Abstract
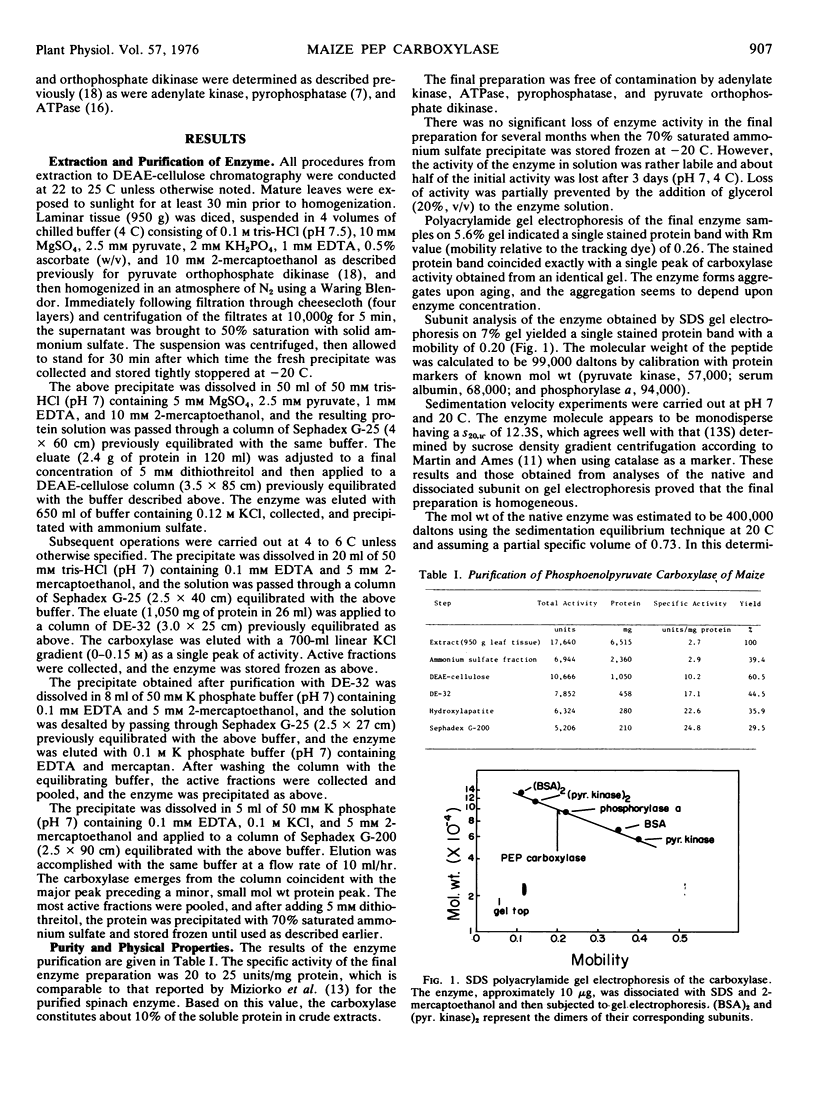
Phosphoenolpyruvate carboxylase has been purified to homogeneity from maize (Zea mays L. var. Golden Cross Bantam T51) leaves. The ratio of specific activities in crude extracts and the purified enzyme suggests that the enzyme is a major soluble protein in the tissue. The enzyme has a sedimentation coefficient (s20,w) of 12.3S and a molecular weight, determined by sedimentation equilibrium, of 400,000 daltons. Dissociation of the enzyme and electrophoresis on dodecyl sulfate polyacrylamide gels yields a single stained band which corresponds to a subunit weight of 99,000 daltons. Thus it appears that the native enzyme is composed of four identical or similar polypeptide chains.
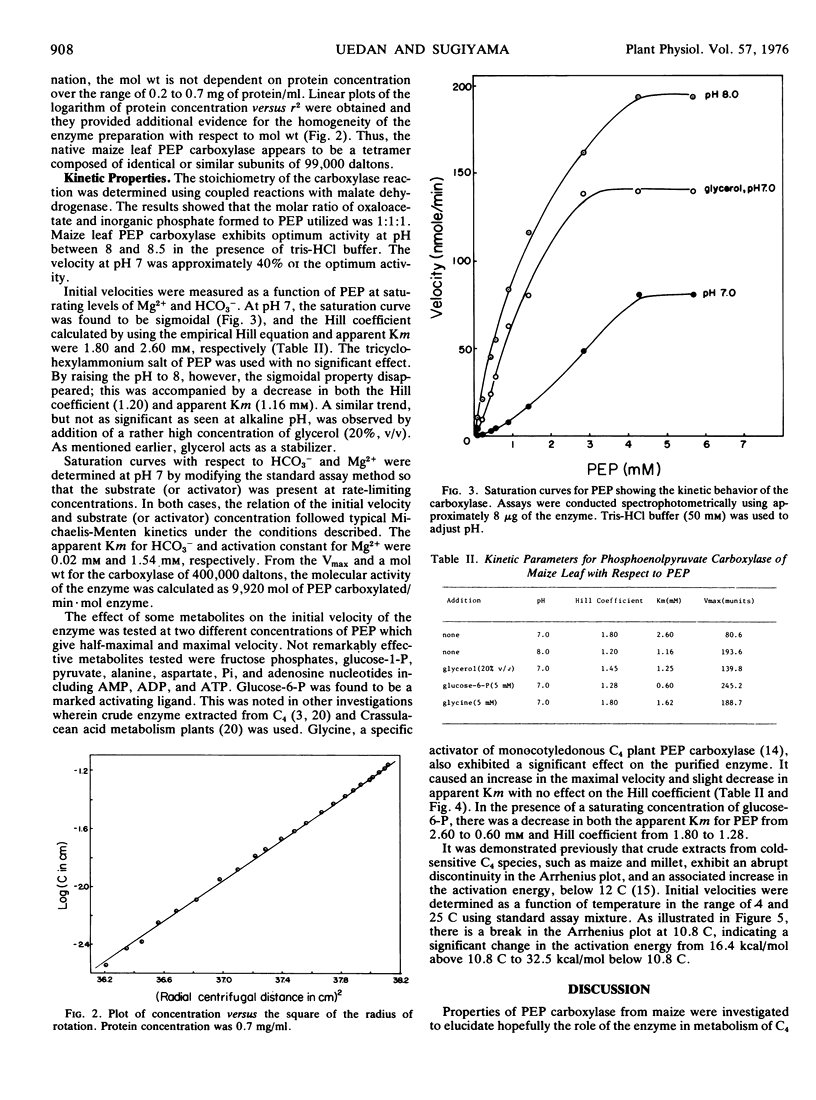
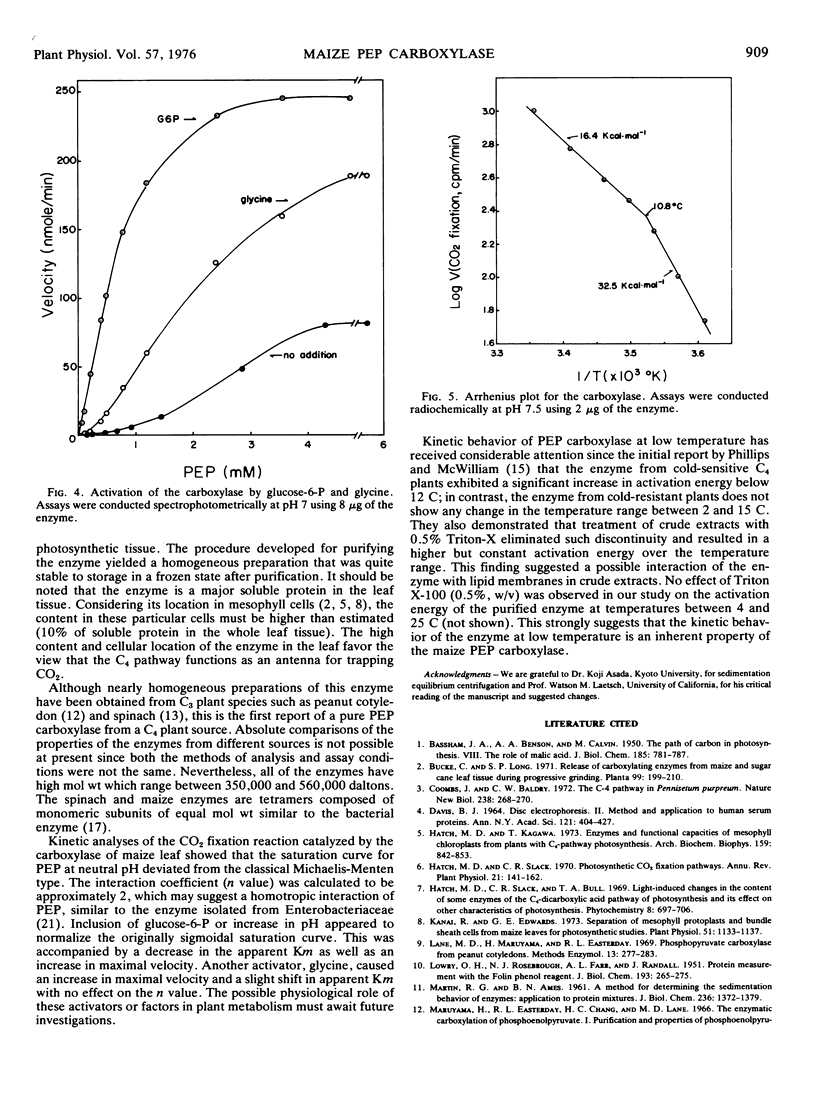
The enzyme yields cooperative rate-concentration plots (Hill number of 2) with phosphoenolpyruvate as the variable substrate at pH 7. This cooperativity disappears in the presence of an activator, glucose-6-P, or by raising the pH of the assay mixture to 8. Glycerol (20%, v/v) exerts a similar effect. The enzyme is also activated in the presence of glycine which causes an increase in Vmax without significant effect on the apparent Km for phosphoenolpyruvate and Hill number. The apparent Km for HCO3− is 0.02 mm, and the activation constant for Mg2+ is 1.54 mm at pH 7. There is an abrupt discontinuity in Arrhenius plots and an associated increase in activation energy below 10.8 C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSHAM J. A., BENSON A. A., CALVIN M. The path of carbon in photosynthesis. J Biol Chem. 1950 Aug;185(2):781–787. [PubMed] [Google Scholar]
- Coombs J., Baldry C. W. C-4 pathway in Pennisetum purpureum. Nat New Biol. 1972 Aug 30;238(87):268–270. doi: 10.1038/newbio238268a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Menzies R. A., Hsu D. S. The pyruvate-phosphate dikinase reaction. The fate of phosphate and the equilibrium. J Biol Chem. 1968 Oct 25;243(20):5486–5491. [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


