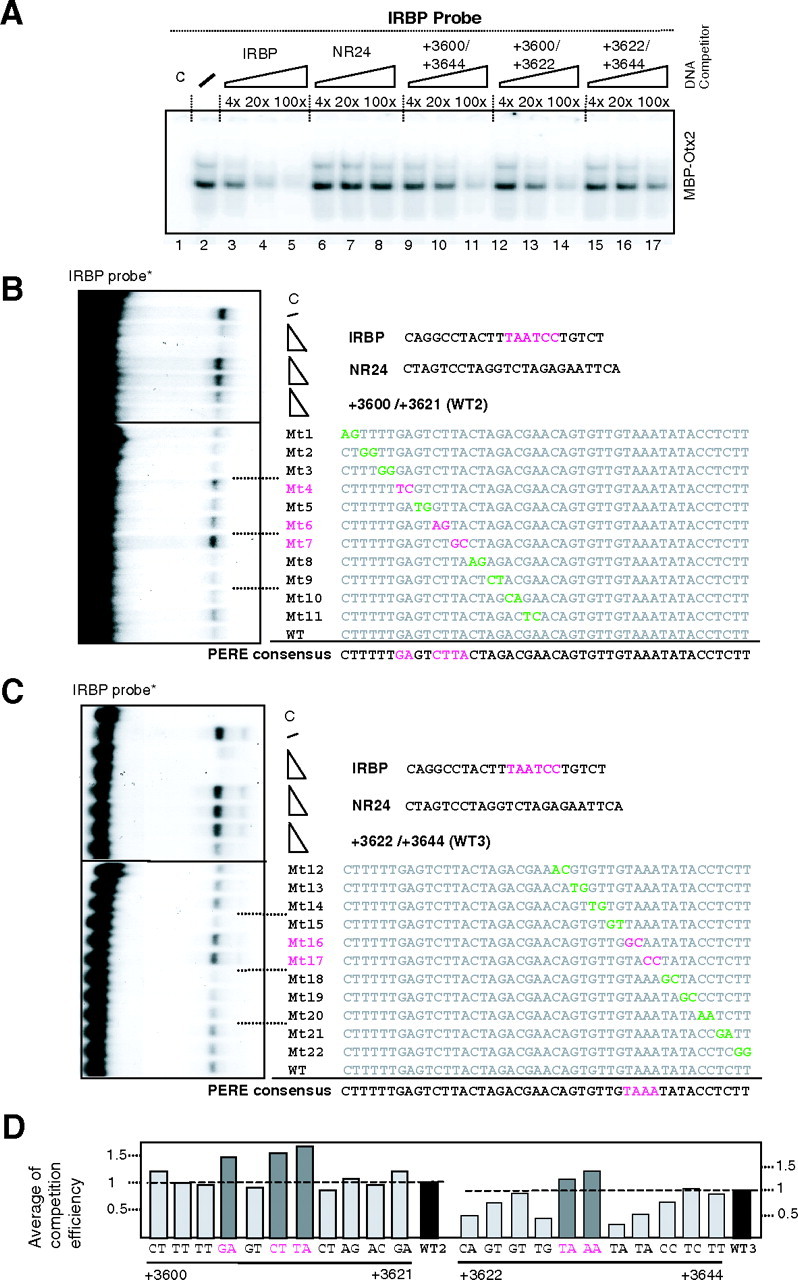
Given that Otx5 was acting through a short region devoid of canonical Otx-binding sites, we determined whether Otx proteins were able to bind to this region. We used EMSA to study whether the binding of Otx proteins to its canonical binding site [such as the one in the interphotoreceptor retinoid binding protein (IRBP) gene (18)] could be competed out by an oligonucleotide encompassing the +3600 to +3644 bp region. As shown in Fig. 2A, a myelin basic protein (MBP)-Otx2 fusion protein binds to the IRBP probe in a specific fashion (compare lane 2 with lanes 3–5 and 6–8). This binding is competed out by the 45-bp region from ZfP2 (3600–3644; lanes 9–11). Competition was more efficient using the 5′-moiety of this region (lanes 12–14) than the 3′-moiety (lanes 15–17) indicating preferential binding to the left part.
Fig. 2.
Determination of the Functional Otx5 Binding Site
Otx5 activates zfRev-erbα promoter within the +3600/+3644 proximal promoter region. A, EMSA competition test with MBP-Otx2 and various competitors. The IRBP gene probe contains the core sequence (TAATCC). Each competitor was added at 4-, 20-, or 100-fold excess. When no specific competitor was added, an equivalent amount of nonspecific competitor was added. IRBP was used as positive control and NR24 was use as a negative control. The slash (lane 2) is the MBP-Otx2 binding without competitor. No addition of MBP-OTX2 to the probe (lane 1). B and C, EMSA of OTX5 binding on IRBP site probe in presence or not of cold competitors used with two doses excess (10- and 20-fold). The region spanning from +3600 to +3644 was mutated systematically and used as two (separated) distinct regions named WT2 and WT3. A 20-fold excess of WT2 region or its mutants (Mt1 to Mt11) was used. The same experiment was done with 20-fold excess of WT3 and its mutants Mt12 to Mt22. Mutations are indicated by colored nucleotides: the green characters are neutral mutations and the pink characters are efficient ones. The upper part is the binding of OTX5 on IRBP site competed with WT2 and its mutants. The bottom part is done with WT3 and its mutants. Lanes (c) are the protein less mixtures and (/) is the OTX-IRBP binding without competition. NR24 is a nonrelevant competitor. D, Densitometer numerial readout of OTX-binding bands of EMSA gels. WT1 and WT2 mean WT regions at positions (+3600/+3621) and (+3622/3644), respectively. WT sequences (WT2 and WT3) are arbitrary considered as a competition level of 1 (black bar). Mutants (dark gray bar) mean lower competition than the WT. Data represent two independent experiments.
