Abstract
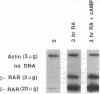
Expression of the retinoic acid receptors alpha and beta (RAR-alpha and RAR-beta) was examined in F9 cells, an embryonal carcinoma cell model established for the study of retinoid metabolism and function. Addition of retinoic acid to F9 cell medium caused a dose-dependent increase in RAR-beta mRNA within 3 hr that reached 5- to 30-fold greater than the constitutively expressed mRNA by 24 hr. The elevation in mRNA resulted from increased transcription, as demonstrated by nuclear run-on transcription, did not require protein synthesis, and required the constant presence of retinoic acid. N6,O2'-Dibutyryl-cAMP attenuated the retinoic acid-induced increase in RAR-beta mRNA by a post-transcriptional mechanism. In contrast, RAR-alpha mRNA in F9 stem cells was affected less (1.2- to 1.4-fold increase) by retinoic acid and decreased 3-fold transiently when fresh serum was added to the medium. Differentiation of F9 cells resulted in increased steady-state levels of RAR-beta mRNA in primitive (4-fold), parietal (3-fold), and visceral (8-fold) endoderm but decreased steady-state levels of RAR-alpha mRNA in primitive (2-fold), parietal (3-fold), and visceral (1.4-fold) endoderm. These data demonstrate that RAR-beta is a primary target gene for retinoic acid in a characterized model of retinoid function, indicate that constitutive expression of both RAR-beta and RAR-alpha is dependent upon the differentiation state, and suggest hormonal modulation of RAR-beta by cAMP and modulation of RAR-alpha by serum factors. These results distinguish the effects of serum, cAMP, and retinoic acid on the expression of RAR from the effects mediated by differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton M. C., Shapiro D. J. Transient administration of estradiol-17 beta establishes an autoregulatory loop permanently inducing estrogen receptor mRNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7119–7123. doi: 10.1073/pnas.85.19.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Carlin B. E., Durkin M. E., Bender B., Jaffe R., Chung A. E. Synthesis of laminin and entactin by F9 cells induced with retinoic acid and dibutyryl cyclic AMP. J Biol Chem. 1983 Jun 25;258(12):7729–7737. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Wald G. THE BIOLOGICAL FUNCTION OF VITAMIN A ACID. Proc Natl Acad Sci U S A. 1960 May;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G., Tickle C., Alberts B. M. Studies on the mechanism of retinoid-induced pattern duplications in the early chick limb bud: temporal and spatial aspects. J Cell Biol. 1985 Nov;101(5 Pt 1):1913–1920. doi: 10.1083/jcb.101.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Hu L., Gudas L. J. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta, and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990 Jan;10(1):391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Kelley D. C., Miles M. F., Milkowski D. M. Cyclic AMP regulation of lactate dehydrogenase. Isoproterenol and N6,O2-dibutyryl cyclic amp increase the rate of transcription and change the stability of lactate dehydrogenase a subunit messenger RNA in rat C6 glioma cells. J Biol Chem. 1983 Apr 25;258(8):5312–5318. [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Calie P., Wicks W. D. Differences in rates of tyrosine aminotransferase deinduction with cyclic AMP and glucocorticoids. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5778–5782. doi: 10.1073/pnas.79.19.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M., Ong D. E., Summerbell D., Chytil F. Spatial distribution of cellular protein binding to retinoic acid in the chick limb bud. Nature. 1988 Oct 20;335(6192):733–735. doi: 10.1038/335733a0. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Napoli J. L. Identification of 5,6-epoxyretinoic acid as an endogenous retinol metabolite. J Biol Chem. 1982 Feb 25;257(4):1730–1735. [PubMed] [Google Scholar]
- Pacha R. F., Condit R. C. Characterization of a temperature-sensitive mutant of vaccinia virus reveals a novel function that prevents virus-induced breakdown of RNA. J Virol. 1985 Nov;56(2):395–403. doi: 10.1128/jvi.56.2.395-403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rees J. L., Daly A. K., Redfern C. P. Differential expression of the alpha and beta retinoic acid receptors in tissues of the rat. Biochem J. 1989 May 1;259(3):917–919. doi: 10.1042/bj2590917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. Retinoic acid receptor. Towards a biochemistry of morphogenesis. Nature. 1987 Dec 3;330(6147):420–421. doi: 10.1038/330420a0. [DOI] [PubMed] [Google Scholar]
- Spielholz C., Schlichter D., Wicks W. D. Cyclic adenonosine monophosphate does not affect the stability of the messenger ribonucleic acid for tyrosine aminotransferase in cultured hepatoma cells. Mol Endocrinol. 1988 Apr;2(4):344–349. doi: 10.1210/mend-2-4-344. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Sawey M. J. Studies on the effect of retinoids on the differentiation of teratocarcinoma stem cells in vitro and in vivo. Dev Biol. 1980 Jul;78(1):76–85. doi: 10.1016/0012-1606(80)90319-x. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. B., Shields C. O., Brettel L. M., Napoli J. L. Assessment of retinoid-induced differentiation of F9 embryonal carcinoma cells with an enzyme-linked immunoadsorbent assay for laminin: statistical comparison of dose-response curves. Anal Biochem. 1987 Feb 1;160(2):267–274. doi: 10.1016/0003-2697(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- de The H., Marchio A., Tiollais P., Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989 Feb;8(2):429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]