Abstract
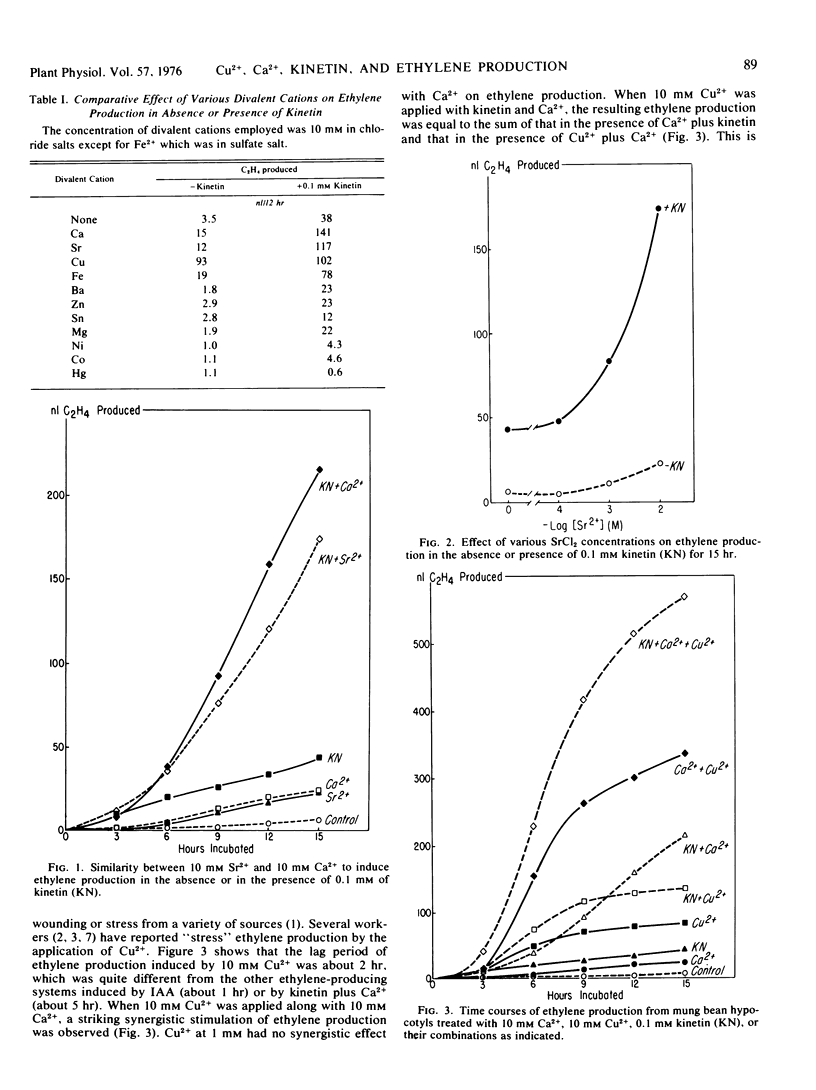
The synergistic stimulation of ethylene production by kinetin and Ca2+ in hypocotyl segments of mung bean (Phaseolus aureus Roxb.) seedling was further studied. The requirement for Ca2+ in this system was specific. Except for Sr2+, which mimicked the effect of Ca2+, none of the following divalent cations, including Ba2+, Mg6+, Cu2+, Hg2+, Co2+, Ni2+, Sn2+, and Zn2+, showed synergism with kinetin on ethylene production. Fe2+, however, showed a slight synergism with kinetin. Some of them (Hg2+, Co2+, and Ni2+) had a strong inhibitory effect, while others (Zn2+, Mg2+, Sn2+, and Ba2+) had a slight or no inhibitory effect on ethylene production in the absence or presence of kinetin.