To identify the nucleotides that were necessary for Otx binding, we systematically mutagenized this sequence using a window of two nucleotides and used the mutated oligonucleotides as competitors in a large EMSA (see Fig. 2). This led us to identify GANNCTTA in the 3600–3621 fragment (Fig. 2B) and TAAA in the 3622–3644 fragment (Fig. 2C) as critical sequences for Otx binding. These results obtained with an MBP-Otx5 fusion protein produced in vitro were confirmed using MBP-Otx2 (data not shown). After a densitometric analysis of the EMSA competitions, we deduced the two parts of the new Otx consensus as summarized in Fig. 2D.
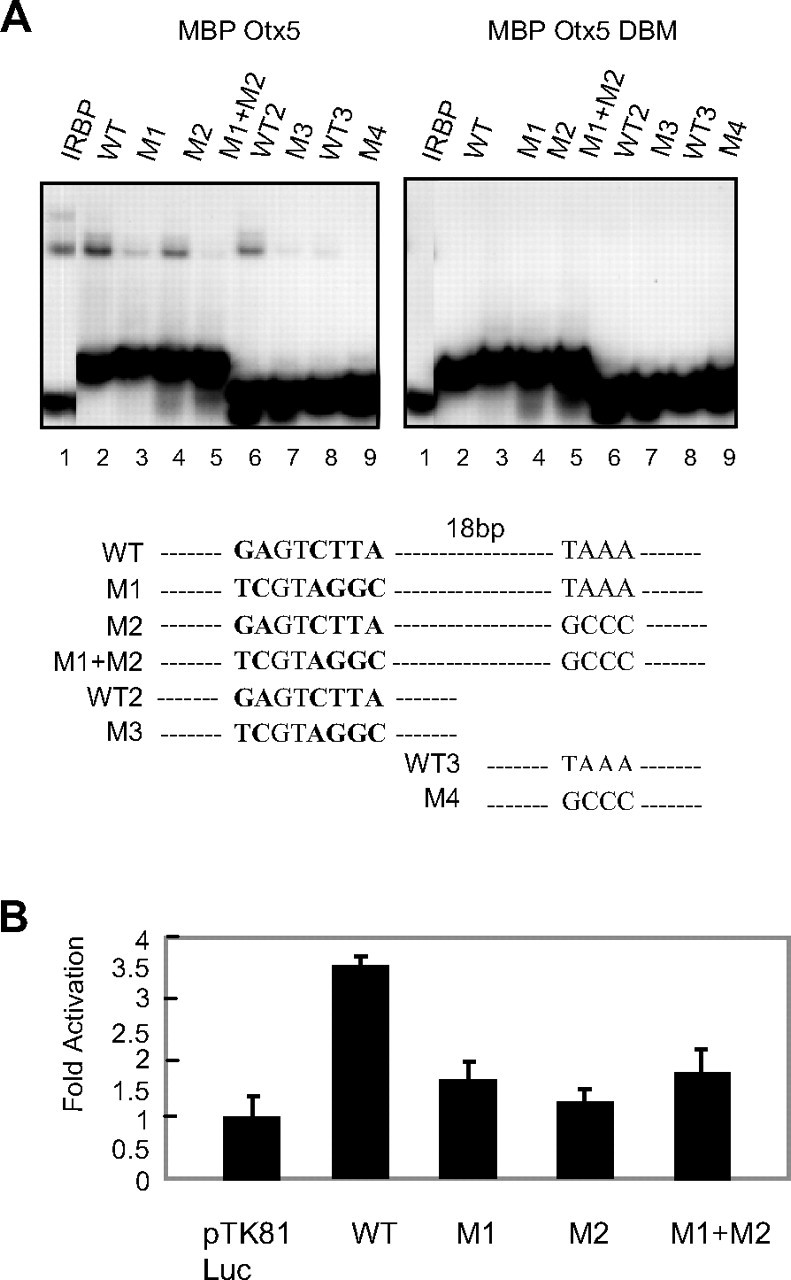
Next, we mutated each of the critical sequences in the context of the whole 45-bp region (M1, M2, or M1+M2 mutants) or of its 5′- or 3′-moiety (M3, M4) (see Fig. 3A and supplemental Table 2). We found that Otx5 is able to bind to the 45-bp region in a single complex [Fig. 3A, wild type (WT) lane 2], whereas the binding was abolished in the M1+M2 mutant (lane 5), much decreased in the M1 (lane 3), and only marginally affected in the M2 mutants (lane 4). In accordance with this result, Otx5 can bind to the 3600–3621 region (WT2, lane 6), and this binding is abolished in the M3 mutant (lane 7), whereas it binds weakly to the 3622–3644 region (WT3, lane 8) and not at all to the M4 mutant (lane 9). Again the results were very similar when Otx2 was used (data not shown). Then, we determined by EMSA the relative affinities of OTX5 for the PERE consensus vs. the classical IRBP site. To do this, we labeled each probe and we performed cross-competition experiments that are shown in supplemental Fig. 2. We observed that IRBP and WT are in the same range of affinity even though IRBP exhibits a 3- to 5-fold better efficiency in competition assays.
