Abstract
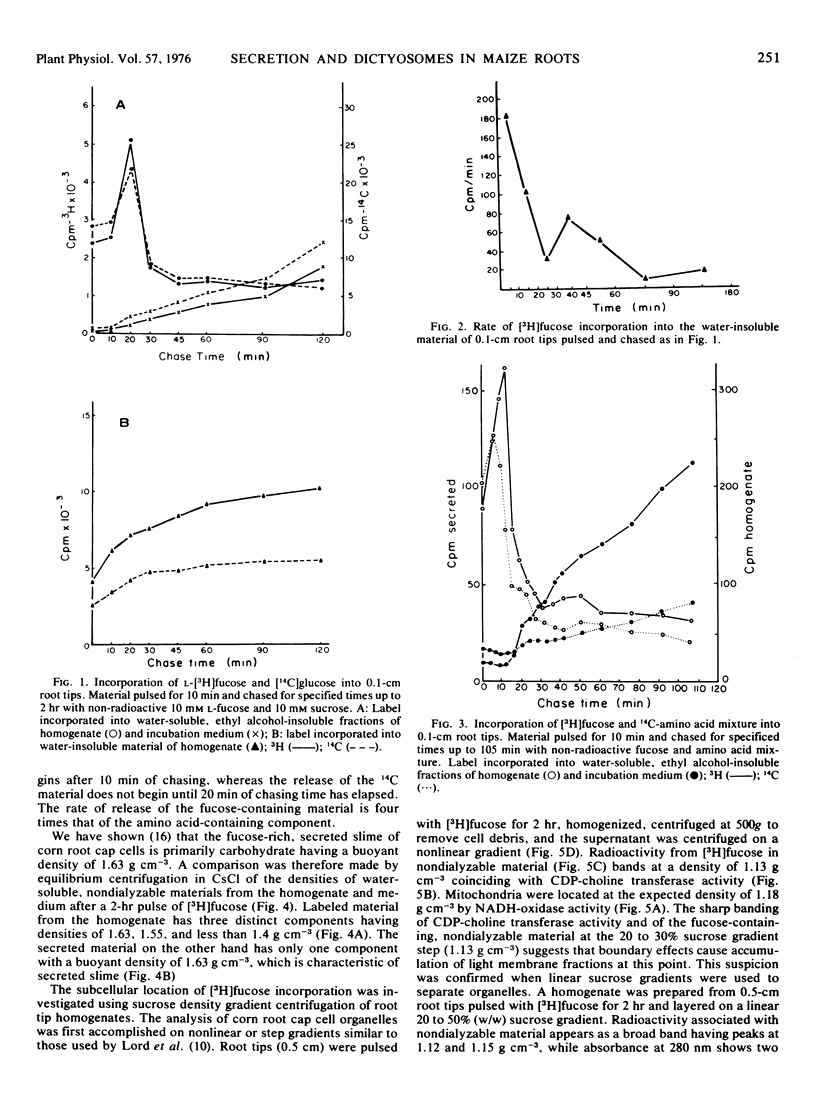
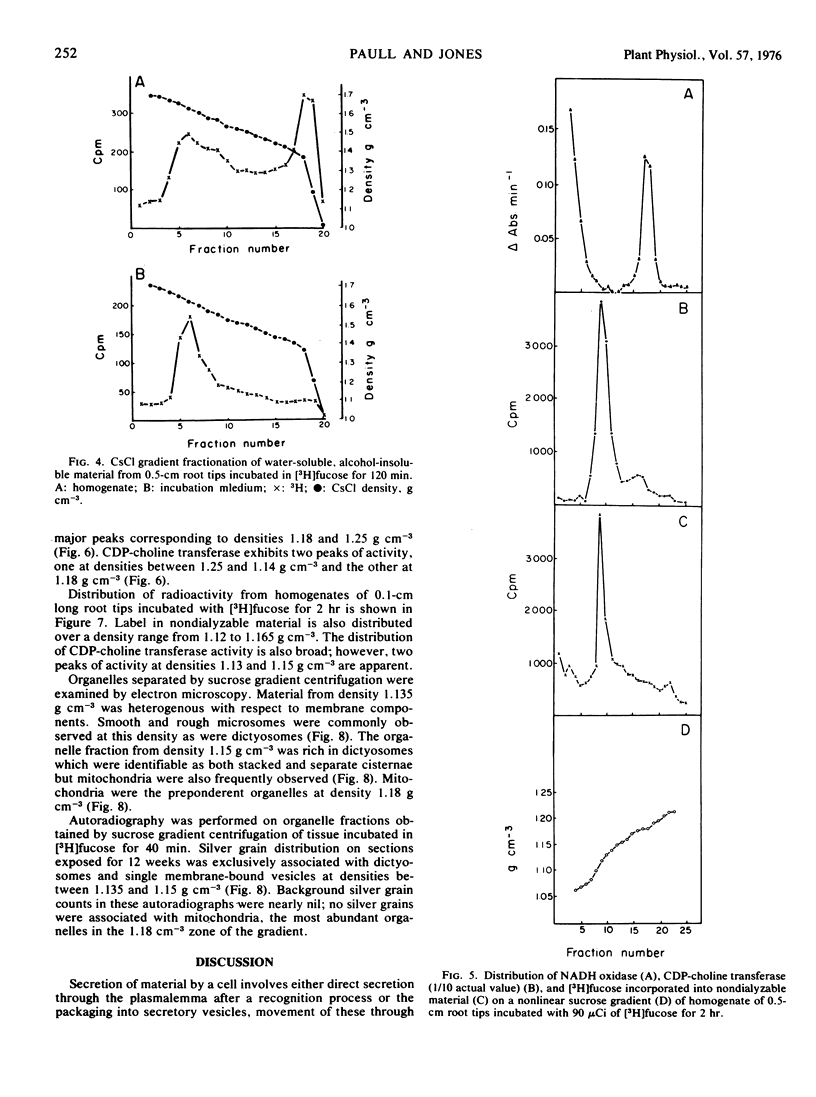
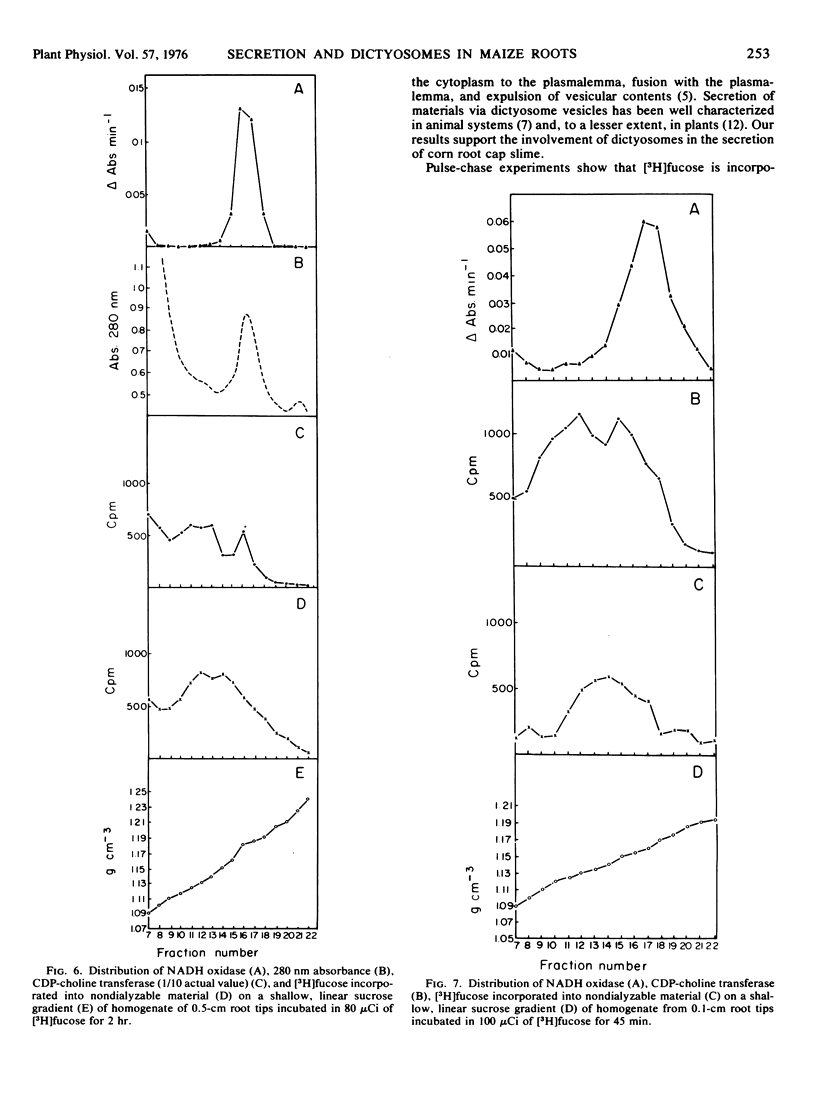
The involvement of dictyosomes and their vesicles in secretion of slime by maize root cap cells is demonstrated by kinetic and organelle fractionation experiments using l-fucose as a specific marker for the secreted slime. Pulse-chase experiments show that l-[1-3H]fucose is incorporated into two distinct fractions of root cap cells. Incorporation into a water-soluble, ethyl alcohol-insoluble fraction of the homogenate has a peak at 20 minutes of chasing followed by rapid loss of label. Seventy per cent of the radioactivity in this fraction is secreted from the tissue during a 2-hour chase period. Incorporation of label from [3H]fucose into a water-insoluble fraction is kinetically different suggesting that in situ incorporation of label is occurring into the cell wall. Labeling of the water-soluble, ethyl alcohol-insoluble fraction with an 14C-amino acid mixture differs from that of [3H]fucose. Thus, while release of the [3H]fucose-containing polymer begins after 10 to 15 