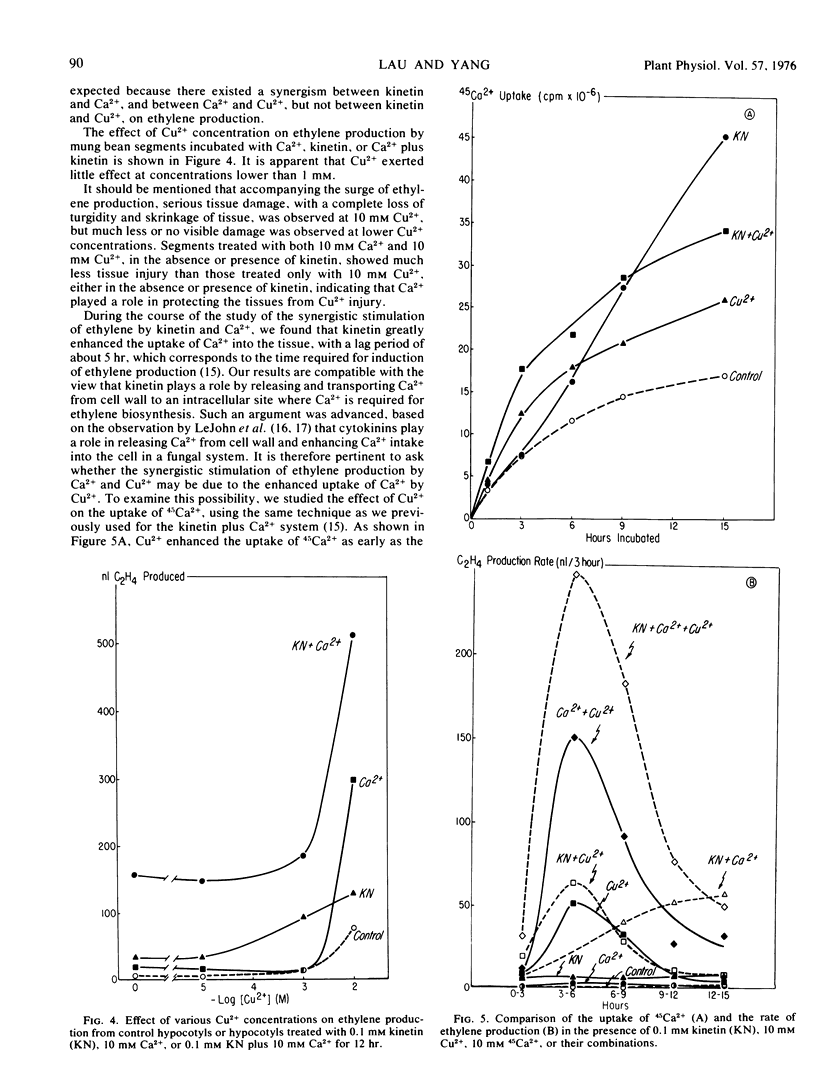
Cu2+ alone, depending on the concentration applied, stimulated ethylene production with a lag period of about 2 hours and had no synergism with kinetin on ethylene production. When Cu2+ was applied with Ca2+, a remarkable synergistic stimulation of ethylene production was observed. Tracer experiments indicated that Cu2+ enhanced the uptake of 45Ca2+ into the tissues during the first few hours of incubation, and this increase of 45Ca2+ uptake paralleled the enhancement of ethylene production. When Ca2+ was applied together with kinetin plus Cu2+, both the ethylene production and the 45Ca2+ uptake were greatly increased over those from the segments treated with Cu2+ or kinetin alone. The increase in ethylene production as a result of kinetin plus Ca2+ plus Cu2+ treatment is equal to the combined increases caused by kinetin plus Ca2+ and Cu2+ plus Ca2+. A possible mechanism accounting for such cooperative effects of Cu2+, Ca2+, and kinetin on ethylene production is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. Biochemical Pathway of Stress-induced Ethylene. Plant Physiol. 1972 Oct;50(4):496–498. doi: 10.1104/pp.50.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoshua S., Biggs R. H. Effects of iron and copper ions in promotion of selective abscission and ethylene production by citrus fruit and the inactivation of indoleacetic Acid. Plant Physiol. 1970 May;45(5):604–607. doi: 10.1104/pp.45.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Cedeno-Maldonado A., Swader J. A. The cupric ion as an inhibitor of photosynthetic electron transport in isolated chloroplasts. Plant Physiol. 1972 Dec;50(6):698–701. doi: 10.1104/pp.50.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Rasmussen G. K., Rogers B. J., Reece P. C., Henry W. H. Control of abscission in agricultural crops and its physiological basis. Plant Physiol. 1968 Sep;43(9 Pt B):1560–1576. [PMC free article] [PubMed] [Google Scholar]
- GURD F. R., WILCOX P. E. Complex formation between metallic cations and proteins, peptides and amino acids. Adv Protein Chem. 1956;11:311–427. doi: 10.1016/s0065-3233(08)60424-6. [DOI] [PubMed] [Google Scholar]
- Gross R. E., Pugno P., Dugger W. M. Observations on the mechanism of copper damage in chlorella. Plant Physiol. 1970 Aug;46(2):183–185. doi: 10.1104/pp.46.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann H. M. Reversal of copper inhibition in chloroplast reactions by manganese. Plant Physiol. 1969 Mar;44(3):331–336. doi: 10.1104/pp.44.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Interaction of kinetin and calcium in relation to their effect on stimulation of ethylene production. Plant Physiol. 1975 Apr;55(4):738–740. doi: 10.1104/pp.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeJohn H. B., Stevenson R. M. Cytokinins and magnesium ions may control the flow of metabolites and calcium ions through fungal cell membranes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1061–1066. doi: 10.1016/0006-291x(73)90801-2. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene production from methionine. Biochem J. 1965 Nov;97(2):449–459. doi: 10.1042/bj0970449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LéJohn H. B., Cameron L. E., Stevenson R. M., Meuser R. U. Influence of cytokinins and sulfhydryl group-reacting agents on calcium transport in fungi. J Biol Chem. 1974 Jul 10;249(13):4016–4020. [PubMed] [Google Scholar]
- O'KELLEY J. C., HERNDON W. R. Effect of strontium replacement for calcium on production of motile cells in Protosiphon. Science. 1959 Sep 18;130(3377):718–718. doi: 10.1126/science.130.3377.718. [DOI] [PubMed] [Google Scholar]
- Queen W. H., Fleming H. W., O'kelley J. C. Effects on Zea mays Seedlings of a Strontium Replacement for Calcium in Nutrient Media. Plant Physiol. 1963 Jul;38(4):410–413. doi: 10.1104/pp.38.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]