Fig. 3.
Analysis of Mutations in the New Consensus
A, EMSA performed with labeled WT and mutated versions of the PERE as probes mixed with MBP-Otx5 or the DNA-binding mutant MBP-Otx5 DBM. The probe sequences are presented at the bottom of the figure. B, Otx5 regulation of WT or mutated PERE-tk-reporter vectors (region spanning from +3600/+3644). Reporter vector (100 ng) and zfOtx5 expression vector (100 ng) were cotransfected into COS-1 cells. Results are presented as fold activation after normalization with an internal control (β-gal activity). Each transfection was conducted in triplicate wells, and data represent the mean ± sd of at least three independent experiments.
These results were further corroborated by transient transfection assays. Consistent with the EMSA results, the 45 bp (WT) alone linked to a minimal thymidine kinase promoter can drive 4-fold transcriptional activation by Otx5 (Fig. 3B). Mutation of the 5′- and/or 3′-part of the binding site virtually abolished this activation. This indicated that, although having different binding affinity, the GANNCTTA and TAAA sites are both required for activation of ZfP2 by Otx5. We thus named this bipartite site PERE for pineal expression related element.
OTX-5 Regulates rev-erbα ZfP2 in Vivo
We next investigated whether Otx5 was able to activate ZfP2 in vivo. Given the genomic organization of the zebrafish rev-erbα gene (see Fig. 1C), a probe encompassing exon 1 can recognize transcripts initiated from ZfP1 but not those emanating from ZfP2 (17). To measure the in vivo activity of both promoters, we thus compared the expression patterns generated by an exon 1-specific probe (Ex1) and by a 3′-probe (Ex2) of the same size, which recognizes both transcripts from ZfP1 and ZfP2. Interestingly, at 48 hpf we found that the exon 1-specific probe generated a very weak signal in the pineal gland whereas the common probe generated a strong signal in the pineal (Fig. 4, A and 4B; see also Ref.17). This suggests that, as expected, ZfP2 controls the expression of rev-erbα in the pineal gland at this developmental stage.
Fig. 4.
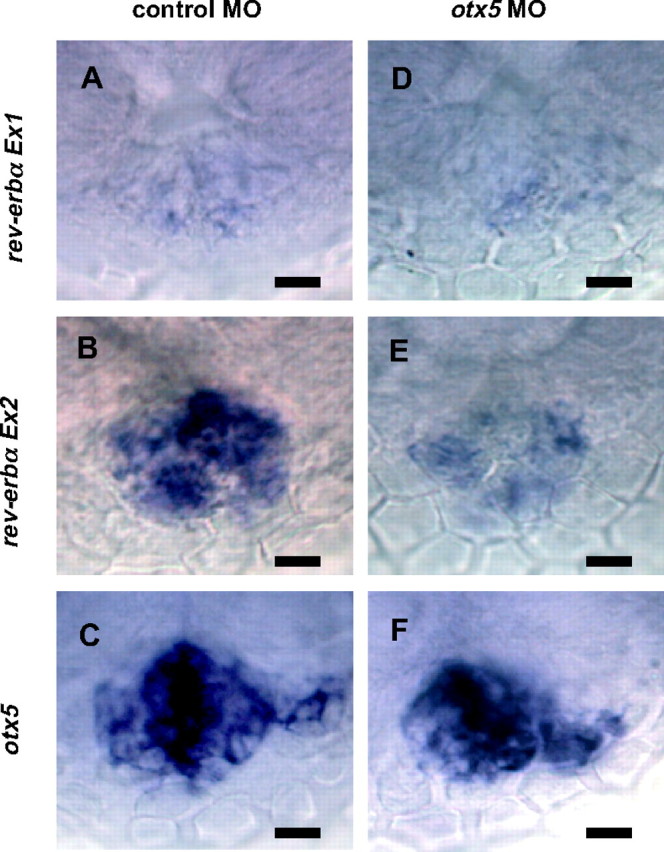
Whole-Mount in Situ Hybridization Analysis of rev-erbα Expression in the Zebrafish Epiphysis at 48 hpf
The expression in embryos injected with the control MO (A–C) or otx5 MO (D–F) are shown. A and D, Expression of transcripts from ZfP1 was analyzed using a probe designed in exon 1. B and E, Expression of transcripts from ZfP1 and ZfP2 analyzed by a 3′-end probe that recognizes both transcripts. C and F, Expression of otx5 at 48 hpf was analyzed as a control for normal pineal gland development. At that stage, no rev-erbα retina expression is observed. All analyses were done on at least 25 embryos. Dorsal views with anterior to the top. Scale bars, 10 μm.
To elucidate the function of Otx5 on rev-erbα expression in vivo, we used a morpholino antisense oligonucleotide (MO) to prevent otx5 mRNA translation (11). The injection of the control MO at the one-cell stage did not modify rev-erbα mRNA expression in the pineal gland at 48 hpf (Fig. 4B). In contrast, the injection of otx5 MO, significantly decreased zfrev-erbα expression (Fig. 4E) in accordance with our in vitro results. These are also consistent with the results reported (11). Interestingly, no detectable effect of the otx5 MO could be observed in the weak expression of rev-erbα1 transcripts detected with exon 1 probe (Fig. 4, A and D), in accordance with the absence of Otx5-mediated activation of ZfP1. This also confirms that at 48 hpf the transcripts detected with the 3′-probe are mainly due to the activity of ZfP2 and not of ZfP1.
