Abstract
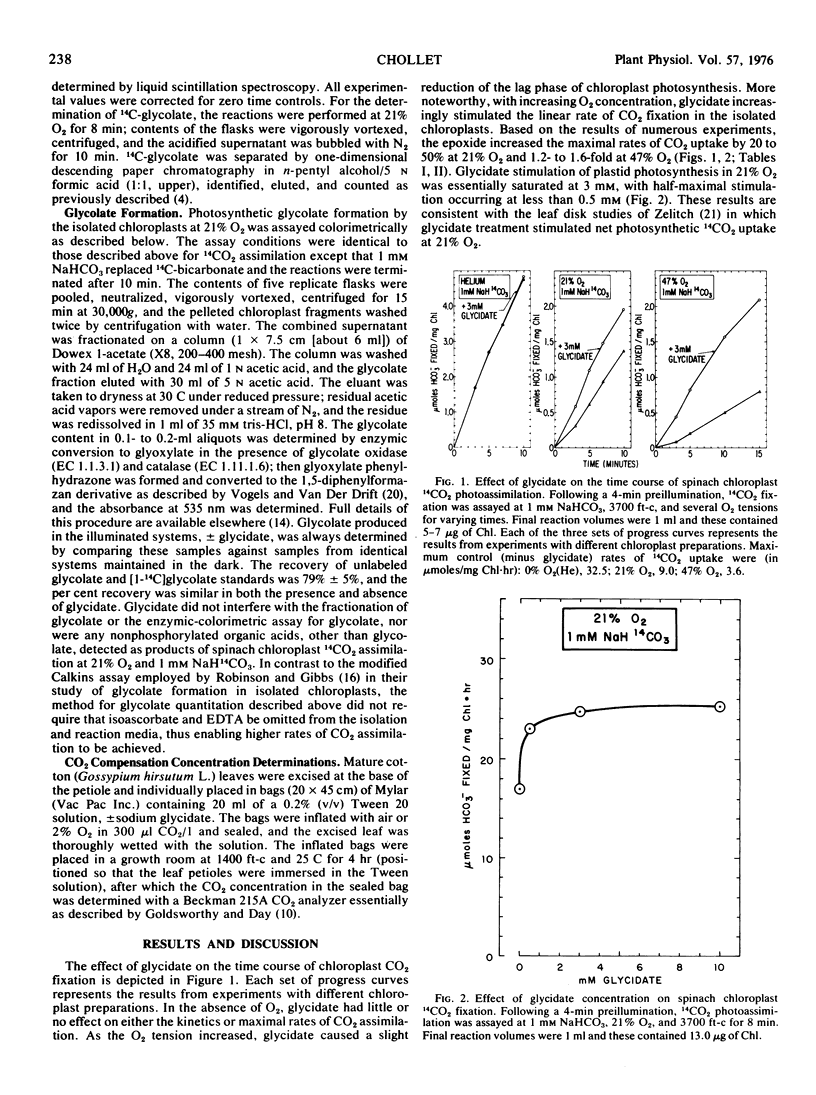
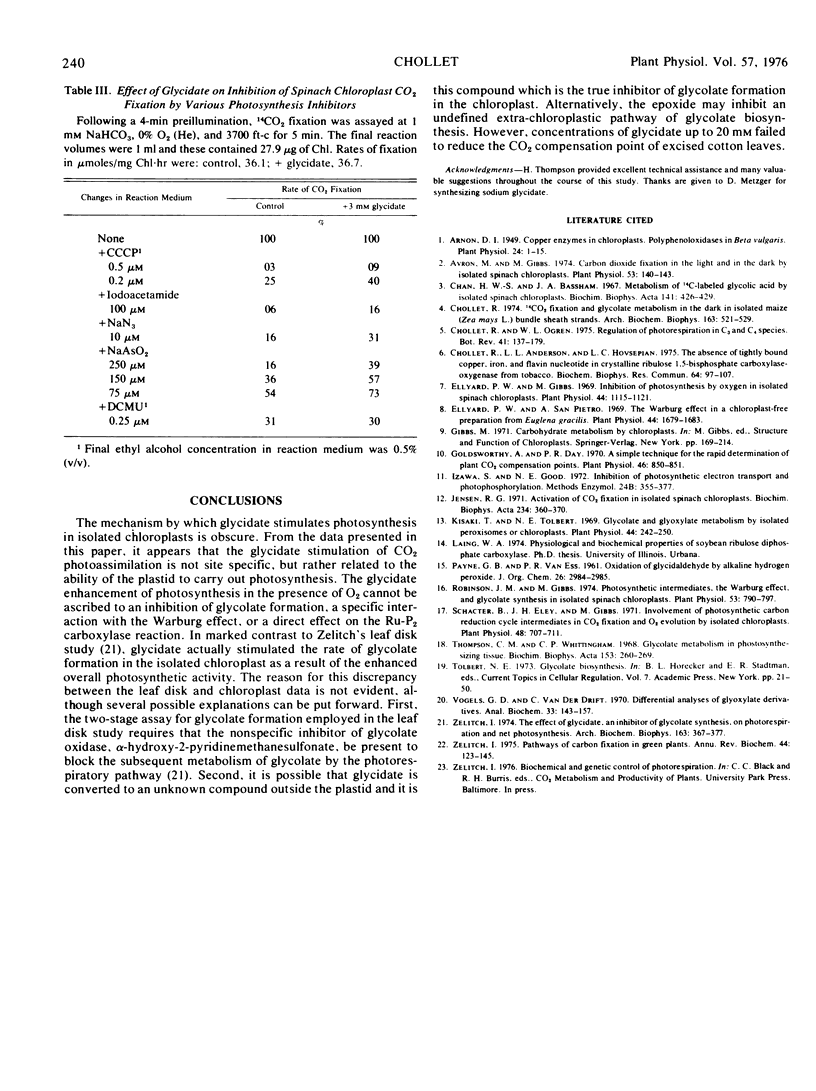
Glycidate (2,3-epoxypropionate) increased CO2 photoassimilation in intact spinach (Spinacia oleracea L.) chloroplasts in the presence of various inhibitors of photosynthesis, including O2, arsenite, azide, iodo-acetamide, and carbonylcyanide 3-chlorophenylhydrazone. Although the mechanism by which glycidate enhances photosynthesis is obscure, the stimulatory effect cannot be ascribed to either an inhibition of glycolate formation, a specific interaction with the O2 inhibition of photosynthesis, or a direct effect on the ribulose 1,5-bisphosphate carboxylase (EC 4.1.1.39) reaction. The lack of a differential effect of glycidate on photosynthesis and glycolate formation in the isolated chloroplast was confirmed in whole leaf studies by the CO2 compensation concentration assay. These results are at variance with the report that glycidate stimulates net photosynthesis in tobacco leaf disks by irreversibly inhibiting glycolate formation and thus photorespiration (Zelitch, I., 1974, Arch. Biochem. Biophys. 163: 367-377).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R. 14CO2 fixation and glycolate metabolism in the dark in isolated maize (Zea mays L.) bundle sheath strands. Arch Biochem Biophys. 1974 Aug;163(2):521–529. doi: 10.1016/0003-9861(74)90510-4. [DOI] [PubMed] [Google Scholar]
- Chollet R., Anderson L. L., Hovsepian L. C. The absence of tightly bound copper, iron, and flavin nucleotide in crystalline ribulose 1,5-bisphosphate carboxylase-oxygenase from tobacco. Biochem Biophys Res Commun. 1975 May 5;64(1):97–107. doi: 10.1016/0006-291x(75)90224-7. [DOI] [PubMed] [Google Scholar]
