Abstract
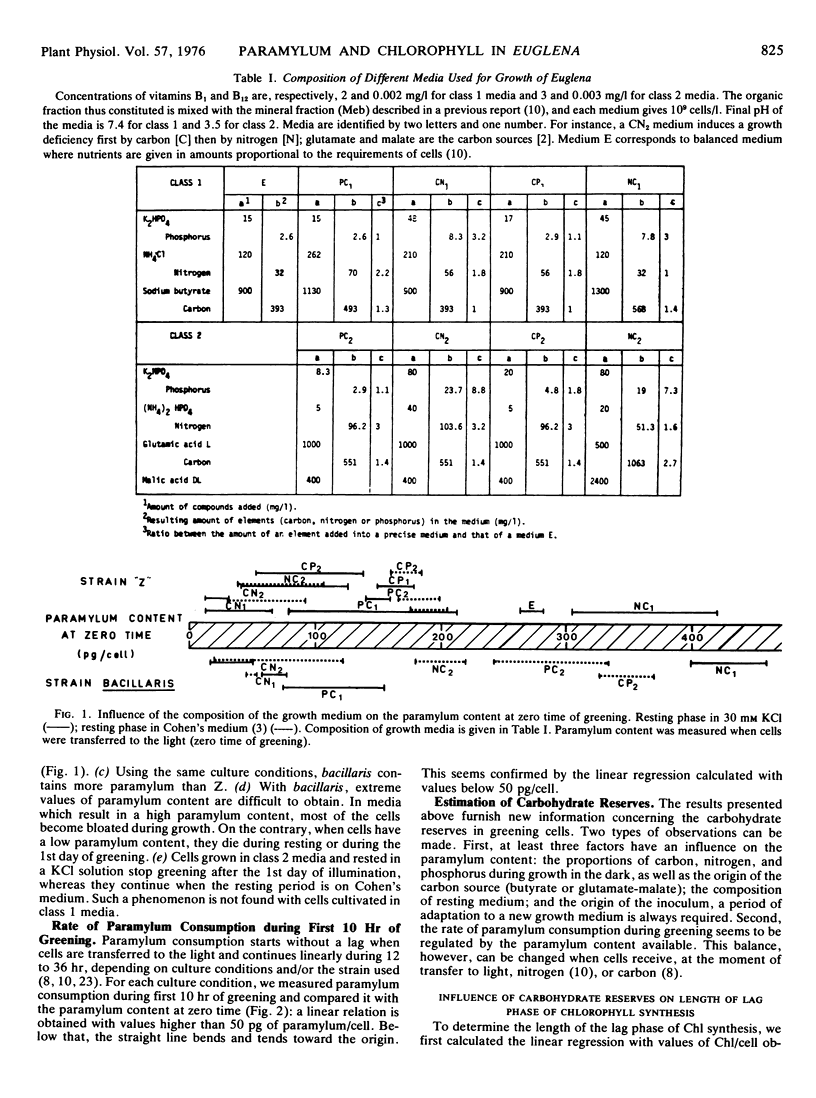
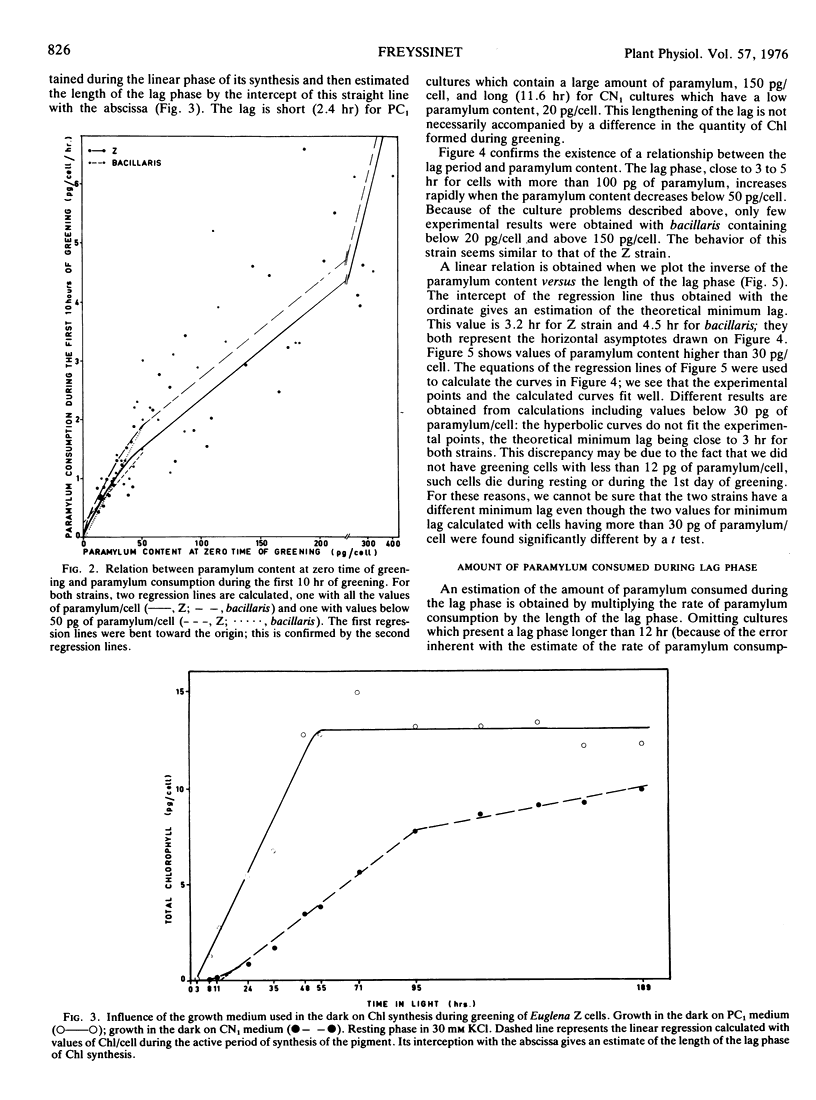
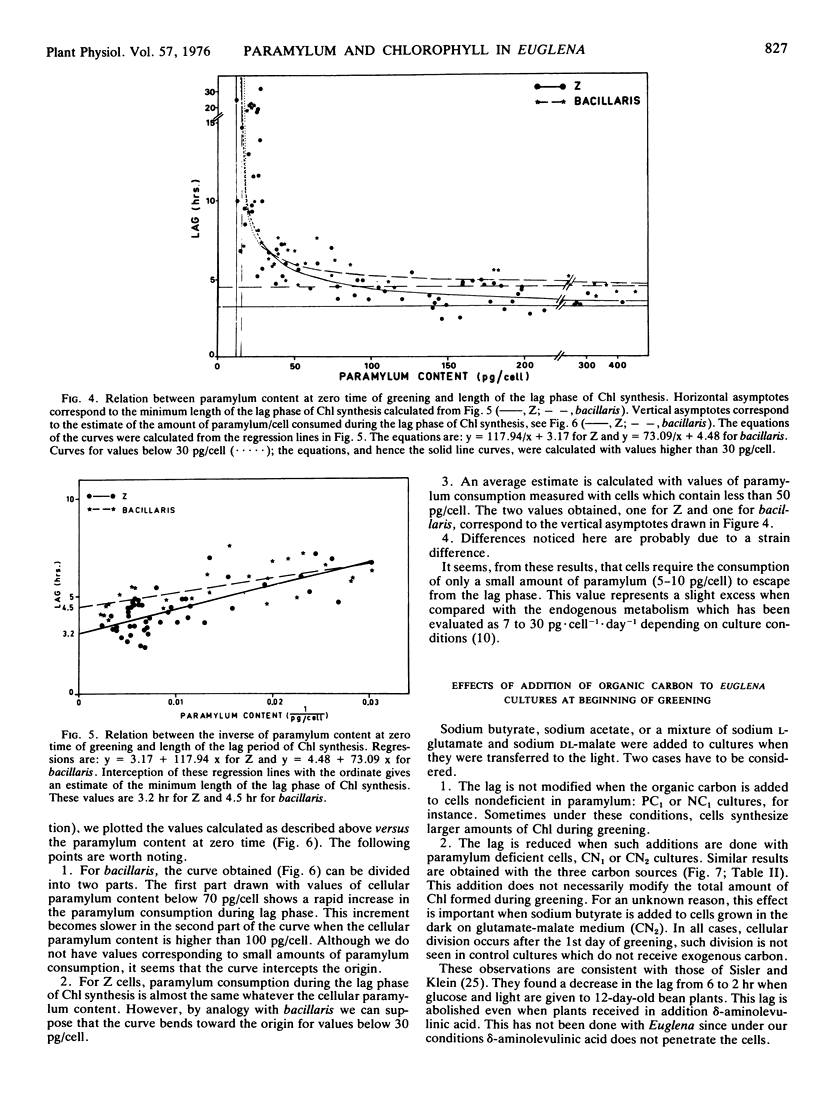
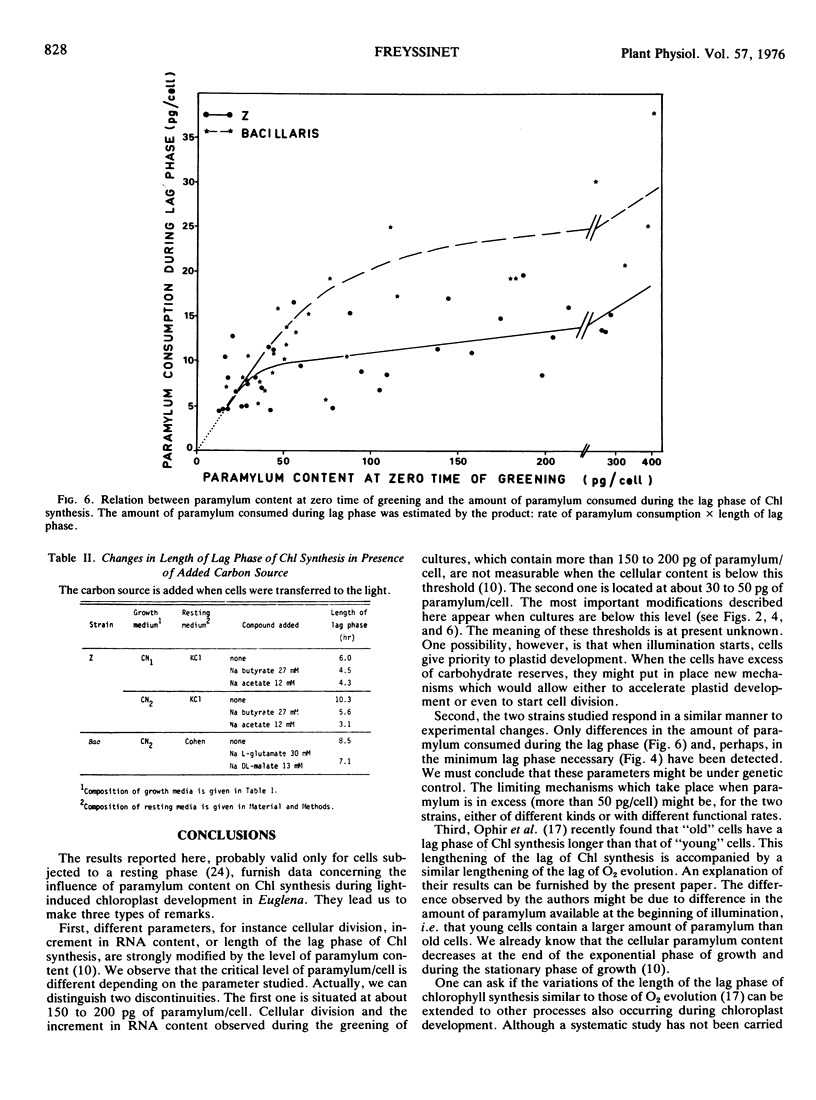
Euglena cells, strains Z and bacillaris, were grown in the dark under various nutritional deficiencies. After 3 days of nondivision, cells were transferred to the light, and the following parameters were measured: the paramylum content at the time of illumination (zero time), the rate of paramylum consumption during the first 10 hours of greening, and the length of the lag phase of chlorophyll synthesis. Similar results were obtained with both strains and can be summarized as follows. (a) The use of various nutritional deficiencies allows the control, to a certain extent, of the amount of paramylum present at zero time. (b) The rate of paramylum consumption is proportional to the cellular paramylum content for values in excess of 50 picograms/cell. (c) The length of the lag phase increases rapidly when the cellular content of paramylum decreases below 50 picograms. This period can be greatly diminished by the addition of an exogenous organíc carbon source. (d) The amount of paramylum (rate of paramylum consumption × length of lag phase) consumed during the lag phase is around 5 to 10 picograms/cell for cells which contain less than 50 picograms of paramylum/cell. It increases when the cellular paramylum content increases, this increment being more rapid for bacillaris than for Z cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Diamond J., Schiff J. A., Kelner A. Photoreactivating enzyme from euglena and the control of its intracellular level. Arch Biochem Biophys. 1975 Apr;167(2):603–614. doi: 10.1016/0003-9861(75)90504-4. [DOI] [PubMed] [Google Scholar]
- Dwyer M. R., Smillie R. M. A light-induced beta-1,3-glucan breakdown associated with the differentiation of chloroplasts in Euglena gracilis. Biochim Biophys Acta. 1970 Sep 1;216(2):392–401. doi: 10.1016/0005-2728(70)90231-8. [DOI] [PubMed] [Google Scholar]
- Dwyer M. R., Smillie R. M. Beta-1,3-glucan: a source of carbon and energy for chloroplast development in Euglena gracilis. Aust J Biol Sci. 1971 Feb;24(1):15–22. doi: 10.1071/bi9710015. [DOI] [PubMed] [Google Scholar]
- Egan J. M., Carell E. F. Studies on Chloroplast Development and Replication in Euglena: III. A Study of the Site of Synthesis of Alkaline Deoxyribonuclease Induced during Chloroplast Development in Euglena gracilis. Plant Physiol. 1972 Sep;50(3):391–395. doi: 10.1104/pp.50.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet G., Schiff J. A. The Chloroplast and Cytoplasmic Ribosomes of Euglena: II. Characterization of Ribosomal Proteins. Plant Physiol. 1974 Apr;53(4):543–554. doi: 10.1104/pp.53.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L. I., Egan J., Reynolds R. J., Nix C. E., Schiff J. A., Barnett W. E. The sites of transcription and translation for Euglena chloroplastic aminoacyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1974 May;71(5):1910–1914. doi: 10.1073/pnas.71.5.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann P. La synthese des RNA ribosomiques au cours de l'eclairement d'Euglenes etiolees. Biochim Biophys Acta. 1974 Jul 11;353(3):301–312. [PubMed] [Google Scholar]
- Heizmann P., Trabuchet G., Verdier G., Freyssinet G., Nigon V. Influence de l'éclairement sur l'évolution des polysomes dans des cultures d'Euglena gracilis étiolées. Biochim Biophys Acta. 1972 Aug 16;277(1):149–160. [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir I., Talmon A., Polak-Charcon S., Ben-Shaul Y. Aspects of structure and photosynthetic competence of Euglena plastids under conditions of greening and degreening. Protoplasma. 1975;84(3-4):283–295. doi: 10.1007/BF01279358. [DOI] [PubMed] [Google Scholar]
- Richard F., Nigon V. La syntheèse de l'acide delta-aminolévulinique et de la chlorophylle lors de l'éclairement d'Euglena gracilis étiolées. Biochim Biophys Acta. 1973 Jun 20;313(1):130–149. [PubMed] [Google Scholar]
- SMILLIE R. M., EVANS W. R., LYMAN H. METABOLIC EVENTS DURING THE FORMATION OF A PHOTOSYNTHETIC FROM A NONPHOTOSYNTHETIC CELL. Brookhaven Symp Biol. 1964 Mar;16:89–108. [PubMed] [Google Scholar]
- Salvador G. F. Evolution du systéme plastidien d'Euglena gracilis etiolée a l'obscurité aprés stimulation lumineuse. Exp Cell Res. 1973 Jun;79(2):479–484. doi: 10.1016/0014-4827(73)90476-x. [DOI] [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A., Goldstein N. H. Events surrounding the early development of euglena chloroplasts: v. Control of paramylum degradation. Plant Physiol. 1975 Aug;56(2):313–317. doi: 10.1104/pp.56.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]