PERE Is Necessary for rev-erbα Pineal-Specific Expression
To investigate whether the rev-erbα regulatory region recapitulates the expression patterns of the gene, we generated a reporter construct, called Full-EGFP, by ligating the enhanced green fluorescent protein (EGFP) gene to ZfP1, exon1, ZfP2, and the 5′-part of exon 2. By injecting this construct in one-cell embryos, we detected pineal expression in 8.5% of injected fish (Fig. 5). The earliest pineal expression was detected around 40 hpf, becoming more intense at 72 hpf and continuing for 14 d (Fig. 5, A–C). We also noticed weak green fluorescent protein (GFP) expression in posterior retina in some embryos at 52 hpf, but retinal signals disappeared by 4 d post fertilization (dpf). This is related to the late expression of zfrev-erbα in retina (15). In contrast neither ZfP1 nor ZfP2 alone induced pineal expression in the same experimental conditions (Fig. 5E and data not shown).
Fig. 5.
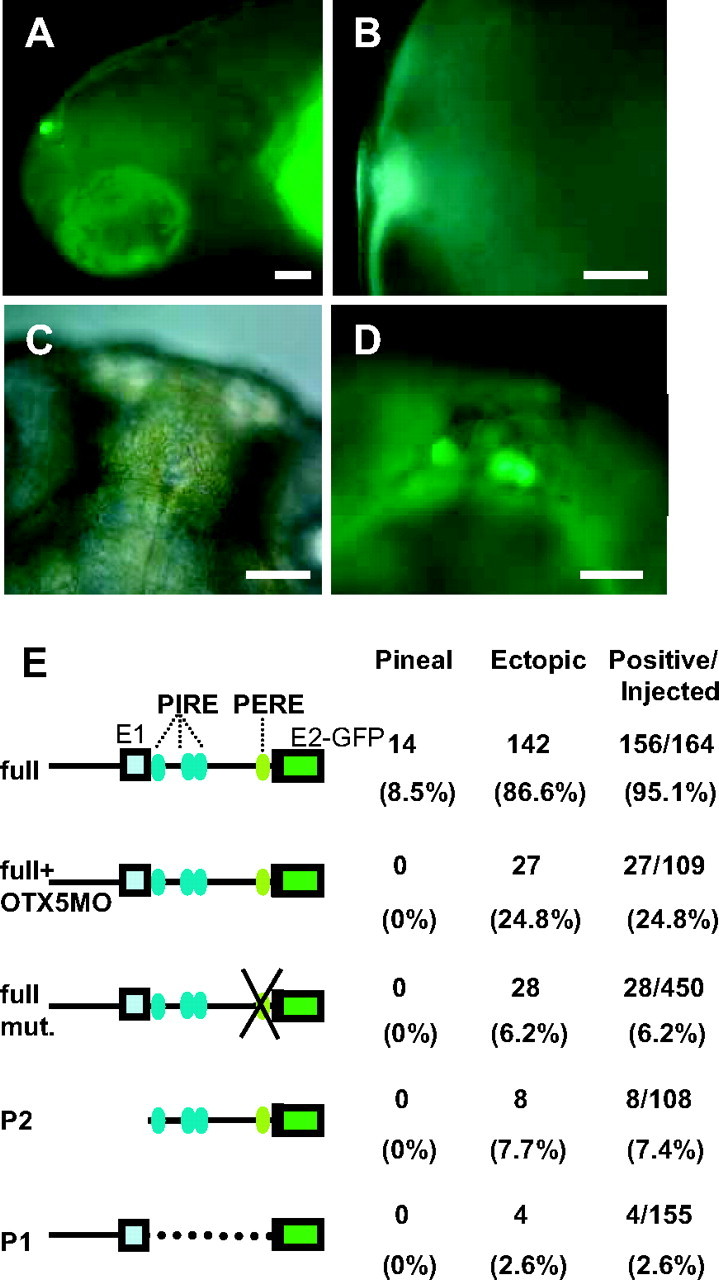
The PERE Controls rev-erbα Expression in the Pineal in Vivo in an Otx5-Dependent Manner
A, Lateral view of a transgenic embryo at 7 dpf showing GFP light in the pineal. B, Fluorescent high-magnification image (lateral view) of pineal gland at 7 dpf. Fluorescence signal was observed specifically in the pineal gland. C, Dorsal view of rev-erbα transgenic embryo at 10 dpf (bright-field image). D, Dorsal view of the embryo presented in panel C showing GFP activity in the pineal. Inset, High-magnification image of the EGFP-positive pineal gland. E, Summary of the injection results. Names (left) and schematic illustrations of reporter constructs containing Rev-erbα full, PERE mutated Rev-erbα full, PERE mutated Rev-erbα full, Rev-erbα ZfP2 alone, Rev-erbα ZfP1 alone. Constructs with normal or mutated and/or deleted Otx-binding site were used. In embryos that were coinjected with otx5 MO, pineal expression is completely blocked, whereas ectopic expression is maintained. The numbers of pineal-specific expression, ectopic expression, and GFP-positive embryos as well as the total number of injected embryos are given in the right part of the panel. Scale bars, 50 μm). mut., Mutated.
