Abstract
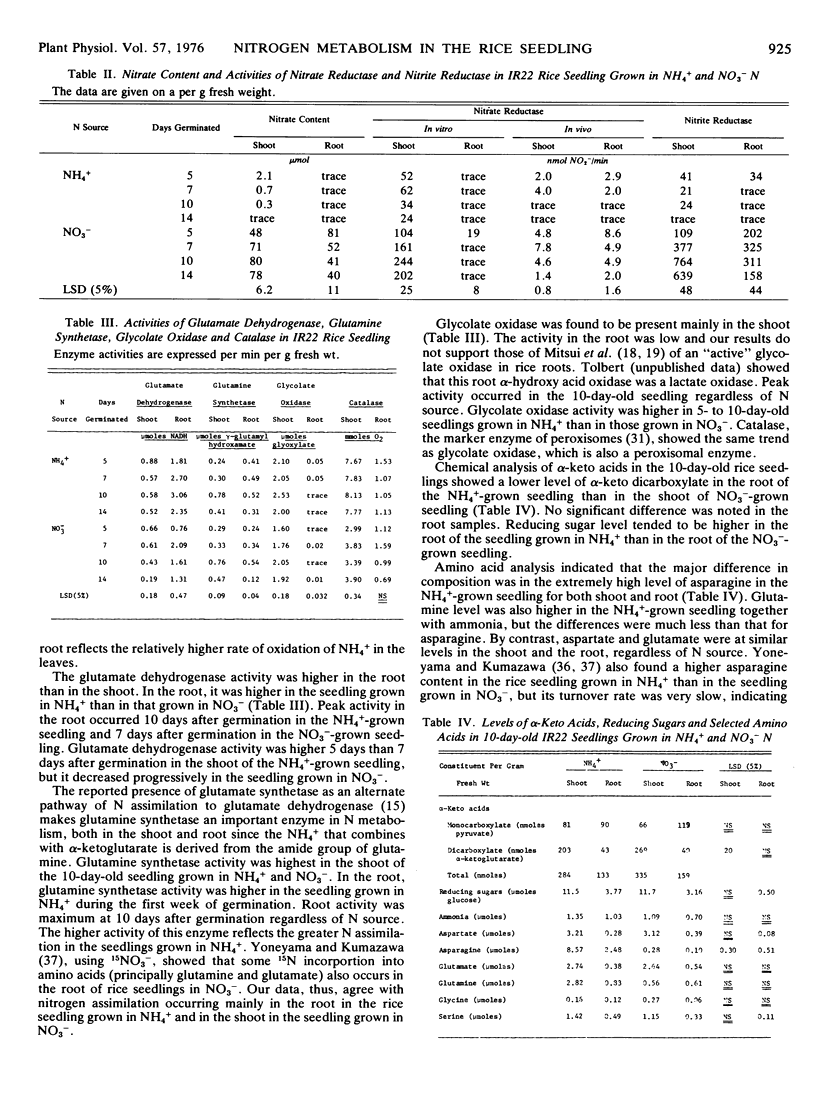
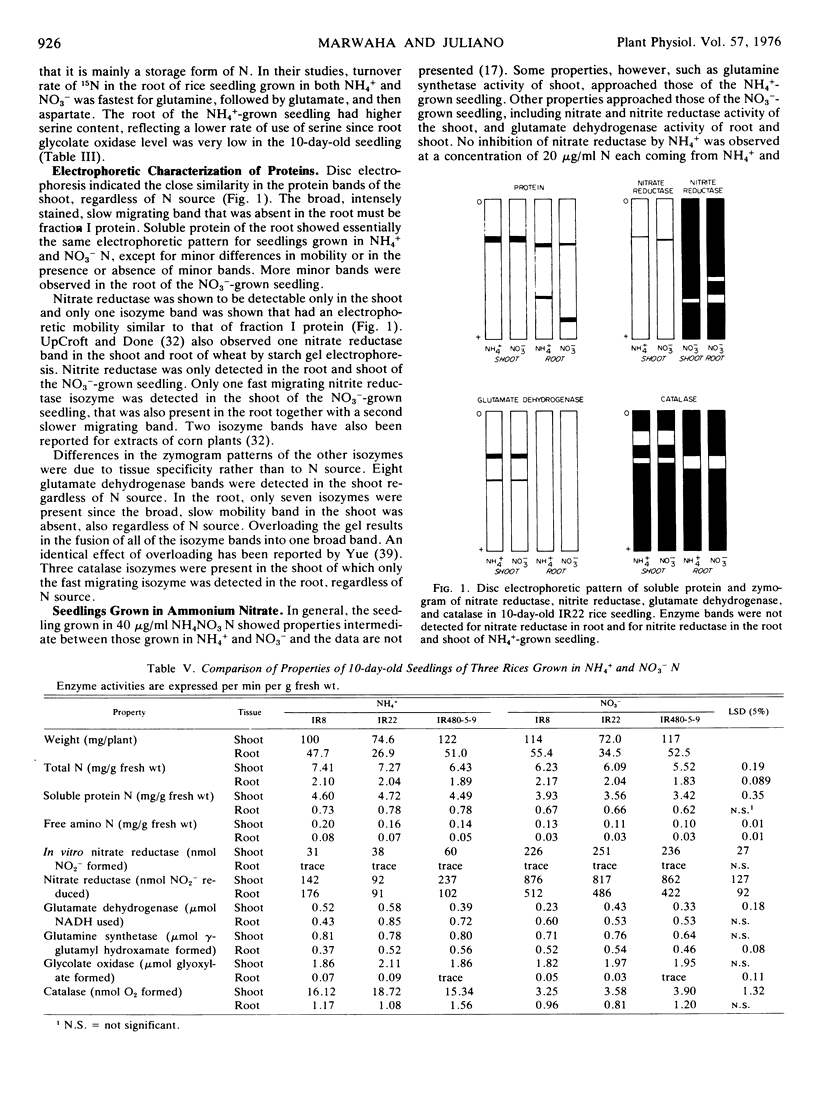
The effects of nitrogen source NO3− or NH4+ on nitrogen metabolism during the first 2 weeks of germination of the rice seedling (Oryza sativa L., var. IR22) grown in nutrient solution containing 40 μg/ml N were studied. Total, soluble protein, and free amino N levels were higher in the NH4+-grown seedling, particularly during the 1st week of germination. Asparagine accounted for most of the difference in free amino acid level, in both the root and the shoot. Nitrate and nitrite reductase activities were present mainly in the shoot and were higher in the NO3−-grown seedling, whereas the activity of glutamate dehydrogenase and glutamine synthetase in the root tended to be lower than that of the NH4+-grown seedling during the 1st week of germination. Glycolate oxidase and catalase activities were present mainly in the shoot. Maximum activity of the above five enzymes occurred 7 to 10 days after germination. Differences in the zymograms of nitrate reductase, glutamate dehydrogenase, and catalase were mainly between shoot and root and not from N source. Nitrite reductase bands were observed only in plants grown in plants grown in NO3−.
Ten-day-old seedlings of three rices differing in level of grain protein did not differ in the level of N fractions and of enzyme activities, which were consistent with their differences in grain protein content.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. H. Isolation of glutamine synthetase and glutamotransferase from green peas. J Biol Chem. 1953 Apr;201(2):661–672. [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano B. O., Antonio A. A., Esmama B. V. Effects of protein content on the distribution and properties of rice protein. J Sci Food Agric. 