Abstract
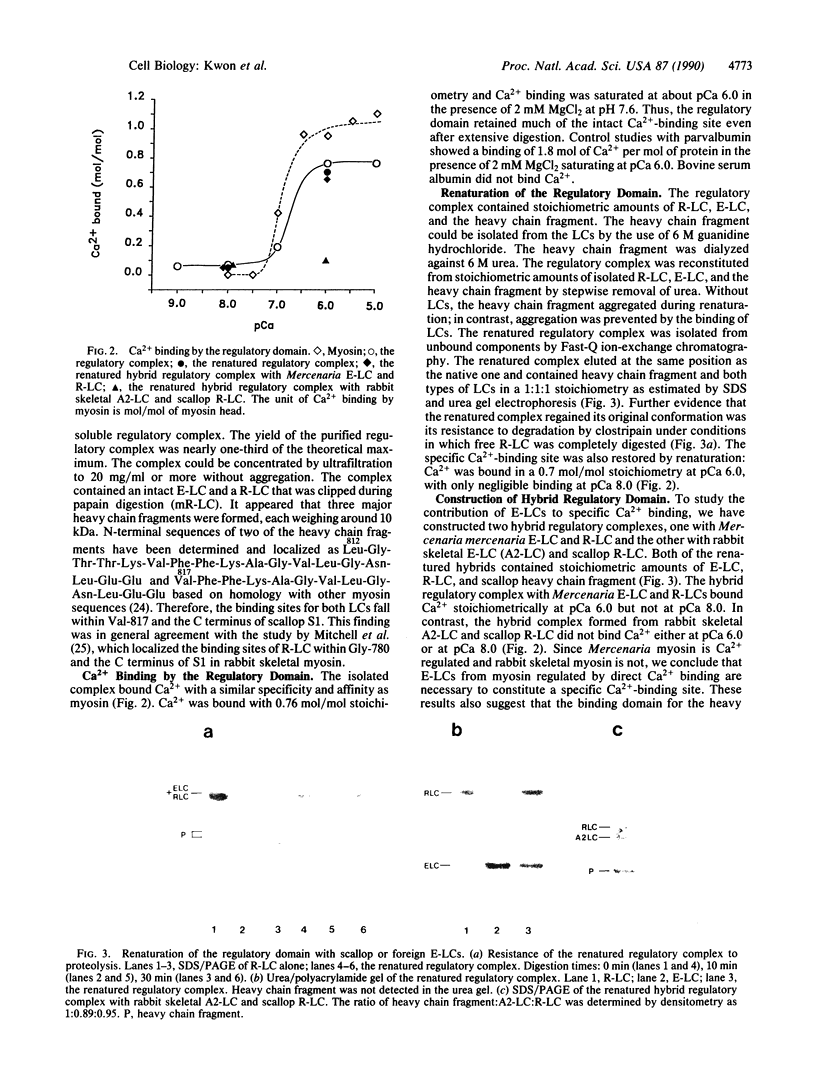
The regulatory domain of scallop myosin, consisting of a regulatory light chain (R-LC), an essential light chain (E-LC), and a portion of heavy chain, occupies the neck region of myosin. This domain is directly involved in the regulation of molluscan muscle contraction, which is triggered by direct Ca2+ binding to myosin. We have isolated a soluble functional complex (regulatory complex) comprised of R-LC, E-LC, and a 10-kDa heavy chain fragment in a 1:1:1 stoichiometry by clostripain digestion of the myosin head (papain subfragment 1). N termini of the heavy chain fragments were either leucine-812 or valine-817. The isolated complex retained the specific Ca2(+)-binding site and bound Ca2+ with a similar affinity and selectivity as myosin. The individual components of the regulatory complex were isolated after complete denaturation with guanidine hydrochloride. The regulatory complex was reconstituted from isolated light chains and the heavy chain fragment. The renatured complex regained Ca2+ binding quantitatively. To elucidate the function of the E-LC in Ca2+ binding, we constructed hybrid regulatory complexes. The hybrid complexes reconstituted with molluscan E-LC and R-LC regained the specific Ca2(+)-binding site, whereas the hybrid complex formed with rabbit skeletal E-LC [alkali LC 2 (A2-LC)] and scallop R-LC did not. The results demonstrate that E-LCs from myosins regulated by direct Ca2+ binding are required for the specific Ca2+ binding in the molluscan muscle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashiba G., Szent-Györgyi A. G. Essential light chain exchange in scallop myosin. Biochemistry. 1985 Nov 5;24(23):6618–6623. doi: 10.1021/bi00344a048. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Kendrick-Jones J. Characterization of homologous divalent metal ion binding sites of vertebrate and molluscan myosins using electron paramagnetic resonance spectroscopy. J Mol Biol. 1979 May 25;130(3):317–336. doi: 10.1016/0022-2836(79)90544-8. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Sellers J. R., Szent-Györgyi A. G. Cooperativity in scallop myosin. Biochemistry. 1981 Jan 6;20(1):210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Jakes R., Kendrick-Jones J., Leszyk J., Barouch W., Theibert J. L., Spiegel J., Szent-Györgyi A. G. Amino acid sequence of myosin essential light chain from the scallop Aquipecten irradians. Biochemistry. 1986 Nov 18;25(23):7651–7656. doi: 10.1021/bi00371a056. [DOI] [PubMed] [Google Scholar]
- Dibb N. J., Maruyama I. N., Krause M., Karn J. Sequence analysis of the complete Caenorhabditis elegans myosin heavy chain gene family. J Mol Biol. 1989 Feb 5;205(3):603–613. doi: 10.1016/0022-2836(89)90229-5. [DOI] [PubMed] [Google Scholar]