To confirm that Otx5 indeed regulates the full-EGFP construct in vivo, we coinjected the reporter construct in the presence of otx5 MOs (Fig. 5E, line 2, and data not shown). As expected, we found no pineal expression in injected fish, whereas we still detected expression in other organs. We also injected the full-EGFP construct mutated in the PERE sequence (Rev-erbα full mut. which corresponds to M1+M2 mutant described in Fig. 3A). As expected, this also resulted in fish that specifically lost all GFP pineal expression. This result confirms our in vitro characterization and shows that the PERE sequence is crucial for pineal expression of rev-erbα in vivo. The mutation of this element also decreases the number of nonspecific GFP-positive fish suggesting that the PERE element may contribute to the overall strength of the promoter.
The PERE Sequence Is Conserved
Our previous analysis of the regulatory region of rev-erbα in both mammals and zebrafish show that although P1 is strikingly conserved, suggesting that conserved factors govern the regulation of this promoter, P2 is divergent between mammals and zebrafish (16, 17). Indeed, we found no trace of the PERE in mammalian P2 promoters, in accordance with their lack of regulation by Otx factors. We nevertheless determined whether ZfP2 and the PERE could be found in other genes (Fig. 6). Interestingly, we found that the Xenopus rev-erbα gene exhibits the same organization as the zebrafish gene and that in the Xenopus P2 promoter the PERE is conserved. During this search we also observed that the zebrafish rev-erbβ-B gene [a fish-specific duplicate of rev-erbβ that exhibits pineal expression (17, 19)] also has a similar organization and conservation of the PERE sequence.
Fig. 6.
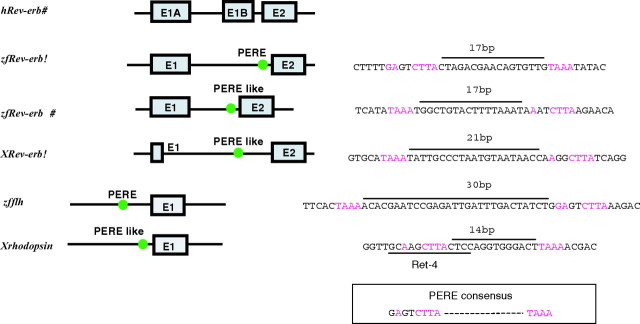
Comparison of Promoter Structures of Vertebrates PERE-Containing Genes: Human rev-erbα (hrev-erbα), Zebrafish rev-erbα (zfrev-erbα), Zebrafish rev-erbβ-B (zfrev-erbβB), Xenopus rev-erbα (Xrev-erbα) Harbor Clear PERE Signatures
Zebrafish floating head (zfflh) that is expressed in the pineal gland and Xenopus rhodopsin (Xrhodopsin) that is expressed in photoreceptor tissues also have PERE or PERE-like sequence. The green circles indicate PERE or PERE-like sequences. PERE consensus sequence (NANNCTTA and TAAA) is indicated in violet. Below the sequence of Xrhodopsin, Ret-4 is an already mapped site that has been shown to be recognized by Crx (29 30 ).
DISCUSSION
In this paper we show that zebrafish Otx5 regulates the early specific expression of the orphan nuclear receptor Rev-erbα in the pineal via a new DNA element that we call PERE. Using in vitro analysis we showed that Otx5 binds to this sequence and activates transcription from ZfP2 in mammalian cells. In addition, we showed that in vivo, during zebrafish development, ZfP2 controls rev-erbα expression in the pineal and that both Otx5 and the PERE are crucial for this effect. Taken together, our analysis reveals a new element controlling gene pineal expression as well as mediating Otx family effects.
Despite a very large number of different biological functions of homeoproteins, nearly all homeodomains from metazoans bind to related sequences containing a TAAT core motif (20, 21). The precise analysis of homeodomain-DNA interactions derived from structural studies suggests a mechanism by which the conserved TAAT motif is specifically recognized by homeodomains (22, 23, 24). For the Otx family, a specific consensus sequence (TAATCC), containing the TAAT core motif, has been defined. Nevertheless, it is clear that many genes regulated by Otx family members are devoid of this core sequence (25). This has prompted questions about the existence of other possible target sequences for Otx family members. Indeed, some homeodomain proteins such as PITX2 and Drosophila Bicoid that are very similar to Otx5 can recognize elements that deviate from the consensus site TAATCC (26, 27). Our findings show that the PERE sequence represents a novel non-consensus binding site for a subset of Otx family members. The PERE sequence, as defined in our analysis, is a complex sequence that can be split into two parts that are separated by 17 bp. It contains a 5′-core TAAGNNTC and a 3′-region TAAA in inverse orientation. From the EMSA data we present it is difficult to ascertain whether Otx members bind as dimers or monomers to this element and whether an additional factor is required for efficient DNA binding. Our results showing that Otx can bind independently to the 5′- and the 3′-core element suggest that two monomers may bind to the two different target sequences (Fig. 2). Therefore, we speculate that two Otx5 molecules bind to GANNCTTA and TAAA separately, and that there might be some direct or indirect interaction between these five molecules, promoting a more efficient binding.
We checked whether PEREs, which we found in several rev-erb genes in zebrafish and Xenopus (Fig. 6), exist in other genes expressed in the pineal gland. We found the same sequence (GAGTCTTA) in the proximal promoter region of zebrafish floating head, a gene expressed from early stages in the pineal gland and required for differentiation of most pineal neurons (28). A TAAA sequence is present 30 bp upstream of the GAGTCTTA element, suggesting an organization similar to the PERE sequence in the zfRev-erbα promoter (Fig. 6). In addition, we also noticed that a PERE-like sequence exists in the proximal promoter region of the Xenopus rhodopsin (Xrhodopsin) gene. In fact, part of the PERE in this promoter corresponds to an already mapped site that has been shown to be recognized by Crx (29, 30). From this comparative analysis, it is tempting to speculate that PEREs are important for regulation of several genes expressed in the pineal gland or photoreceptor tissues.