1973 Mar;24(3):295–306. doi: 10.1002/jsfa.2740240306. [DOI] [PubMed] [Google Scholar]
- Kedenburg C. P. A lithium buffer system for accelerated single-column amino acid analysis in physiological fluids. Anal Biochem. 1971 Mar;40(1):35–42. doi: 10.1016/0003-2697(71)90081-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Palmiano E. P., Juliano B. O. Changes in the Activity of Some Hydrolases, Peroxidase, and Catalase in the Rice Seed during Germination. Plant Physiol. 1973 Sep;52(3):274–277. doi: 10.1104/pp.52.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C. M., Cagampang G. B., Esmama B. V., Monserrate R. U., Juliano B. O. Protein metabolism in leaves and developing grains of rices differing in grain protein content. Plant Physiol. 1973 Mar;51(3):537–542. doi: 10.1104/pp.51.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Cataldo D. A., Peterson D. M. Use of protein in extraction and stabilization of nitrate reductase. Plant Physiol. 1974 May;53(5):688–690. doi: 10.1104/pp.53.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Shen T. C. The induction of nitrate reductase and the preferential assimilation of ammonium in germinating rice seedlings. Plant Physiol. 1969 Nov;44(11):1650–1655. doi: 10.1104/pp.44.11.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Yue S. B. Isoenzymes of glutamate dehydrogenase in plants. Plant Physiol. 1969 Mar;44(3):453–457. doi: 10.1104/pp.44.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]