- Flicker P. F., Wallimann T., Vibert P. Electron microscopy of scallop myosin. Location of regulatory light chains. J Mol Biol. 1983 Sep 25;169(3):723–741. doi: 10.1016/s0022-2836(83)80167-3. [DOI] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Wallimann T., Szent-Györgyi A. G. Regulatory and essential light-chain interactions in scallop myosin. I. Protection of essential light-chain thiol groups by regulatory light-chains. J Mol Biol. 1982 Mar 25;156(1):141–152. doi: 10.1016/0022-2836(82)90463-6. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J. Role of myosin light chains in calcium regulation. Nature. 1974 Jun 14;249(458):631–634. doi: 10.1038/249631a0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Margossian S. S., Bhan A. K., Slayter H. S. Role of the regulatory light chains in skeletal muscle actomyosin ATPase and in minifilament formation. J Biol Chem. 1983 Nov 10;258(21):13359–13369. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mitchell E. J., Jakes R., Kendrick-Jones J. Localisation of light chain and actin binding sites on myosin. Eur J Biochem. 1986 Nov 17;161(1):25–35. doi: 10.1111/j.1432-1033.1986.tb10120.x. [DOI] [PubMed] [Google Scholar]
- Pope B. J., Wagner P. D., Weeds A. G. Heterogeneity of myosin heavy chains in subfragment-1 isoenzymes rabbit skeletal myosin. J Mol Biol. 1977 Jan 25;109(3):470–473. doi: 10.1016/s0022-2836(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Nagai K., Kendrick-Jones J. Site-directed mutagenesis of the regulatory light-chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986 Jul 3;322(6074):80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. The role of myosin light chains in regulating actin-myosin interaction. Biochimie. 1981 Apr;63(4):255–271. doi: 10.1016/s0300-9084(81)80115-0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Chantler P. D., Szent-Györgyi A. G. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980 Dec 15;144(3):223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. Reversible loss of calcium control of tension in scallop striated muscle associated with the removal of regulatory light chains. Nature. 1978 May 4;273(5657):62–64. doi: 10.1038/273062a0. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Szentkiralyi E. M. Tryptic digestion of scallop S1: evidence for a complex between the two light-chains and a heavy-chain peptide. J Muscle Res Cell Motil. 1984 Apr;5(2):147–164. doi: 10.1007/BF00712153. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Szent-Györgyi A. G. An immunological approach to myosin light-chain function in thick filament linked regulation. 2. Effects of anti-scallop myosin light-chain antibodies. Possible regulatory role for the essential light chain. Biochemistry. 1981 Mar 3;20(5):1188–1197. doi: 10.1021/bi00508a021. [DOI] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann D. A., Almeda S., Vibert P., Cohen C. A new myosin fragment: visualization of the regulatory domain. Nature. 1984 Feb 23;307(5953):758–760. doi: 10.1038/307758a0. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S., Press J. L. Monoclonal antibodies localize changes on myosin heavy chain isozymes during avian myogenesis. Cell. 1983 Aug;34(1):295–306. doi: 10.1016/0092-8674(83)90160-5. [DOI] [PubMed] [Google Scholar]