The effect of otx5 MOs and the deletion of the PERE sequence highlight the importance of this element for pineal expression. Nevertheless, we obtain pineal expression only by combining ZfP1 and ZfP2 in a unique construct. Why the ZfP2-GFP construct alone is unable to generate pineal expression is still unclear. One hypothesis is that this promoter alone is too weak to induce a visible GFP expression in the pineal, a possibility still open, given the strong mosaicism obtained after injection of reporter constructs in zebrafish eggs (31). This observation could also suggest that a cooperation between ZfP1 and ZfP2 is necessary to achieve pineal expression in a PERE-dependant manner. Of note, during early development, ZfP1 is active in the pineal gland, albeit at a low level (see Fig. 4A and Ref.17). It has recently been shown that Otx5 and CLOCK-BMAL are synergistically implicated in the pineal expression of the AANAT2 gene in zebrafish (14). Given that ZfP1 is regulated by CLOCK-BMAL1 (16), it is thus possible that, although in two different promoters such a synergistic cis-regulation also occurs for rev-erbα gene pineal expression.
We have previously shown that ZfP2 is divergent when compared with the mammalian P2 (17). This is in sharp contrast to mammalian P1 and ZfP1, which are about 60% identical on the sequence level and share conserved functional motifs. In accordance with this divergence we have demonstrated that mammalian P2 and ZfP2 are regulated by different factors (17). For example, the mammalian P2 promoter is regulated by REVERB/retinoid-related orphan receptor (ROR), whereas ZfP2 is not. In contrast, only ZfP2 is regulated by Otx factors but not the mammalian P2. These observations are further reinforced by the observation that zfRev-erbα exhibits a developmentally regulated expression pattern (15), whereas mammalian rev-erbα is expressed ubiquitously in the anterior part of the mouse embryos (data not shown).
In contrast, by comparing rev-erbα-regulatory regions of several vertebrates (Fig. 6) we found a PERE-like (AAGGCTTA and TAAA) sequence in the putative P2 promoter region of Xenopus rev-erbα as well as in the zebrafish rev-erbβ-B gene. This conservation suggests that the PERE and the pineal expression of rev-erb factors governed by a specific promoter are important in teleost fish and amphibians. From this observation it is tempting to suggest that the PERE-mediated Otx regulation of rev-erbα and the pineal function of Rev-erbα is conserved in all early vertebrates and has been lost in mammals. We propose that Rev-erbα expression in the pineal experienced a major functional shift in the mammalian lineage. Interestingly, it is known that in most nonmammalian vertebrates the pineal organ is a photosensitive organ that contains functional photoreceptors, whereas the pineal organ of mammals is a nonsensory neuroendocrine organ under the control of photoperiod (32). Given that Otx family members control the photoreceptor cell fate in the mouse retina (33, 34), and because in mice the knock-out of rev-erbα induces a major hypersensitivity to light pulses of the circadian clock (32), it is tempting to speculate that the Otx/Rev-erb connection described in this paper, and the evolutionary shift in rev-erbα regulatory region, has played a role in the evolution of pineal function.
MATERIALS AND METHODS
Plasmid Constructs and Vectors
ZfP1 and ZfP2 have been described in Kakizawa et al. (17). Accession number of the sequence of zfRev-erbα promoter obtained from GenBank is AY336123. The 3079 bp upstream of exon 1 and 3683 bp located between exon 1 and exon 2 correspond to ZfP1 and ZfP2 (see supplemental Fig. 1), respectively. ZfP1 and ZfP2 were subcloned into pGL2basic and pd2EGFP (CLONTECH, Palo Alto, CA) vectors as XhoI-Bgl II and XhoI-BamHI fragments, respectively. These constructs were termed “pGL2Rev-erbα full” and “pd2EGFPRev-erbα full.” Using PCR amplification, a series of internal deletion constructs (P2D-1 to P2D-12) was generated by PCR and subcloned. A mutated construct with substitutions was produced by PCR as described in Ref.17 . The promoters regions within each modified plasmid were sequenced to confirm the absence of PCR errors, whereas the vector backbone was replaced by an intact one excised from pd2EGFPRev-erbα full plasmid by Pst and BamHI digestion to eliminate possible PCR errors. The pTK81Luc Rev-erbα WT and mutated constructs were done by PCR using oligonucleotides bearing BamHI sites. Then the amplified products were digested by BamHI and subcloned into the BamHI site of pTK81Luc to generate pTK81WT, pTK81M1, pTK81M2, and pTKr1M1+M2. Details of the primers used are available in supplemental Table 1.
Cell Culture and Transient Transfections and Reporter Assays in Mammalian Cells
Transient transfection experiments in COS-1 cells with Exgene 500 transfection reagent (Euromedex, Souffelweyersheim, France) were performed according to Ref.17 . Luciferase activity was measured in a Monolight 2010 luminometer (Analytical Luminescence Laboratory, Sparks, Md.). To study the in vitro function of Otx5 on zfRev-erbα promoters, 50 ng pCDNA3zfOtx5 expression vector was cotransfected with 100 ng pGL2Rev-erbα vector and pCDNA3-βgal vector (16) to allow normalization. The DBMs of Otx5 were produced by PCR using the oligonucleotides given in supplemental Table 1. Each construct was sequenced on both strands. Zebrafish or rat Rev-erbα P1 and P2 promoter luciferase reporter vectors (100 ng) and 50 ng Otx5 or Otx family members expression vector (pSG5mOtx1, pSG5mOtx2, pCDNA3ZfCrx) was cotransfected into COS1 cells. Luciferase activity was measured 42 h after transfection. Each transfection was conducted in triplicate samples, and the data represent the mean ± sd of more than three independent experiments.