- Ellyard P. W., San Pietro A. The Warburg effect in a chloroplast-free preparation from Euglena gracilis. Plant Physiol. 1969 Dec;44(12):1679–1683. doi: 10.1104/pp.44.12.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy A., Day P. R. A Simple Technique for the Rapid Determination of Plant CO(2) Compensation Points. Plant Physiol. 1970 Dec;46(6):850–851. doi: 10.1104/pp.46.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G. Activation of CO 2 fixation in isolated spinach chloroplasts. Biochim Biophys Acta. 1971 Jun 15;234(3):360–370. doi: 10.1016/0005-2728(71)90203-9. [DOI] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B., Eley J. H., Gibbs M. Involvement of Photosynthetic Carbon Reduction Cycle Intermediates in CO(2) Fixation and O(2) Evolution by Isolated Chloroplasts. Plant Physiol. 1971 Dec;48(6):707–711. doi: 10.1104/pp.48.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Glycollate metabolism in photosynthesising tissue. Biochim Biophys Acta. 1968 Jan 15;153(1):260–269. doi: 10.1016/0005-2728(68)90168-0. [DOI] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]
- Zelitch I. The effect of glycidate, an inhibitor of glycolate synthesis, on photorespiration and net photosynthesis. Arch Biochem Biophys. 1974 Jul;163(1):367–377. doi: 10.1016/0003-9861(74)90488-3. [DOI] [PubMed] [Google Scholar]
- Zelitch I. The effect of glycidate, an inhibitor of glycolate synthesis, on photorespiration and net photosynthesis. Arch Biochem Biophys. 1974 Jul;163(1):367–377. doi: 10.1016/0003-9861(74)90488-3. [DOI] [PubMed] [Google Scholar]