minutes of chasing, the release of the 14C-amino acid polymer is delayed an additional 5 to 10 minutes and occurs at a lower rate. Cesium chloride density gradient centrifugation of secreted material labeled with radioactivity from [3H]fucose indicates the presence of only one major component having a buoyant density similar to that of purified root cap slime (1.63 g cm−3). Sucrose density gradient centrifugation of homogenates of [3H]fucose-labeled root cap tissue shows that radioactivity in nondialyzable material occurs as a broad band between densities 1.12 and 1.18 g cm−3 with a peak at density 1.15 g cm−3, the same density at which dictyosomes were localized by electron microscopy. Autoradiography of organelle fractions shows that radioactivity was associated almost exclusively with dictyosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L. Electron Transport and Cytochromes of Sub-Cellular Particles from Cauliflower Buds. Plant Physiol. 1957 Nov;32(6):619–625. doi: 10.1104/pp.32.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MACKLER B. Studies on the electron transport system. II. On the "opening" phenomenon. Biochim Biophys Acta. 1956 Jul;21(1):1–6. doi: 10.1016/0006-3002(56)90086-5. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E., Morré D. J. Cytomembrane differentiation in the endoplasmic reticulum-Golgi apparatus-vesicle complex. Science. 1968 Jul 12;161(3837):171–173. doi: 10.1126/science.161.3837.171. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Nyquist S., Rivera E. Lecithin Biosynthetic Enzymes of Onion Stem and the Distribution of Phosphorylcholine-Cytidyl Transferase among Cell Fractions. Plant Physiol. 1970 Jun;45(6):800–804. doi: 10.1104/pp.45.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull R. E., Johnson C. M., Jones R. L. Studies on the secretion of maize root cap slime: I. Some properties of the secreted polymer. Plant Physiol. 1975 Aug;56(2):300–306. doi: 10.1104/pp.56.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull R. E., Jones R. L. Studies on the Secretion of Maize Root Cap Slime: II. Localization of Slime Production. Plant Physiol. 1975 Aug;56(2):307–312. doi: 10.1104/pp.56.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]