EMSAs
WT cDNA of Otx2 and Otx5 or their mutated sequences were subcloned into pMal-c2 plasmid (New England Biolabs, Beverly, MA) and expressed as MBP-Otx fusion proteins in Escherichia coli BL21 cells. Proteins were affinity purified onto an amylose resin according to the manufacturer’s instructions. EMSA experiments were performed as described in Ref.35 . All oligonucleotides used are summarized in supplemental Table 1.
Fish Care, Microinjection, and Whole-Mount in Situ Hybridization
Zebrafish (Danio rerio) were kept at 28 C in a 14-h light/10-h dark cycle. Embryos were collected after spawning to perform the morpholino injection. To prevent pigmentation, 0.2 mm 1-phenyl-2-thiourea (Sigma Chemical Co., St. Louis, MO) was added to the water at 12 hpf. Microinjection and whole-mount in situ hybridization were done according to Ref.36 . Plasmid DNA for microinjection in fish was purified by using a plasmid isolation kit (QIAGEN, Chatsworth, CA). MOs were designed and purchased from GeneTools. The MO sequences for Otx5 were taken from Ref.11 . Control MO sequences are described in Ref.17 . We injected one- or two-cell stage WT embryos. The zebrafish Rev-erbα exon 1 and 3′-end probes for in situ hybridization were amplified by PCR and subcloned into pCSII+ vector. Oligonucleotides are given in supplemental Table 1.
Fluorescent Microscopy
Embryos and larvae were anesthetized by immersing them into 0.017% 3-aminobenzoic acid ethyl ester methanesulfonate salt (Sigma) solution and then mounted in 3% methyl cellulose (Sigma) or 4% low-melting-point agarose (Invitrogen, Carlsbad, CA) on glass slides. They were observed under a Zeiss upright fluorescence microscope (Axioplan2; Carl Zeiss, Thornwood, NY) equipped with a Zeiss filterset 10. Images with higher magnifications were obtained by using a Leica confocal laser scanning microscope (Leica Corp., Deerfield, IL).
Acknowledgments
We thank François Bonneton and Michael Schubert for critical reading of the manuscript.
NURSA Molecule Pages:
Nuclear Receptors: REV-ERBα.
Footnotes
This work was supported by grants from the Région Rhone-Alpes as well as from the Centre National de la Recherche Scientifique, Association pour la Recherche sur le Cancer, and Ministère de l’Education National de la Recherche et de la Technologie. T.K. was supported by the Région Rhone-Alpes.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 13, 2007
S.N and T.K. should be considered as equal first authors.
Abbreviations: BMAL, Brain and muscle ARNT-like; CLOCK, circadian locomoter output cycles kaput; DBM, DNA-binding mutant; dpf, days post fertilization; EGFP, enhanced GFP; GFP, green fluorescent protein; hpf, hours post fertilization; IRBP, interphotoreceptor retinoid binding protein; MBP, myelin basic protein; MO, morpholino antisense oligonucleotide; Otx, orthodenticle homeobox; PERE, pineal expression-related element; PIRE, pineal regulatory element; WT, wild type.
References
- 1.Falcon J 1999. Cellular circadian clocks in the pineal. Prog Neurobiol 58:121–162 [DOI] [PubMed] [Google Scholar]
- 2.Pu GA, Dowling JE 1981. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus J Neurophysiol 46:1018–1038 [DOI] [PubMed] [Google Scholar]
- 3.Cole WC, Youson JH 1982. Morphology of the pineal complex of the anadromous sea lamprey, Petromyzon marinus L. Am J Anat 165:131–163 [DOI] [PubMed] [Google Scholar]
- 4.Tamotsu S, Morita Y 1986. Photoreception in pineal organs of larval and adult lampreys, Lampetra japonica J Comp Physiol 159:1–5 [DOI] [PubMed] [Google Scholar]
- 5.Meissl H 1997. Photic regulation of pineal function. Analogies between retinal and pineal photoreception. Biol Cell 89:549–554 [PubMed] [Google Scholar]
- 6.Kusmic C, Gualtieri P 2000. Morphology and spectral sensitivities of retinal and extraretinal photoreceptors in freshwater teleosts. Micron 31:183–200 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Chen S, Wang Q, Zack DJ, Snyder SH, Borjigin J 1998. A pineal regulatory element (PIRE) mediates transactivation by the pineal/retina-specific transcription factor CRX. Proc Natl Acad Sci USA 95:1876–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard M, Dinet V, Voisin P 2001. Transcriptional regulation of the chicken hydroxyindole-O-methyltransferase gene by the cone-rod homeobox-containing protein. J Neurochem 79:248–257 [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum L, Gothilf Y 2006. Mechanism of pineal-specific gene expression: the role of E-box and photoreceptor conserved elements. Mol Cell Endocrinol 252:27–33 [DOI] [PubMed] [Google Scholar]
- 10.Asaoka Y, Mano H, Kojima D, Fukada Y 2002. Pineal expression-promoting element (PIPE), a cis-acting element, directs pineal-specific gene expression in zebrafish. Proc Natl Acad Sci USA 99:15456–15461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO 2002. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet 30:117–121 [DOI] [PubMed] [Google Scholar]
- 12.Plouhinec JL, Sauka-Spengler T, Germot A, Le Mentec C, Cabana T, arrison G, Pieau C, Sire JY, Veron G, Mazan S 2003. The mammalian Crx genes are highly divergent representatives of the Otx5 gene family, a gnathostome orthology class of orthodenticle-related homeogenes involved in the differentiation of retinal photoreceptors and circadian entrainment. Mol Biol Evol 20:513–521 [DOI] [PubMed] [Google Scholar]
- 13.Appelbaum L, Toyama R, Dawid IB, Klein DC, Baler R, Gothilf Y 2004. Zebrafish serotonin-N-acetyltransferase-2 gene regulation: pineal-restrictive downstream module contains a functional E-box and three photoreceptor conserved elements. Mol Endocrinol 18:1210–1221 [DOI] [PubMed] [Google Scholar]
- 14.Appelbaum L, Anzulovich A, Baler R, Gothilf Y 2005. Homeobox-clock protein interaction in zebrafish. A shared mechanism for pineal-specific and circadian gene expression. J Biol Chem 280:11544–11551 [DOI] [PubMed] [Google Scholar]
- 15.Delaunay F, Thisse C, Marchand O, Laudet V, Thisse B 2000. An inherited functional circadian clock in zebrafish embryos. Science 289:297–300 [DOI] [PubMed] [Google Scholar]
- 16.Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V 2004. The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker. J Mol Endocrinol 33:585–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakizawa T, Nishio S, Triqueneaux G, Bertrand S, Rambaud J, Laudet V 2007. Two differentially active alternative promoters control the expression of zebrafish orphan nuclear receptor gene Rev-erbα. J Mol Endocrinol 38:555–568 [DOI] [PubMed] [Google Scholar]
- 18.Bobola N, Briata P, Ilengo C, Rosatto N, Craft C, Corte G, Ravazzolo R 1999. OTX2 homeodomain protein binds a DNA element necessary for interphotoreceptor retinoid binding protein gene expression. Mech Dev 82:165–169 [DOI] [PubMed] [Google Scholar]
- 19.Bertrand S, Brunet FG, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M 2004. Evolutionary genomics of nuclear receptors: from twenty-five ancestral genes to derived endocrine systems. Mol Biol Evol 21:1923–1937 [DOI] [PubMed] [Google Scholar]
- 20.Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, Affolter M, Otting G, Wuthrich K 1994. Homeodomain-DNA recognition. Cell 78:211–223 [DOI] [PubMed] [Google Scholar]
- 21.Nasevicius A, Ekker SC 2000. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26:216–220 [DOI] [PubMed] [Google Scholar]
- 22.Otting G, Qian YQ, Billeter M, Muller M, Affolter M, Gehring WJ, Wuthrich K 1990. Protein-DNA contacts in the structure of a homeodomain-DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J 9:3085–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemm JD, Rould MA, Aurora R, Herr W, Pabo CO 1994. Crystal structure of the Oct-1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Cell 77:21–32 [DOI] [PubMed] [Google Scholar]
- 24.Wilson, DS, ShengG, Jun S, Desplan C 1996. Conservation and diversification in homeodomain-DNA interactions: a comparative genetic analysis. Proc Natl Acad Sci USA 93:6886–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boncinelli E, Morgan R 2001. Downstream of Otx2, or how to get a head. Trends Genet 17:633–636 [DOI] [PubMed] [Google Scholar]
- 26.Dave V, Zhao C, Yang F, Tung CS, Ma J 2000. Reprogrammable recognition codes in bicoid homeodomain-DNA interaction. Mol Cell Biol 20:7673–7684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Dave V, Yang F, Scarborough T, Ma J 2000. Target selectivity of bicoid is dependent on nonconsensus site recognition and protein-protein interaction. Mol Cell Biol 20:8112–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masai I, Heisenberg CP, Barth KA, Macdonald R, Adamek S, Wilson SW 1997. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron 18:43–57 [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ 1997. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19:1017–1030 [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Zack DJ 1996. Ret 4, a positive acting rhodopsin regulatory element identified using a bovine retina in vitro transcription system. J Biol Chem 271:28549–28557 [DOI] [PubMed] [Google Scholar]
- 31.Gibbs PD, Schmale MC 2000. GFP as a genetic marker scorable throughout the life cycle of transgenic zebra fish. Mar Biotechnol (NY) 2:107–125 [DOI] [PubMed] [Google Scholar]
- 32.Ekstrom P, Meissl H 2003. Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors. Philos Trans R Soc Lond B Biol Sci 358:1679–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL 1999. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet 23:466–470 [DOI] [PubMed] [Google Scholar]
- 34.Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T 2003. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci 6:1255–1263 [DOI] [PubMed] [Google Scholar]
- 35.Chatelain G, Fossat N, Brun G, Lamonerie T 2006. Molecular dissection reveals decreased activity and not dominant negative effect in human OTX2 mutants. J Mol Med 84:604–615 [DOI] [PubMed] [Google Scholar]
- 36.Thisse B, Heyer V, Lux A, Alunni V, Degrave A, Seiliez I, Kirchner J, Parkhill JP, Thisse C 2004. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol 77:505–519 [DOI] [PubMed] [Google Scholar]